Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6235
Peer-review started: March 24, 2016
First decision: May 12, 2016
Revised: May 25, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 21, 2016
AIM: To investigate the role of activating transcription factor 4 (ATF4) in glucose deprivation (GD) induced colorectal cancer (CRC) drug resistance and the mechanism involved.
METHODS: Chemosensitivity and apoptosis were measured under the GD condition. Inhibition of ATF4 using short hairpin RNA in CRC cells under the GD condition and in ATF4-overexpressing CRC cells was performed to identify the role of ATF4 in the GD induced chemoresistance. Quantitative real-time RT-PCR and Western blot were used to detect the mRNA and protein expression of drug resistance gene 1 (MDR1), respectively.
RESULTS: GD protected CRC cells from drug-induced apoptosis (oxaliplatin and 5-fluorouracil) and induced the expression of ATF4, a key gene of the unfolded protein response. Depletion of ATF4 in CRC cells under the GD condition can induce apoptosis and drug re-sensitization. Similarly, inhibition of ATF4 in the ATF4-overexpressing CRC cells reintroduced therapeutic sensitivity and apoptosis. In addition, increased MDR1 expression was observed in GD-treated CRC cells.
CONCLUSION: These data indicate that GD promotes chemoresistance in CRC cells through up-regulating ATF4 expression.
Core tip: In this work, we demonstrated that glucose deprivation induces chemoresistance in colorectal cancer (CRC) cells through up-regulating ATF4 expression, and ATF4 is an attractive therapeutic target to combat therapeutic resistance in CRC cells.
- Citation: Hu YL, Yin Y, Liu HY, Feng YY, Bian ZH, Zhou LY, Zhang JW, Fei BJ, Wang YG, Huang ZH. Glucose deprivation induces chemoresistance in colorectal cancer cells by increasing ATF4 expression. World J Gastroenterol 2016; 22(27): 6235-6245
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6235
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death worldwide[1]. Chemotherapy is one of the basic treatments for CRC. However, more than half of CRC patients did not respond to conventional chemotherapy due to drug resistance. Multiple factors contribute to the failure of CRC chemotherapy, including multidrug resistance (MDR) and tumor heterogeneity[2]. Cancer cells with MDR phenotype simultaneously become resistant to multiple drugs with different structures or cellular targets[3]. The development of MDR is commonly mediated by multiple factors, including accelerated drug efflux, drug activation and inactivation, alterations in the drug target, repair of drug-induced damage, and escape from apoptosis[4].
Recent data showed that tumor microenvironment plays a key role in tumor MDR[5]. In the tumor microenvironment, the abnormal development of vasculature results in insufficient blood supply, which is a key reason for the tumor progression and has been associated with glucose deprivation (GD), chronic hypoxia and other nutrient stress. Increasing evidence indicates that GD promotes tumor cell survival and angiogenesis and induces drug resistance by inducing complex signaling pathways, including unfolded protein response (UPR)[6-11]. However, the molecular mechanisms by which cancer cells adapt to GD condition and inhibit drug-induced apoptosis remain poorly understood. Recent studies have indicated that the activating transcription factor 4 (ATF4) pathway, a key player in UPR signaling, is important in regulating malignant phenotypes in various types of human cancers, including breast cancer[12], CRC[13], and head and neck squamous cell carcinoma[14]. In cellular adaptation to tumor GD, the GD activates cell survival through PERK-dependent ATF4 expression. In addition, our previous work revealed that GD and amino acid deprivation promote tumor angiogenesis through activating ATF4[8,9]. Accumulated data strongly suggest that ATF4 is an important gene in regulating tumor survival under stress conditions, but the functional relationships among cell drug resistance, ATF4 and GD in CRC have not been fully elucidated.
In this study, we investigated whether and how GD affects drug resistance and apoptosis in CRC cells, and revealed that GD induces drug resistance and apoptosis inhibition by activating PERK/ATF4 signaling pathway.
Human CRC cell lines HCT116 and LoVo were obtained from ATCC. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 23.05 mmol/L glucose (Hyclone) supplemented with 10% fetal bovine serum (FBS; Gibco) and penicillin/streptomycin at 37 °C in a humidified incubator containing 5% CO2.
To mimic the GD condition of tumor microenvironment, HCT116 and LoVo cells were incubated for 48 h in DMEM containing 1.5 mmol/L glucose and 10% FBS containing about 0.5 mmol/L glucose at 37 °C in a humidified atmosphere containing 5% CO2.
For the cell proliferation assay, 1000 CRC cells were plated in 96-well plates and incubated for different time periods (24, 48, 72, and 96 h), and then the cell growth was detected with the Cell Counting Kit-8 (CCK-8, Dojindo, Japan) according to the manufacturer’s instructions. For the cell chemotherapy sensitivity assay, 2000 LoVo or HCT116 cells were plated in 96-well plates and treated with oxaliplatin (LOHP; range, 0-16 μg/mL) and 5-fluorouracil (5-FU; range, 0-1.6 μg/mL) for 48 h, and cell inhibition was then assessed by the CCK-8 assay.
Hoechst Staining was performed according to the manufacturer’s protocol (Beyotime, China). Cells were visualized with a DP70 inverted immunofluorescence microscope (Olympus). Cells with condensed and fragmented nuclei were judged to be apoptotic.
Total RNA was prepared from cultures on day 10, using the RNAiso reagent (TaKaRa, Japan) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using the HiFiScript cDNA Kit (CWBIO, China). Quantitative real-time RT-PCR analysis was performed to detect mRNA expression using UltraSYBR Mixture (CWBIO), with β-actin as an internal control. The sequences of primers used in this study were as follows: 5’-CGTGGTCTTTGCTTGGG TG-3’ and 5’-TGCGGTGCTTTGCTGGAAT-3’ for ATF4; 5’-GACCTATTGGGGTGTTTCG-3’ and 5’-CCTCAGCGGTTTCTTTCAT-3’ for Grp78; 5’-ATAGTGATAAAGGTTTCGGTT-3’ and 5’-ACAGGAGTTCTGGAAGGAG-3’ for PERK; 5’-AGTGTGACGTGGACATCCGCAAAG-3’ and 5’-ATCCACATCTGCTGGAAGGTGG AC-3’ for β-actin.
Cells were lysed with RIPA buffer and incubated on ice for 30 min. After centrifugation, protein concentration was measured with BCA Protein Assay Reagent (CWBIO). Cell lysates dissolved in sample buffer were separated using SDS-PAGE and transferred to a polyvinylidene fluoride membrane. After blocking with Tris-buffered saline containing Tween-20 containing 5% milk, the membrane was immunoblotted with appropriate primary antibodies, including anti-Grp78 (Santa Cruz, United States), anti-PERK (Cell Signaling, United States), anti-ATF4 (Santa Cruz), anti-MDR1 (Santa Cruz) and anti-β-actin (Abcam, United States), followed by incubation with goat anti-mouse immunoglobulin (Ig) or anti-rabbit Ig conjugated with horseradish peroxidase. After washing, the membrane was developed using Chemiluminescent Substrate (CWBIO).
Green fluorescent protein-expressing lentiviral plasmids expressing short hairpin RNA (shRNA) against human ATF4 (Vehicle-shATF4) were obtained from Open Biosystems (Carlsbad, CA), and ATF4 lentiviral plasmid (Vehicle-ATF4) was constructed as described in our previous work[8]. The Vehicle, Vehicle-shATF4, Vehicle and Vehicle-ATF4 plasmids were cotransfected into HEK-293T cells along with the packaging plasmid ps-PAX2 and the envelope plasmid pMD2G using Lipofectamine 2000 (Invitrogen). Virus particles were harvested 48 h after cotransfection. Then, the particles were individually used to infect HCT116 and LoVo cells. The cells were then harvested 3 d after infection for Western blot and qRT-PCR validation.
LoVo or HCT116 cells were plated in 6-well plates and treated with LOHP (0.1 μg/mL) and 5-FU (0.05 μg/mL) for 48 h. The cells were then harvested and subjected to apoptosis analysis using an Annexin V/7-AAD Apoptosis Detection Kit (CWBIO).
Each experiment was repeated at least three times. The data are presented as the mean ± SD. Differences between groups were analyzed with Student’s t test. All statistical analyses were performed using GraphPad Prism 5 software. The significance level was set at 0.05.
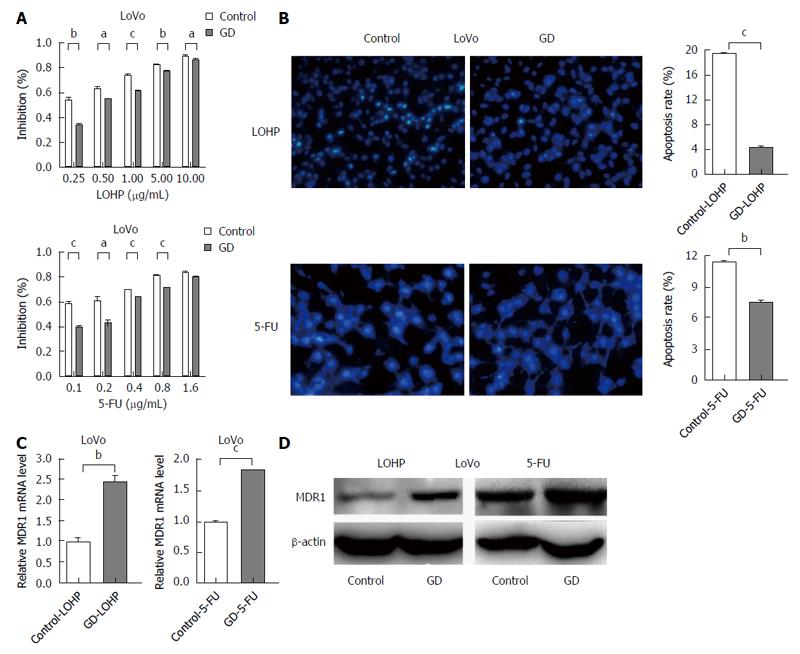
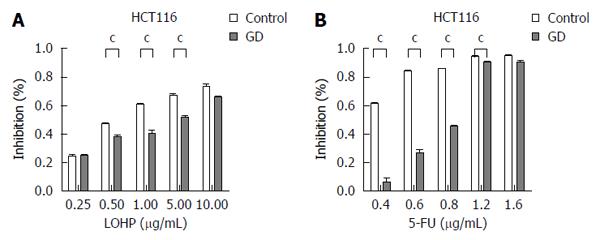
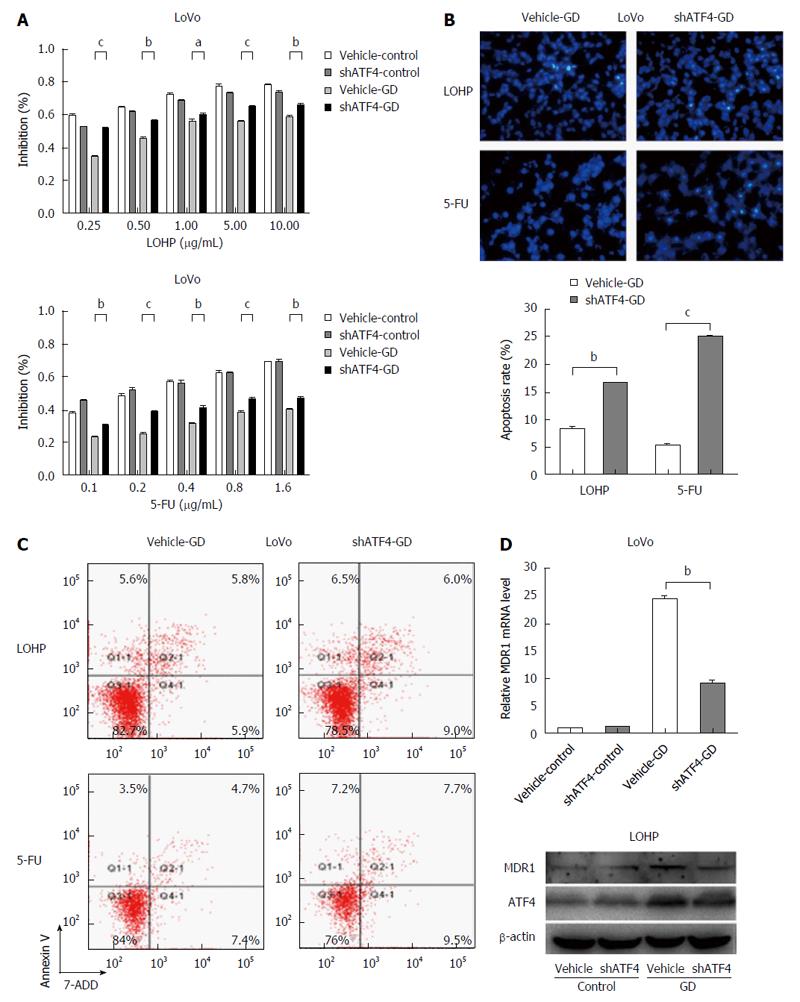
To investigate whether the surviving CRC cells under GD could acquire drug resistance, we assessed the potential effect of GD on the sensitivity of CRC cells to LOHP and 5-FU, two of the most commonly used drugs for CRC treatment[15]. The results revealed that the IC50 values of GD-treated HCT116/LoVo cells were significantly higher than those of their corresponding control cells (Figure 1A and Figure 2), suggesting that GD strongly decreases the sensitivity of CRC cells to LOHP and 5-FU. These data indicate that GD induces a MDR phenotype in CRC cells. Next, to determine whether GD inhibits chemotherapy-induced apoptosis in CRC cells, we used Hoechst staining to investigate the apoptotic rates. After incubation under GD condition for 24 h, CRC cells were treated with LOHP or 5-FU for subsequent 48 h under normal culture conditions. These cells were then subjected to Hoechst staining. The results revealed that the apoptotic rates were much lower in the GD-treated CRC cells than in the control cells (Figure 1B). To confirm the MDR phenotype of the GD-treated CRC cells, we examined the expression levels of multidrug resistance gene 1 (MDR1), a major marker of MDR. As shown in Figure 1C and D, both the mRNA and protein expression levels of MDR1 were increased in the GD-treated CRC cells as compared to the control cells. Taken together, these observations suggest that GD, through inhibiting apoptosis, significantly decreases the sensitivity of CRC cells to chemotherapy.
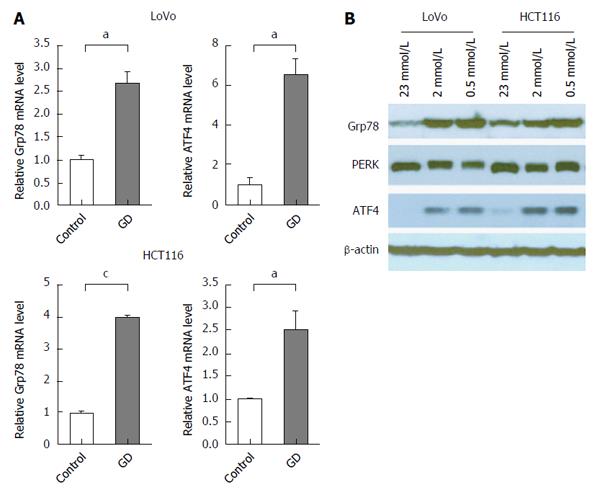
Our previous work showed that GD induces tumor growth and angiogenesis by activating PERK/ATF4 arm of UPR signaling. To investigate the role of PERK/ATF4 pathway in GD-induced MDR in CRC cells, we examined the mRNA and protein expression of UPR markers (Grp78, PERK and ATF4), which are well-known to be induced by stressful microenvironments such as GD and hypoxia[8,16]. As expected, the mRNA levels of Grp78 and ATF4 were significantly increased in GD-treated CRC cells. Although the mRNA and protein expression of PERK was not significantly increased as that of Grp78 and ATF4, the phosphorylation (activation) of PERK (upward shift in the bands) was clearly observed in GD-treated CRC cells (Figure 3A and B). These data suggest the activation of UPR upon GD treatment and the potential key role of Grp78/PERK/ATF4 pathway in GD-induced MDR phenotype in CRC cells.
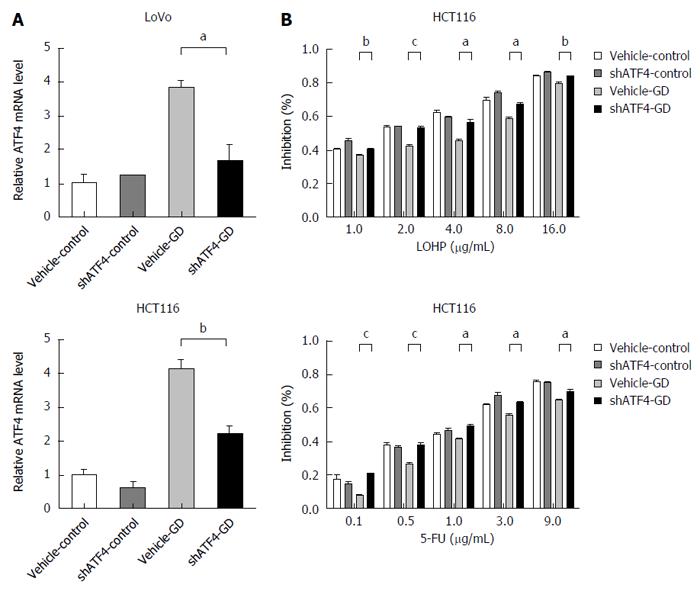
To explore whether the acquisition of anti-apoptotic property in glucose-depleted CRC cells was due to the activation of ATF4, we silenced the expression of ATF4 using shATF4 in the GD-treated LoVo and HCT116 cells (Figure 4A). The results showed that silencing ATF4 expression counteracted GD-induced drug resistance of CRC cells to both drugs (LOHP and 5-FU) compared with the control cells (Figure 4B and Figure 5A). Moreover, both Hoechst nuclear staining (Figure 5B) and Annexin V/7-AAD staining assays (Figure 5C) showed that ATF4 knockdown significantly increased apoptotic rates of GD-treated CRC cells compared with the control cells. These results suggest that GD inhibits apoptotic activity in CRC cells by activating ATF4 expression. In addition, down-regulation of MDR1 was observed in the ATF4-depleted CRC cells treated with LOHP compared with the control cells, suggesting that ATF4 may mediate GD-induced MDR effect in CRC cells by up-regulating MDR1 expression (Figure 5D). Collectively, these results suggest that the activation of ATF4 plays a crucial role in the GD-induced MDR phenotype in CRC cells.
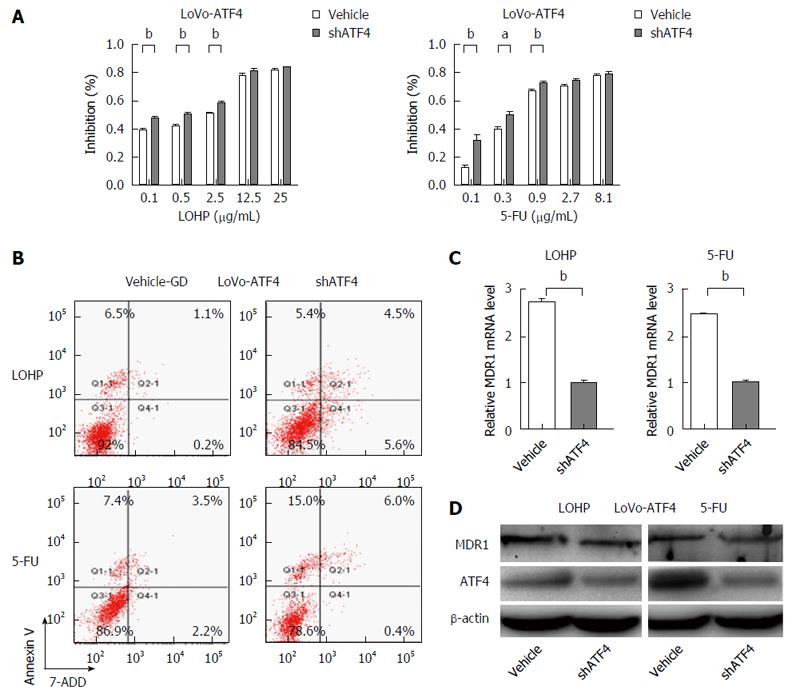
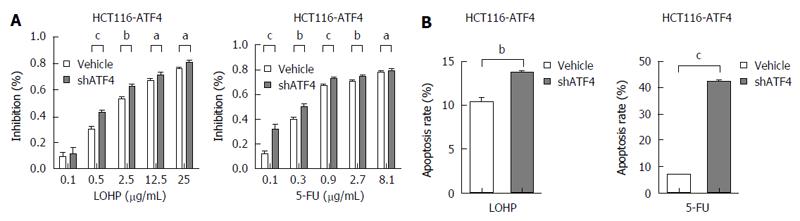
To further investigate the role of ATF4 in the drug resistance of CRC cells, forced expression of ATF4 was induced in LoVo and HCT116 cells (LoVo-ATF4 and HCT116-ATF4) using lentivirus transduction. ATF4-overexpressing CRC cells were co-treated with shATF4 and therapeutic drugs, and the results demonstrated that inhibition of ATF4 increased the sensitivity of LoVo-ATF4 and HCT116-ATF4 cells to chemotherapy (Figure 6A and Figure 7A). Moreover, we detected the apoptosis in ATF4-overexpressing cells treated with shATF4, and revealed that the apoptotic rates were much higher compared with the control cells (Figure 6B and Figure 7B). Meanwhile, qRT-PCR and Western blot results also showed the decreased expression of MDR1 (Figure 6C and D). These findings further demonstrate that ATF4 contributes to the induction of chemoresistance in CRC cells.
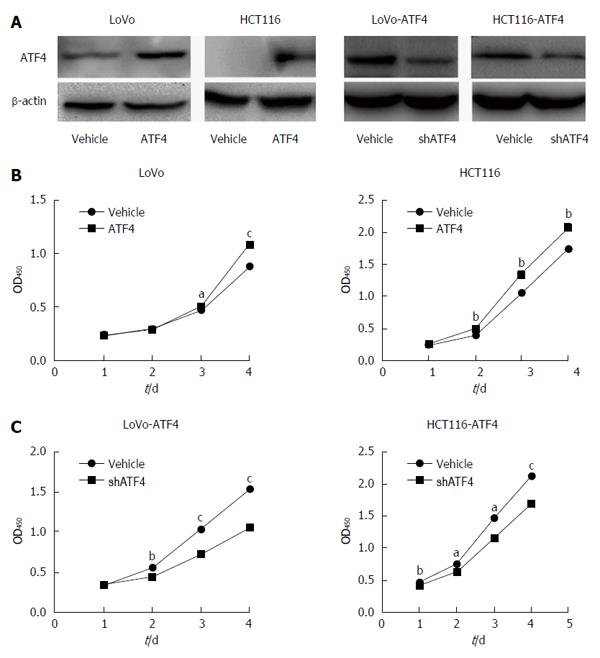
Previous studies have proved the role of ATF4 in tumor proliferation. To investigate the proliferation-prompting function of ATF4 in CRC, we overexpressed ATF4 in LoVo and HCT116 cells and then inhibited ATF4 in these cells or their control cells, respectively (As shown in Figure 8A). ATF4 overexpression significantly increased the growth rates of HCT116 and LoVo cells compared to the vector control (Figure 8B). In contrast, inhibition of ATF4 in the ATF4-overexpressing CRC cells significantly decreased the growth rates compared to the control cells (Figure 8C). The results suggest that ATF4 may play multiple roles in CRC progression.
Therapeutic resistance remains a major cause of tumor chemotherapy failure. Its mechanisms are very complicated. Recently, GD has been reported to promote cell proliferation, migration, invasion, angiogenesis and drug resistance in a variety of human cancers through different mechanisms, suggesting its extensive function in tumor development and progression[6-10,17,18]. UPR is an important mechanism by which GD regulates malignant phenotypes of tumor cells. Our previous work showed that GD contributes to tumor angiogenesis by increasing expression of multiple proangiogenic factors through the PERK/ATF4 signaling, a key signaling pathway in UPR[8]. In this study, we revealed that GD can decrease the sensitivity of CRC cells to two most commonly used chemotherapeutic drugs (LOHP and 5-FU) in CRC cells by activating PERK/ATF4 pathway. Further analysis showed that silencing ATF4 expression could counteract the inhibitory effect of GD on drug-induced apoptosis, suggesting the key role of ATF4 in GD-induced chemoresistance in CRC.
Due to their rapid and uncontrollable growth, tumors are frequently exposed to extracellular environments that are deficient in nutrients and oxygen, resulting in the disruption of homeostasis in the endoplasmic reticulum (ER) and leading to the activation of UPR. UPR serves to decrease the detrimental effects of accumulated unfolded proteins by increasing protein degradation and decreasing protein synthesis. However, UPR can induce apoptosis in normal cells encountering prolonged stress conditions. Accumulating evidence indicates that UPR contributes to the cancer development, affecting angiogenesis, cell growth, cell differentiation, cell migration, and the inflammatory microenvironment. In addition, recent studies also show that UPR activation can alter the sensitivity of tumor cells to a variety of chemotherapeutic agents. As a common stressful microenvironment in tumor, GD can regulate a variety of tumor phenotypes, mainly by activating UPR pathway. In this study, we revealed that GD induced MDR phenotype by inhibiting 5-FU/LOHP-induced apoptosis in CRC cells. A recent study also reported that COLO-320 colon cancer cells adapted to GD could acquire resistance to doxorubicin-induced apoptosis[10]. These data demonstrate the key role of GD microenvironment in regulating the MDR phenotype of CRC.
To elucidate the mechanism by which GD induces MDR phenotype in CRC cell, we checked the PERK/ATF4 arm of UPR pathway and revealed its activation under GD condition. In view of the potential role of ATF4 in the UPR and drug resistance in cancer cells, we tried to reveal its potential influence on the MDR phenotype inducing by GD in CRC cells. As a member of the CREB protein family, ATF4 participates in many intracellular physiological and biochemical processes and has been suggested as an important target of cancer therapy[19-26]. For example, ATF4 is the main transcriptional regulator of the cellular hypoxic response to UPR signaling and activates genes that promote restoration of normal ER function and survival under hypoxia condition[23]. Recently, ATF4 was reported to promote drug resistance in several types of tumors, including breast cancer[27], lung cancer[28], liver cancer[29], and gastric cancer[3]. Similarly, we revealed that the MDR was reversed when we inhibited the GD-induced up-reguation of ATF4 using shATF4. Moreover, we revealed that inhibition of ATF4 by shRNA in the ATF4-overexpressing CRC cells also reintroduced therapeutic sensitivity and apoptosis in CRC cells. These data indicate that GD induces MDR mainly by activating ATF4. Nishimoto et al[10] showed that GD could induce acquire resistance to doxorubicin-induced apoptosis in CRC cells, suggesting that multiple downstream targets mediate the MDR-inducing function of GD.
Ledoux et al[30] reported that GD enhances expression of MDR1 through c-Jun activation in hepatoma cells. Our data also observed increased MDR1 expression in GD-treated CRC cells. In addition, our data indicated that ATF4 knockdown significantly decreased MDR1 expression in the GD-treated CRC cells compared with the control cells. These results imply that ATF4 contribute to chemoresistance in CRC partly via regulating MDR1.
In conclusion, our data clearly identify that GD induces the MDR phenotype of CRC cells by activating PEKR/ATF4 signaling, and targeting the ATF4 pathway may provide a clinical perspective for treating drug resistance of CRC cells to conventional therapy. Further studies are necessary to identify key molecules that enhance the effect of ATF4 knockdown on CRC cells resistant to conventional chemotherapy. Therefore, interventions based on the disruption of GD-induced ATF4 expression may be effective in reversing drug resistance in CRC cells.
Chemoresistance is an important reason for clinical chemotherapy failure. Recent studies suggest that that tumor microenvironment is an important determinant of malignant progression and chemoresistance. Changes in the tumor microenvironment, such as hypoxia and glucose deprivation (GD), can prompt tumor progression and drug resistance. However, the role and mechanism of GD in colorectal cancer (CRC) drug resistance are unknown.
GD has been found to be involved in the regulation of multiple pathological processes that contribute to tumorigenesis and metastasis, such as tumor cell proliferation, multidrug resistance, and autophagy. Activating transcription factor 4 (ATF4) is a key player in UPR signaling. Many studies recently revealed that ATF4 participates in drug resistance of cancer. However, the role of ATF4 in GD-induced CRC drug resistance remains unclear and needs further exploration.
The authors clearly identified that GD induces the MDR phenotype of CRC cells by activating PEKR/ATF4 signaling and targeting the ATF4 pathway may provide a clinical perspective for treating drug resistant of CRC to conventional therapy.
ATF4 may be a therapeutic target to combat therapeutic resistance in CRC cells.
GD: In the tumor microenvironment, the abnormal development of vasculature results in insufficient blood supply, such as hypoxia and GD. Changes in the tumor microenvironment can promote tumor progression and drug resistance.
This is a very interesting study which explored the role and mechanism of GD in the chemoresistance of CRC. The results suggest that ATF4 may be a new target for overcoming CRC resistance to conventional chemotherapy.
Manuscript source: Invited manuscript
P- Reviewer: Nishiyama M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Yin Y, Zhang B, Wang W, Fei B, Quan C, Zhang J, Song M, Bian Z, Wang Q, Ni S. miR-204-5p inhibits proliferation and invasion and enhances chemotherapeutic sensitivity of colorectal cancer cells by downregulating RAB22A. Clin Cancer Res. 2014;20:6187-6199. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037-1050. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Zhu H, Xia L, Zhang Y, Wang H, Xu W, Hu H, Wang J, Xin J, Gang Y, Sha S. Activating transcription factor 4 confers a multidrug resistance phenotype to gastric cancer cells through transactivation of SIRT1 expression. PLoS One. 2012;7:e31431. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Zhu H, Chen X, Chen B, Chen B, Fan J, Song W, Xie Z, Jiang D, Li Q, Zhou M. Activating transcription factor 4 mediates a multidrug resistance phenotype of esophageal squamous cell carcinoma cells through transactivation of STAT3 expression. Cancer Lett. 2014;354:142-152. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Chen F, Qi X, Qian M, Dai Y, Sun Y. Tackling the tumor microenvironment: what challenge does it pose to anticancer therapies? Protein Cell. 2014;5:816-826. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Ameri K, Jahangiri A, Rajah AM, Tormos KV, Nagarajan R, Pekmezci M, Nguyen V, Wheeler ML, Murphy MP, Sanders TA. HIGD1A Regulates Oxygen Consumption, ROS Production, and AMPK Activity during Glucose Deprivation to Modulate Cell Survival and Tumor Growth. Cell Rep. 2015; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Mathews EH, Stander BA, Joubert AM, Liebenberg L. Tumor cell culture survival following glucose and glutamine deprivation at typical physiological concentrations. Nutrition. 2014;30:218-227. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nör JE, Polverini PJ. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res. 2012;72:5396-5406. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Wang Y, Ning Y, Alam GN, Jankowski BM, Dong Z, Nör JE, Polverini PJ. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia. 2013;15:989-997. [PubMed] [Cited in This Article: ] |
| 10. | Nishimoto A, Kugimiya N, Hosoyama T, Enoki T, Li TS, Hamano K. HIF-1α activation under glucose deprivation plays a central role in the acquisition of anti-apoptosis in human colon cancer cells. Int J Oncol. 2014;44:2077-2084. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Igarashi T, Izumi H, Uchiumi T, Nishio K, Arao T, Tanabe M, Uramoto H, Sugio K, Yasumoto K, Sasaguri Y. Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene. 2007;26:4749-4760. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, Span PN. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Lee SH, Min KW, Zhang X, Baek SJ. 3,3’-diindolylmethane induces activating transcription factor 3 (ATF3) via ATF4 in human colorectal cancer cells. J Nutr Biochem. 2013;24:664-671. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Nagelkerke A, Sweep FC, Stegeman H, Grénman R, Kaanders JH, Bussink J, Span PN. Hypoxic regulation of the PERK/ATF4/LAMP3-arm of the unfolded protein response in head and neck squamous cell carcinoma. Head Neck. 2015;37:896-905. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Hecht JR, Mitchell EP, Yoshino T, Welslau M, Lin X, Chow Maneval E, Paolini J, Lechuga MJ, Kretzschmar A. 5-Fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) plus sunitinib or bevacizumab as first-line treatment for metastatic colorectal cancer: a randomized Phase IIb study. Cancer Manag Res. 2015;7:165-173. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Goldenberg-Cohen N, Raiter A, Gaydar V, Dratviman-Storobinsky O, Goldstein T, Weizman A, Hardy B. Peptide-binding GRP78 protects neurons from hypoxia-induced apoptosis. Apoptosis. 2012;17:278-288. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Örd T, Örd D, Adler P, Vilo J, Örd T. TRIB3 enhances cell viability during glucose deprivation in HEK293-derived cells by upregulating IGFBP2, a novel nutrient deficiency survival factor. Biochim Biophys Acta. 2015;1853:2492-2505. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Redzic ZB, Rabie T, Sutherland BA, Buchan AM. Differential effects of paracrine factors on the survival of cells of the neurovascular unit during oxygen glucose deprivation. Int J Stroke. 2015;10:407-414. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM, Grandis JR, Li C, Johnson DE. The next generation proteasome inhibitors carfilzomib and oprozomib activate prosurvival autophagy via induction of the unfolded protein response and ATF4. Autophagy. 2012;8:1873-1874. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Walter F, Schmid J, Düssmann H, Concannon CG, Prehn JH. Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival. Cell Death Differ. 2015;22:1502-1516. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Rzymski T, Milani M, Singleton DC, Harris AL. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle. 2009;8:3838-3847. [PubMed] [Cited in This Article: ] |
| 22. | Dey S, Sayers CM, Verginadis II, Lehman SL, Cheng Y, Cerniglia GJ, Tuttle SW, Feldman MD, Zhang PJ, Fuchs SY. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Invest. 2015;125:2592-2608. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Notte A, Rebucci M, Fransolet M, Roegiers E, Genin M, Tellier C, Watillon K, Fattaccioli A, Arnould T, Michiels C. Taxol-induced unfolded protein response activation in breast cancer cells exposed to hypoxia: ATF4 activation regulates autophagy and inhibits apoptosis. Int J Biochem Cell Biol. 2015;62:1-14. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Singleton DC, Harris AL. Targeting the ATF4 pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:1189-1202. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Ye P, Mimura J, Okada T, Sato H, Liu T, Maruyama A, Ohyama C, Itoh K. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol. 2014;34:3421-3434. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Jiang Q, Li F, Shi K, Wu P, An J, Yang Y, Xu C. Involvement of p38 in signal switching from autophagy to apoptosis via the PERK/eIF2α/ATF4 axis in selenite-treated NB4 cells. Cell Death Dis. 2014;5:e1270. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, Harris AL. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009;69:4415-4423. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Fan CF, Miao Y, Lin XY, Zhang D, Wang EH. Expression of a phosphorylated form of ATF4 in lung and non-small cell lung cancer tissues. Tumour Biol. 2014;35:765-771. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Zhang Z, Yin J, Zhang C, Liang N, Bai N, Chang A, Liu Y, Li Z, Tan X, Li N. Activating transcription factor 4 increases chemotherapeutics resistance of human hepatocellular carcinoma. Cancer Biol Ther. 2012;13:435-442. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003;63:7284-7290. [PubMed] [Cited in This Article: ] |