Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6876
Peer-review started: March 18, 2016
First decision: May 12, 2016
Revised: May 24, 2016
Accepted: June 13, 2016
Article in press: June 13, 2016
Published online: August 14, 2016
Drug resistance develops in nearly all patients with colon cancer, leading to a decrease in the therapeutic efficacies of anticancer agents. This review provides an up-to-date summary on over-expression of ATP-binding cassette (ABC) transporters and evasion of apoptosis, two representatives of transport-based and non-transport-based mechanisms of drug resistance, as well as their therapeutic strategies. Different ABC transporters were found to be up-regulated in colon cancer, which can facilitate the efflux of anticancer drugs out of cancer cells and decrease their therapeutic effects. Inhibition of ABC transporters by suppressing their protein expressions or co-administration of modulators has been proven as an effective approach to sensitize drug-resistant cancer cells to anticancer drugs in vitro. On the other hand, evasion of apoptosis observed in drug-resistant cancers also results in drug resistance to anticancer agents, especially to apoptosis inducers. Restoration of apoptotic signals by BH3 mimetics or epidermal growth factor receptor inhibitors and inhibition of cancer cell growth by alternative cell death pathways, such as autophagy, are effective means to treat such resistant cancer types. Given that the drug resistance mechanisms are different among colon cancer patients and may change even in a single patient at different stages, personalized and specific combination therapy is proposed to be more effective and safer for the reversal of drug resistance in clinics.
Core tip: Drug resistance in colon cancer is still an obstacle to successful chemotherapy. This review focuses on over-expression of ATP-binding cassette transporters and evasion of apoptosis, two representatives of transport-based and non-transport-based mechanisms of drug resistance, as well as their therapeutic strategies. Given that the drug resistance mechanisms are different among colon cancer patients and may change even in a single patient at different stages, personalized and specific combination therapy is proposed to be more effective and safer for the reversal of drug resistance in the clinical setting.
- Citation: Hu T, Li Z, Gao CY, Cho CH. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol 2016; 22(30): 6876-6889
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6876.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6876
Colon cancer, a disease during which malignant tumors form in the tissues of colon, is the third most frequently diagnosed cancer and one of the leading causes of cancer-related deaths worldwide[1-3]. Currently, surgery and chemotherapy are the two main treatment options for colon cancer, depending on the cancer stages and tumor location at diagnosis, as well as individual characteristics of the patients[4]. Generally, chemotherapy can be used at different stages during the treatment and is often given after surgery as an adjuvant therapy for patients with advanced colon cancer. It is also used before surgery as neoadjuvant chemotherapy to shrink the tumor before removal[5]. Due to the availability of various chemotherapy regimens, the overall survival of patients with advanced colon cancer has been improved over the past decades. However, even though the response rate to current systemic chemotherapies can reach up to 50%, drug resistance reportedly develops in nearly all patients with colon cancer and limits the therapeutic efficacies of anticancer agents and finally leads to chemotherapy failure[6].
Drug resistance is the reduction in effectiveness of drugs, including antibiotics, antiviral and chemotherapeutic agents, during the treatment of various diseases[7]. Cancer drug resistance has been extensively investigated since the discovery of a novel type of resistance correlated with P-glycoprotein (P-gp) in several Chinese hamster ovary cell lines in 1976[8]. It refers to resistance to a variety of structurally and functionally unrelated chemotherapeutic agents after exposure to a single cytotoxic compound. Till now, multidrug resistance in cancer is still an obstacle to successful chemotherapy[9]. In fact, most cancer-related deaths are due to chemotherapy failure caused by drug resistance that occurs during the course of cancer progression and chemotherapy. Thus, investigation of the mechanisms of drug resistance and their reversal strategies plays an important role in the success of cancer chemotherapy.
A number of underlying mechanisms conferring drug resistance have been described in the past decades, which have been broadly classified into two categories: the non-cellular and cellular resistance mechanisms. Non-cellular mechanisms refer to the extracellular factors, such as limited vascular accessibility and tumor microenvironment[10]. Cellular mechanisms, on the other hand, are mainly concerned with the drug targets, enzymes and transport systems inside the cancer cells[11], which are further divided into two categories: classical/transport-based and non-classical/non-transport-based mechanisms[11]. Since the experimental models can be easily generated by in vitro selection with cytotoxic compounds, cellular mechanisms of drug resistance in cancer have been intensively studied so far[12]. Given the accumulating literature regarding this field, the present review will focus on cellular mechanisms of drug resistance in colon cancer and its reversal strategies, with an emphasis on the over-expression of drug efflux transporters and evasion of apoptosis, two representatives of transport-based and non-transport-based cellular mechanisms, respectively.
The transport-based cellular mechanisms of drug resistance mainly refer to the efflux of drugs out of cancer cells through a variety of membrane transporters, thereby leading to decreased intracellular accumulation of anticancer drugs and chemotherapy failure. Membrane transporters are a group of membrane-associated proteins that control the transport of their substrates into and out of the cells[13]. To date, more than 400 membrane transporters have been annotated in the human genome, and they are divided into two major superfamilies: ATP-binding cassette (ABC) and solute carrier (SLC) transporters. Representative ABC transporters include P-gp, breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs); whereas, transporters such as the organic anion transporters, organic cation transporters and organic anion transporting polypeptides belong to the SLC superfamily[13,14]. In fact, the most commonly observed mechanism conferring drug resistance in cancer cells is the over-expression of ABC transporters on plasma membrane[15].
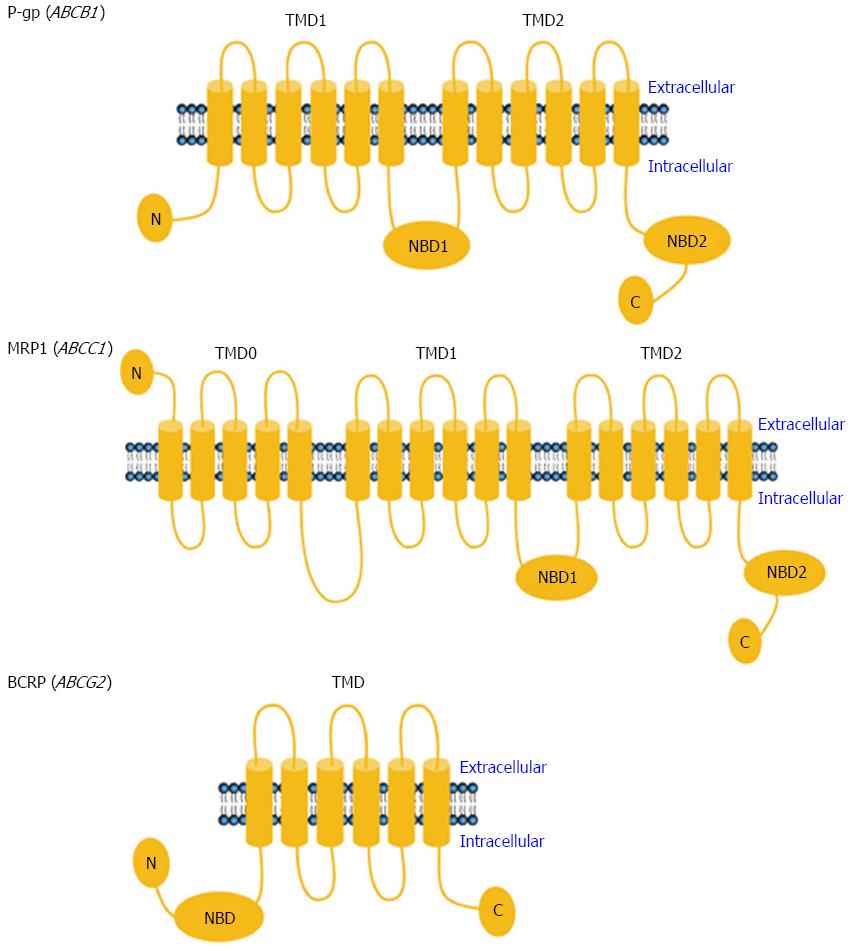
The ABC transporter superfamily includes a number of transporters located on the cellular plasma membrane that mediate the efflux of endogenous and exogenous substances using energy provided by ATP hydrolysis[13]. There are at least 48 known human ABC transporters. Based on their amino acid sequences, they are grouped into 7 subfamilies, designated A though G[13]. It has been recognized that several members of three ABC subfamilies - in particular P-gp of the ABCB subfamily, MRP1 of the ABCC subfamily and BCRP of the ABCG subfamily - play pivotal roles in the transport of anticancer drugs out of cells, as well as in the development of drug resistance.
P-gp, a 170-kDa protein encoded by the human ABCB1 gene, is one of the most well characterized ABC transporters. As an ATP-dependent drug efflux pump, the functional unit of P-gp consists of two nucleotide-binding domains (NBDs) and two transmembrane domains (TMDs) containing 12 (2 × 6) membrane-spanning alpha helices (Figure 1)[16]. The two NBDs form a common binding site, where the energy of ATP is harvested to promote the efflux of substrates through a pore that is delineated by the transmembrane helices[17]. P-gp preferentially transports relatively large, lipophilic and positively charged molecules[13]. The 190-kDa MRP1, encoded by ABCC1 in humans, has a P-gp-like core structure containing two NBDs and two TMDs, and an additional third TMD (TMD0) with five predicted transmembrane segments and an extra N-terminus (Figure 1)[18]. Generally, the substrates of MRP1 are unconjugated and conjugated organic anions. The conjugation of drugs with glutathione, glucuronate, phosphate or sulfate by phase II drug-metabolizing enzymes usually makes them better substrates of MRP1[13]. Unlike P-gp and MRP1, however, BCRP is a 72-kDa “half transporter” encoded by ABCG2 in humans and consisting of only one NBD and one TMD (Figure 1)[19]. BCRP also transports a broad range of endogenous and exogenous substrates across the cellular plasma membrane[13].
Physiologically, ABC transporters are expressed in important biological barriers in the body, such as small intestine, liver, kidney, blood-brain barrier, choroid plexus, testis and placenta, functioning to pump their substrates out of the cells and protecting the body against endogenous toxins and xenobiotics[13]. These biological barriers are also important tissues involved in the disposition of various drugs in the body. Thus, from a pharmacokinetic point of view, ABC transporters play pivotal roles in the absorption, distribution and excretion of anticancer drugs, and thereby affect their efficacy and safety profiles.
In addition to their physiological roles in host detoxification and pharmacokinetics, dysregulation of ABC transporters is associated with a variety of diseases. ABC transporters, in particular the P-gp, MRP1 and BCRP, have been reported to be up-regulated in different tumors and over-expressed in various cancer cells cultured under specific microenvironments, such as conditions of insult by different cytotoxic agents[20-22].
The involvement of P-gp in clinical tumors has been extensively characterized. Approximately 50% of human cancers express P-gp at levels sufficient to confer drug resistance[23]. Colon cancer is insensitive to most chemotherapeutic agents from the beginning of therapy. Indeed, high expression of P-gp has been observed at the time of colon cancer diagnosis, which is associated with the intrinsic resistance of various colon cancer cell lines to anticancer drugs derived from natural products[24-26]. P-gp expression is also inducible by chemotherapeutic agents in cancer cells. For instance, its expression level was up-regulated by 5-fold in Caco-2 cells after chronic exposure to imatinib[27]. Using HCT15 colon cancer cells, nuclear factor- kappaB (NF-κB) activation was reported to induce P-gp expression, and inhibition of NF-κB or P-gp to increase the level of apoptosis induced by daunomycin[28]. Hypoxia-inducible factor-1alpha (HIF-1α) was also reported to be associated with the expression of P-gp in human colon carcinoma tissues and colon cell lines, including HCT116, HT29, LoVo and SW480[29]. Down-regulation of P-gp by HIF-1α inhibition reversed the drug resistance in LoVo multicellular spheroids[30]. Besides, suppression of P-gp via inhibition of transient receptor potential channel 5 also reversed the resistance of human colon cancer HCT8 and LoVo cells to 5-fluorouracil (5-FU), the first-line drug used for colon cancer therapy[31]. Thus, multiple signaling pathways are involved in the regulation of P-gp in cancer cells.
Increased expression of MRP1 also occurs early in colorectal carcinogenesis in humans[32]. Up-regulation of MRP1 was found during the development of drug resistance in the HT29 colon cancer cells[33]. Besides, MRP1 and MRP3 were induced by non-steroidal anti-inflammatory drugs in the human colon cancer cells HCT15, HT29 and HCA7[34]. The activities of MRP1 and BCRP were increased in HT29 cells treated with hypericin and in turn affected the accumulation of hypericin in cancer cells[35]. Like P-gp, the MRP1 gene can also be induced by HIF-1α in LoVo cells[36]. Induction of MRP1 gene expression by interleukin-1beta in HT29 cells was demonstrated to be related to nitric oxide-related signaling pathways[37].
Induction of BCRP was also observed during the acquirement of resistance to anticancer drugs. In fact, it was cloned from human colon carcinoma cells S1-M1-80 after selection with mitoxantrone[38]. Dramatically over-expressed BCRP mRNA was also detected in HT29 cells selected with mitoxantrone[39]. The resistance of HT29 cells to doxorubicin was also shown to be mediated by elevated BCRP expression, which was associated with c-MET/PI3K signaling[40]. In Caco-2 cells after chronic exposure to imatinib, BCRP expression was found to be up-regulated by 17-fold[27]. The mRNA expression of BCRP in SW1116 cells resistant to hydroxycamptothecin, a topoisomerase I inhibitor, was 200-fold higher than its level in the parental SW1116 cells[41]. Up-regulation of MRP2 and BCRP was also involved in the cisplatin-induced drug resistance in colon cancer cells Caco-2 and LS174T[42]. In HCT8 cells with BCRP expression, the intracellular accumulation of CI1033, a tyrosine kinase inhibitor and BCRP substrate, was reduced. By inhibition of BCRP-mediated drug efflux, CI1033 enhanced the cytotoxicities of SN-38 and topotecan in HCT8 cells[43]. Besides, expression of P-gp and BCRP was significantly higher in side population (SP) colon cancer cells than in non-SP cells, which conferred the higher resistance of SP cells to 5-FU and irinotecan[44].
Besides P-gp, MRP1 and BCRP, several other ABC transporters could also be induced by anticancer drugs in colon cancer cells and play roles in drug resistance. For instance, ABCB5 expression was substantially increased in clinical colorectal cancers after 5-FU-based chemotherapy and contributed to the development of resistance to 5-FU[45]. Based on an analysis of 45 patients, MRP2 was reported to be important for the resistance of colon cancer to cisplatin treatment[46], and its level was also increased in SW620 and LoVo cells selected by oxaliplatin[20]. MRP4 and MRP5 were also induced in WiDr and COLO-205 cells treated with celecoxib at a clinically relevant concentration[47]. Taken together, these findings suggest that over-expression of ABC transporters could be induced by various anticancer drugs and that ABC transporters contribute to both intrinsic and acquired drug resistance in colon cancer.
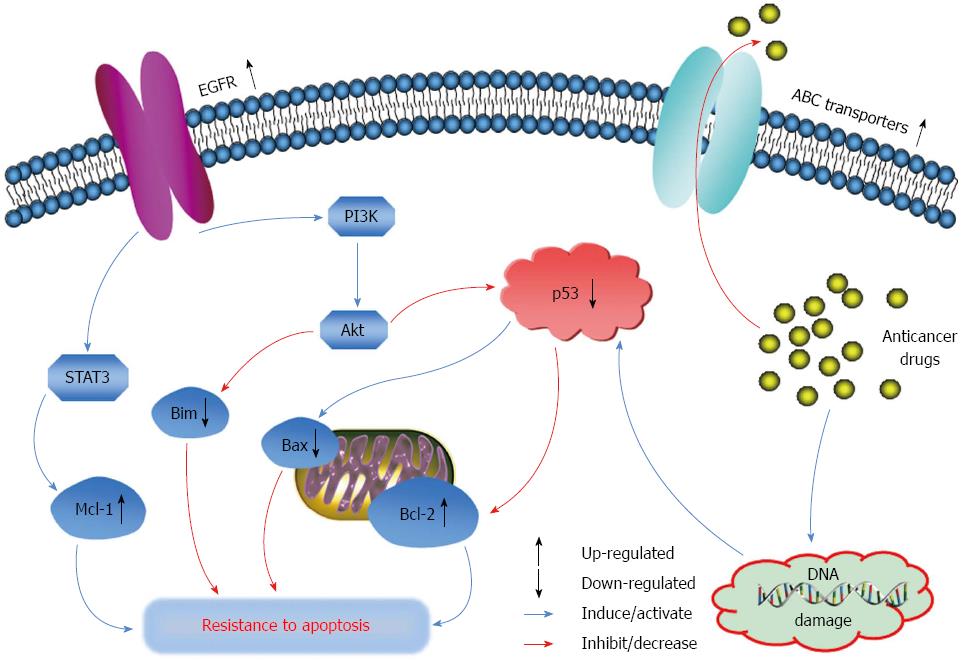
As shown in Figure 2, the over-expression of ABC transporters can facilitate the efflux of their substrate anticancer drugs out of cancer cells, which consequently decreases the drug intracellular concentrations and therapeutic effects, giving rise to drug resistance[48]. So far, a number of clinically used anticancer drugs have been identified as the substrates of ABC transporters. A non-exhaustive list is shown in Table 1. Some of the anticancer drugs such as 5-FU, oxaliplatin and irinotecan are often used alone or as combination therapy for the treatment of advanced colon cancer. Thus, efflux of these drugs by ABC transporters can decrease their therapeutic efficacies for colon cancer. In terms of the transport of chemotherapeutic agents, of note, the substrate specificity of ABC transporters overlaps extensively, which increases the barrier function of these efflux transporters and makes the chemotherapy of drug-resistant cancer more difficult.
| Transporters | Anticancer drug substrates | Inhibitors | Ref. |
| P-gp (ABCB1) | Actinomycin D, bisantrene, colchicine, daunorubicin, dasatinib, docetaxel, doxorubicin, epirubicin, etoposide, imatinib, irinotecan, mitoxantrone, nilotinib, paclitaxel, saquinavir, teniposide, topotecan, vinblastine, vincristine, vindesine, vinorelbine | Biricodar, chloroquine, cryptotanshinone, curcumin, cyclosporin A, dexverapamil, dihydrotanshinone, dofequidar, laniquidar, nifedipine, quinidine, sipholenol A, tamoxifen, tariquidar, valspodar, verapamil, zosuquidar | [12,66-68,132,133] |
| MRP1 (ABCC1) | Colchicine, doxorubicin, etoposide, imatinib, irinotecan, methotrexate, mitoxantrone, saquinavir, topotecan, vinblastine, vincristine | Biricodar, celecoxib, curcumin, dinaciclib, dofequidar, flavonoids, ibrutinib, myricetin, sulindac, tariquidar | [133-141] |
| BCRP (ABCG2) | Bisantrene, daunorubicin, doxorubicin, etoposide, gefitinib, imatinib, irinotecan, methotrexate, mitoxantrone, SN-38, teniposide, topotecan, vincristine | Biricodar, corticosterone, curcumin, cyclosporin A, elacridar, gefitinib, imatinib, ketoconazole, lopinavir, nifedipine, quercetin, rotenoids, stilbenoids, tariquidar, tectochrysin | [12,142-146] |
Given that over-expression of ABC transporters is one of the most commonly observed mechanisms contributing to drug resistance in cancer cells, inhibition of these transporters is proposed to be an effective approach to sensitize drug-resistant cancer cells to chemotherapeutic agents[15]. One method to suppress the ABC transporters is to regulate their expression levels. Different antisense oligonucleotides, ribozymes and small interfering RNAs have been reported to successfully reduce the expression levels of ABC transporters and reverse the drug resistance in cancer cells over-expressing these transporters[49-53]. Besides the regulation of protein expression, another important method to inhibit ABC transporters is the co-administration of their inhibitors, of which the P-gp inhibitors will be discussed in detail in this review.
Since the discovery of P-gp inhibition by verapamil in 1981, at least three generations of P-gp inhibitors have been identified[54]. The first-generation P-gp inhibitors, including quinidine, verapamil and cyclosporine A, have relatively low affinity for P-gp, which requires the treatment of high doses and leads to severe side effects when co-administrated with anticancer drugs[54]. Most of the second-generation P-gp inhibitors are derivatives of the first-generation modulators, such as dexverapamil, valspodar and biricodar. As compared with the first generation, the second-generation inhibitors are more specific for P-gp, with greater potency and less toxicity. However, they also inhibit the metabolism and excretion of co-administrated drugs, resulting in unpredictable pharmacokinetic interactions[54]. To overcome this drawback, the third generation of P-gp inhibitors was investigated, most of which have been developed by combinatorial chemistry. Some representatives include tariquidar, zosuquidar and laniquidar, and a number of new tariquidar derivatives have also shown potent inhibition on P-gp in cell-based studies[55]. They are more specific for P-gp without affecting the activity of cytochrome P450 enzymes[54,56].
All three generations of P-gp inhibitors have been reported to dramatically sensitize various drug-resistant cancer cells to the known P-gp substrate anticancer drugs in vitro[57]. Some of them, such as verapamil, cyclosporine A, dexverapamil, valspodar and tariquidar, have also been studied as chemosensitizers in clinical trials[58-62]. However, so far, none of them has been used in the clinical setting because of such undesirable drawbacks as poor selectivity, low potency, high toxicity and unpredictable pharmacokinetic interactions[54]. In order to develop novel P-gp inhibitors with good safety and efficacy profiles, nowadays, a lot of research work is focused on natural products and subsequent structural modifications, owing to their versatile applications and relatively low toxicities[63].
The P-gp modulators from natural products belong to the fourth generation of P-gp inhibitors[64]. In fact, more than 70% of the inhibitors reported in the last decade were natural products and their synthetic derivatives[65]. Some of them were able to sensitize drug-resistant colon cancer cells. For instance, cryptotanshinone and dihydrotanshinone, the two tanshinones from Salvia miltiorrhiza, were reported to inhibit P-gp function and enhance the cytotoxicities of doxorubicin and irinotecan in SW620 Ad300 cells over-expressing P-gp[66]. Sipholenol A, a marine-derived triterpene, also specifically reversed P-gp-mediated drug resistance in SW620 Ad300 cells[67]. Using an in situ cancerous colon perfusion model in rat, curcumin was shown to increase the permeability of irinotecan via inhibition of P-gp function[68].
In addition to P-gp inhibitors, modulators of other ABC transporters have also been identified and shown as capable of sensitizing drug-resistant cancer cells. Selective inhibitors of P-gp, MRP1 and BCRP are summarized in Table 1. However, most of the current findings were obtained from cell-based studies, and the in vivo effects of these potential candidates have not been well investigated[69]. Thus, pre-clinical and clinical trials including both pharmacodynamic and pharmacokinetic studies should be carried out for their further development as chemosensitizers. Nevertheless, the development of novel inhibitors of ABC transporters is an important approach to overcoming drug resistance in various cancers, including colon cancer.
The non-transport based mechanisms of drug resistance are often associated with altered activities of specific enzymes and alterations in various cell death signaling pathways. For instance, over-expression of glutathione S-transferases (GSTs), the phase II metabolic enzymes involved in drug metabolism, can facilitate the anticancer drug detoxification in cancer cells and decrease their therapeutic effects[70,71]. Down-regulation of topoisomerases, enzymes that regulate the process of DNA replication, can also cause drug resistance of cancer cells to such anticancer drugs as doxorubicin and etoposide[72]. In addition to the change of enzymes, another important non-transport-based mechanism of drug resistance is the alterations in cell death signaling pathways, in particular apoptosis, the type I programmed cell death[73]. This type of drug resistance develops with the over-expression of proteins that inhibit cell death and/or with the loss of proteins required for cell death[74-76]. Most of the conventional anticancer drugs such as doxorubicin, cisplatin, oxaliplatin and cyclophosphamide are apoptosis inducers[77], therefore, defects in the apoptotic signaling pathways could protect cancer cells from this type of programmed cell death, leading to drug resistance to chemotherapy in the clinical setting.
Programmed cell death, which has been recognized since the 1960s, is any type of cell death in which the cell uses specialized intracellular machinery to kill itself[78]. Apoptosis, the best-described type of programmed cell death, is triggered by different extracellular and intracellular signals and characterized by cell shrinkage, chromatin condensation, DNA laddering and nuclear fragmentation[79]. The extracellular signals include hormones, nitric oxide, growth factors, cytokines, toxins and chemotherapeutic agents; whereas, the intracellular apoptotic signals are often initiated in response to various stresses, such as radiation, heat, hypoxia, viral infection, nutrient deprivation and increased intracellular calcium concentration[79]. Through elimination of damaged, unnecessary and old cells, apoptosis plays an important role in the body growth and development as well as in maintaining the health of the body. Impaired apoptosis is involved in a diverse range of diseases such as viral infections, inflammatory diseases, autoimmune diseases and cancers[80,81].
Macroautophagy (hereafter referred to as autophagy), type II programmed cell death, is characterized by the degradation of cellular components including Golgi apparatus, mitochondria, polyribosomes and endoplasmic reticulum as well as the formation of numerous autophagosomes[82]. Autophagy is activated in response to stressful stimuli, including starvation, hypoxia and high temperature or intracellular stress such as damaged organelles and mutant proteins. During the cellular process of autophagy, the redundant, damaged or aged organelles and cells are sequestered, degraded and recycled[83]. One important function of autophagy is to overcome stress conditions and maintain cellular homeostasis. Conversely, excessive activation of autophagy may lead to cell death by destroying major proportions of the cytoplasm[84]. Impaired autophagy is also involved in various diseases including neurodegeneration, cardiovascular diseases, autoimmune diseases, aging, rheumatoid arthritis, infection and cancers[85]. Currently, the role of autophagy in tumorigenesis is still controversial. Autophagy can promote the survival of rapidly growing cancer cells by targeting damaged or aged organelles for degradation and recycling. On the other hand, its death-promoting effect may lead to growth inhibition of cancer cells and suppress tumorigenesis[86]. As a double-edged sword in cancer, the function of autophagy may differ at different stages of cancers[87].
Evasion of apoptosis, one of the hallmarks of human cancers, contributes to carcinogenesis and tumor progression, as well as drug resistance in cancer[73]. Indeed, suppression of apoptosis has been observed in drug-resistant cancer cells, leading to drug resistance to chemotherapeutic agents, especially to apoptosis inducers[88,89]. Resistance to apoptosis in cancer cells is often associated with increased expression of anti-apoptotic genes and proteins, as well as decreased expression of pro-apoptotic genes and proteins (Figure 2)[90]. For instance, Bcl-2, Bcl-XL, Mcl-1 and X-linked inhibitor of apoptosis protein have been found to be over-expressed in various cancers, whereas p53, Bax, Bim, p53 up-regulated modulator of apoptosis and apoptotic protease activating factor 1 are mutated or suppressed[90]. Indeed, in colon cancer SW620 Ad300 cells over-expressing P-gp, which were selected by doxorubicin and resistant to apoptosis, Bcl-2 protein level was significantly up-regulated as compared to the parental SW620 cells, whereas Bax and p53 levels were down-regulated[91]. Loss of Bax expression was found to reduce the sensitivity of colon cancer cells HCT116 to apoptosis induced by 5-FU and oxaliplatin[92]. Besides, epidermal growth factor receptor (EGFR), a protein tyrosine kinase, was also over-expressed in colorectal tumors[93]. Through its regulation on the anti-apoptotic signaling pathways including PI3K/Akt and signal transducer and activator of transcription (STAT), over-expression of EGFR also contributes to the resistance of cancer cells to apoptosis (Figure 2)[94,95]. Nevertheless, these altered genes and proteins are potential targets for the development of novel anticancer drugs and successful chemotherapy for cancers resistant to apoptosis.
As the most frequent mutant gene in cancer, tumor suppressor p53 plays a pivotal role in the regulation of apoptosis and in the protection of the body against cancer. The p53 protein is a transcriptional factor activated and stabilized by post-translational modifications following DNA damage (Figure 2)[96]. Usually, p53 executes its function through the transactivation of target genes that are mainly involved in the regulation of cell cycle arrest and apoptosis[97,98]. When DNA is slightly damaged, activation of p53 results in G1 phase cell cycle arrest by targeting p21 and the subsequent inhibition of cyclin-dependent kinases, to allow DNA repair to proceed. However, if DNA damage is severe and cannot be repaired successfully, p53 triggers apoptosis through targeting of Bax, which is essential for mitochondrial outer membrane permeabilization, cytochrome c release and caspase activation[99]. In addition, the anti-apoptotic protein Bcl-2 is also suppressed by wild-type p53, and its down-regulation by p53 promotes apoptosis[100].
Mutated p53 has been found in more than 50% of all types of human cancers[101]. In fact, loss of p53 function was found in approximately 80% of colorectal cancers[102]. The majority (> 75% in colorectal carcinomas) of the mutations are missense mutations consisting of single amino acid substitutions, which affect the responses of cancer cells to chemotherapeutic agents[103]. In contrast to wild-type p53, mutant p53 attenuates its pro-apoptotic function and inhibits wild-type p53 function. As a result, the suppression or loss of wild-type p53 function could decrease the sensitivity of cancer cells to DNA-damaging agents and facilitate evasion of p53-mediated apoptosis (Figure 2)[104]. Although the apoptotic signaling pathways are not totally inactivated in p53 mutant cancer cells, the cells become insensitive to DNA damage, thereby increasing the threshold required for DNA damage to activate apoptosis and finally giving rise to drug resistance to apoptosis inducers[105].
Accumulating evidence has demonstrated that p53 mutant or null cancer cells tend to be more resistant to a range of cytotoxic drugs, including DNA cross-linking agents, antimitotic agents, antimetabolites and topoisomerase I/II inhibitors, as compared with their respective p53 wild-type cells[106,107]. It has been reported that apoptosis induced by 5-FU and oxaliplatin was significantly reduced in p53 mutant HCT116 cells, when compared with the p53 wild-type cells[108]. Disruption of p53 function also led to the resistance of human colon cancer cells to 5-FU both in vitro and in xenograft tumors in nude mice[109]. Besides, clinical study showed that colorectal tumors with mutant p53 had weak or no response to 5-FU treatment, and patients with wild-type p53 colorectal tumors had longer survival than those with mutant p53 tumors[110]. Of note, the extent of resistance for different agents is different, partly depending on the cancer cell lines, the p53 gene status and the mechanisms of action of the agents[107]. For instance, the sensitivity of colon cancer cells to irinotecan is independent of p53 status in xeno-transplanted colorectal tumors[111].
Since apoptosis is suppressed in drug-resistant cancer cells, restoration of apoptotic signals and inhibition of cancer cell growth by alternative cell death pathways are proposed to be effective means to treat such resistant cancers.
To restore the impaired apoptotic signals in cancer cells, BH3 mimetics, small molecules that mimic the BH3-only proteins by inserting their BH3 domain into the hydrophobic groove of the Bcl-2 proteins, were developed to inhibit the function of Bcl-2 proteins and induce apoptosis[112]. A number of BH3 mimetics were reported to induce apoptosis and sensitize the apoptosis-resistant colon cancer cells to anticancer drugs. For example, combination treatment of carfilzomib and ABT-263, a BH3 mimetic, synergistically enhanced apoptosis in colon cancer cells with mutant KRAS-mediated apoptosis resistance[113]. Another BH3 mimetic, obatoclax, was shown to reduce HIF-1α level in colon cancer cells HT29, HCT8 and HCT116 and to sensitize the hypoxic cells to apoptosis induced by 5-FU[114]. BH3 mimetic ABT-737 was able to overcome resistance to immunotoxin-mediated apoptosis in colon cancer DLD1 cells. It also increased the level of apoptosis in suspended SW480 cells and sensitized the metastatic SW620 cells to anoikis[115,116]. In addition to BH3 mimetics, EGFR tyrosine kinase inhibitors are also used to restore the apoptosis function in cancer cells. Some inhibitors including cetuximab and panitumumab have already been approved by the United States’ Food and Drug Administration for the treatment of advanced colon cancer as monotherapy or adjuvant therapy. Combination treatment of irinotecan with cetuximab could overcome resistance to irinotecan through abrogating drug efflux, restoring apoptosis and impairing DNA-repair activity[117]. This combination of treatment was also used to treat patients with metastatic colorectal cancer resistant to fluoropyrimidine and oxaliplatin[118]. A list of BH3 mimetics and EGFR inhibitors with the activity to restore apoptotic signals is presented in Table 2.
| Restoration of apoptotic signals | Alternative cell death | Ref. | ||
| BH3 mimetics | EGFR inhibitors | Autophagy inducers | Agents with p53-independent toxicity | |
| ABT-199 (venetoclax), ABT-263 (navitoclax), ABT-737, apogossypol, apogossypolone, gossypol, maritoclax, obatoclax, sabutoclax | Afatinib, cetuximab, dacomitinib, erlotinib, gefitinib, lapatinib, matuzumab, neratinib, nimotuzumab, panitumumab, zalutumumab | Clonidine, cryptotanshinone, curcumin, dihydrotanshinone, evodiamine, genistein, helenalin, monascuspiloin, oridonin, paclitaxel, quercetin, rapamycin, resveratrol, sodium valproate, verapamil | Betulinic acid, crocetin, cryptotanshinone, dihydrotanshinone, epigallocatechic-3-gallate, genistein, a-iso-cubebene, resveratrol, triptolide, thymoquinone, ursolic acid | [91,112-115,117,120,121,124,127-129,147-156] |
In terms of alternative cell death pathways, despite the finding that autophagy can protect cancer cells against apoptosis in response to chemotherapy, it can also lead to cell death in cancer cells, especially in the apoptosis-resistant cancer cells[119]. The pro-cell death function of autophagy suggests that treatment of autophagy inducers may be a novel therapeutic approach to overcome resistance to apoptosis in cancer cells. As reported, the DNA-alkylating agent temozolomide, and rapamycin, an inhibitor of the mammalian target of rapamycin, induced autophagy but not apoptosis in malignant glioma cells that highly express Bcl-2[120,121]. Histone deacetylase inhibitors sodium butyrate and suberoylanilide hydroxamic acid induced autophagy in HeLa cervical cancer cells over-expressing Bcl-XL, but induced apoptosis in parental HeLa cells[122,123]. Cryptotanshinone and dihydrotanshinone also induced more autophagic cell death in apoptosis-resistant colon cancer cells than that in the parental cancer cells[91]. In addition, a number of structurally different natural products, such as curcumin, resveratrol, paclitaxel and quercetin, have been shown to activate autophagic signaling pathways and cause cell death in various cancer cell lines, including colon cancer cells (Table 2)[124-127]. Thus, through the induction of type II programmed cell death, autophagy inducers may be further developed for sensitizing the apoptosis-resistant cancer cells to chemotherapy.
As cancer cells with mutant p53 generally have greater resistance to chemotherapy than those with wild-type p53, cytotoxic agents that kill cancer cells p53-independently should be promising candidates to overcome drug resistance caused by p53 mutations. It has been reported that natural products such as triptolide, resveratrol and dihydrotanshinone could inhibit cell proliferation and induce p53-independent apoptosis in different cancer cell lines especially in p53-deficient cancer cells (Table 2)[127-129]. Hence, the apoptosis-resistant cancer cells with mutant p53 should be relatively sensitive to this kind of cytotoxic agent in terms of cell death.
Taken together, agents such as the BH3 mimetics and EGFR inhibitors can restore the apoptotic signaling pathways in colon cancer cells. They are effective drugs for sensitizing apoptosis-resistant cancers to apoptosis induced by anticancer drugs. Moreover, pharmacological compounds that can induce autophagic cell death or p53-independent cytotoxicity are also promising candidates to overcome resistance of colon cancer cells to apoptosis.
Combination treatments of conventional anticancer drugs with inhibitors of ABC transporters, BH3 mimetics, EGFR inhibitors or autophagy inducers have been proven effective approaches for the circumvention of drug resistance in colon cancer in pre-clinical studies. A few agents, such as cetuximab and panitumumab, have been successfully approved as drugs for colon cancer therapy in clinics. However, most of the combination of treatments failed to reverse drug resistance in clinical studies, suggesting that targeting a single mechanism is not sufficient to reverse drug resistance in patients with cancer.
Of note, polymorphisms in genes related to drug resistance, including ABCB1, ABCC1, ABCG2 and TP53, have been recognized[130,131], leading to the interindividual differences in tumorigenesis, drug resistance mechanisms and outcome of treatments. Besides, as the defense mechanism of cancer cells, drug resistance continues to develop during tumorigenesis and drug treatments. Thus, the mechanisms of drug resistance may vary at different cancer stages, as well as at different phases of therapies. Given that the mechanisms of drug resistance are different among cancer patients and may change even in a single patient during the progression of cancer, personalized and specific combination therapy should be more effective and safer for achieving reversal of drug resistance in the clinical setting.
In summary, drug resistance in colon cancer is still an obstacle to successful chemotherapy and novel therapeutic strategies are urgently needed. Hence, investigation on the underlying mechanisms conferring drug resistance, as well as development of safe and effective reversing agents by targeting these mechanisms, will play a pivotal role in the successful chemotherapy for colon cancer.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aravalli RN S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma S
| 1. | Gill S, Thomas RR, Goldberg RM. Review article: colorectal cancer chemotherapy. Aliment Pharmacol Ther. 2003;18:683-692. [PubMed] [Cited in This Article: ] |
| 2. | Hu T, Li LF, Shen J, Zhang L, Cho CH. Chronic inflammation and colorectal cancer: the role of vascular endothelial growth factor. Curr Pharm Des. 2015;21:2960-2967. [PubMed] [Cited in This Article: ] |
| 3. | Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu WK, Wang L, Hu T, Li MX, Cho CH. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review). Int J Mol Med. 2014;34:372-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Stein A, Atanackovic D, Bokemeyer C. Current standards and new trends in the primary treatment of colorectal cancer. Eur J Cancer. 2011;47 Suppl 3:S312-S314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1112] [Cited by in F6Publishing: 1147] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 6. | Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 7. | White NJ. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301-308. [PubMed] [Cited in This Article: ] |
| 8. | Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152-162. [PubMed] [Cited in This Article: ] |
| 9. | Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159-165. [PubMed] [Cited in This Article: ] |
| 10. | Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3291] [Cited by in F6Publishing: 3084] [Article Influence: 280.4] [Reference Citation Analysis (0)] |
| 11. | Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265-283. [PubMed] [Cited in This Article: ] |
| 12. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4043] [Cited by in F6Publishing: 3969] [Article Influence: 180.4] [Reference Citation Analysis (0)] |
| 13. | Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2418] [Cited by in F6Publishing: 2385] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 14. | Duan H, Hu T, Foti RS, Pan Y, Swaan PW, Wang J. Potent and Selective Inhibition of Plasma Membrane Monoamine Transporter by HIV Protease Inhibitors. Drug Metab Dispos. 2015;43:1773-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2565] [Cited by in F6Publishing: 2637] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 16. | Sharom FJ. Shedding light on drug transport: structure and function of the P-glycoprotein multidrug transporter (ABCB1). Biochem Cell Biol. 2006;84:979-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Ward AB, Szewczyk P, Grimard V, Lee CW, Martinez L, Doshi R, Caya A, Villaluz M, Pardon E, Cregger C. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc Natl Acad Sci USA. 2013;110:13386-13391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs). Essays Biochem. 2011;50:179-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Liu Z, Qiu M, Tang QL, Liu M, Lang N, Bi F. Establishment and biological characteristics of oxaliplatin-resistant human colon cancer cell lines. Chin J Cancer. 2010;29:661-667. [PubMed] [Cited in This Article: ] |
| 21. | Ekblad L, Kjellström J, Johnsson A. Reduced drug accumulation is more important in acquired resistance against oxaliplatin than against cisplatin in isogenic colon cancer cells. Anticancer Drugs. 2010;21:523-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Yuan J, Lv H, Peng B, Wang C, Yu Y, He Z. Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009;63:1103-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Fu D, Arias IM. Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol. 2012;44:461-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Spoelstra EC, Dekker H, Schuurhuis GJ, Broxterman HJ, Lankelma J. P-glycoprotein drug efflux pump involved in the mechanisms of intrinsic drug resistance in various colon cancer cell lines. Evidence for a saturation of active daunorubicin transport. Biochem Pharmacol. 1991;41:349-359. [PubMed] [Cited in This Article: ] |
| 25. | Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84:265-269. [PubMed] [Cited in This Article: ] |
| 26. | Meschini S, Calcabrini A, Monti E, Del Bufalo D, Stringaro A, Dolfini E, Arancia G. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int J Cancer. 2000;87:615-628. [PubMed] [Cited in This Article: ] |
| 27. | Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, de Boeck G, Sparreboom A, Verweij J, Nooter K. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4:747-752. [PubMed] [Cited in This Article: ] |
| 28. | Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 332] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 29. | Ding Z, Yang L, Xie X, Xie F, Pan F, Li J, He J, Liang H. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J Cancer Res Clin Oncol. 2010;136:1697-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Chen J, Ding Z, Peng Y, Pan F, Li J, Zou L, Zhang Y, Liang H. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One. 2014;9:e98882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Wang T, Chen Z, Zhu Y, Pan Q, Liu Y, Qi X, Jin L, Jin J, Ma X, Hua D. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J Biol Chem. 2015;290:448-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Meijer GA, Schroeijers AB, Flens MJ, Meuwissen SG, van der Valk P, Baak JP, Scheper RJ. Increased expression of multidrug resistance related proteins Pgp, MRP1, and LRP/MVP occurs early in colorectal carcinogenesis. J Clin Pathol. 1999;52:450-454. [PubMed] [Cited in This Article: ] |
| 33. | Klappe K, Hinrichs JW, Kroesen BJ, Sietsma H, Kok JW. MRP1 and glucosylceramide are coordinately over expressed and enriched in rafts during multidrug resistance acquisition in colon cancer cells. Int J Cancer. 2004;110:511-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Tatebe S, Sinicrope FA, Kuo MT. Induction of multidrug resistance proteins MRP1 and MRP3 and gamma-glutamylcysteine synthetase gene expression by nonsteroidal anti-inflammatory drugs in human colon cancer cells. Biochem Biophys Res Commun. 2002;290:1427-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Jendzelovský R, Mikes J, Koval’ J, Soucek K, Procházková J, Kello M, Sacková V, Hofmanová J, Kozubík A, Fedorocko P. Drug efflux transporters, MRP1 and BCRP, affect the outcome of hypericin-mediated photodynamic therapy in HT-29 adenocarcinoma cells. Photochem Photobiol Sci. 2009;8:1716-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Lv Y, Zhao S, Han J, Zheng L, Yang Z, Zhao L. Hypoxia-inducible factor-1α induces multidrug resistance protein in colon cancer. Onco Targets Ther. 2015;8:1941-1948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Ikegami Y, Tatebe S, Lin-Lee YC, Xie QW, Ishikawa T, Kuo MT. Induction of MRP1 and gamma-glutamylcysteine synthetase gene expression by interleukin 1beta is mediated by nitric oxide-related signalings in human colorectal cancer cells. J Cell Physiol. 2000;185:293-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 38. | Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8-13. [PubMed] [Cited in This Article: ] |
| 39. | Ross DD, Yang W, Abruzzo LV, Dalton WS, Schneider E, Lage H, Dietel M, Greenberger L, Cole SP, Doyle LA. Atypical multidrug resistance: breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. J Natl Cancer Inst. 1999;91:429-433. [PubMed] [Cited in This Article: ] |
| 40. | Jung KA, Choi BH, Kwak MK. The c-MET/PI3K signaling is associated with cancer resistance to doxorubicin and photodynamic therapy by elevating BCRP/ABCG2 expression. Mol Pharmacol. 2015;87:465-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Zhu MM, Tong JL, Xu Q, Nie F, Xu XT, Xiao SD, Ran ZH. Increased JNK1 signaling pathway is responsible for ABCG2-mediated multidrug resistance in human colon cancer. PLoS One. 2012;7:e41763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Herraez E, Gonzalez-Sanchez E, Vaquero J, Romero MR, Serrano MA, Marin JJ, Briz O. Cisplatin-induced chemoresistance in colon cancer cells involves FXR-dependent and FXR-independent up-regulation of ABC proteins. Mol Pharm. 2012;9:2565-2576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Erlichman C, Boerner SA, Hallgren CG, Spieker R, Wang XY, James CD, Scheffer GL, Maliepaard M, Ross DD, Bible KC. The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res. 2001;61:739-748. [PubMed] [Cited in This Article: ] |
| 44. | Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, Katano M. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30:2041-2048. [PubMed] [Cited in This Article: ] |
| 45. | Wilson BJ, Schatton T, Zhan Q, Gasser M, Ma J, Saab KR, Schanche R, Waaga-Gasser AM, Gold JS, Huang Q. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71:5307-5316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Hinoshita E, Uchiumi T, Taguchi K, Kinukawa N, Tsuneyoshi M, Maehara Y, Sugimachi K, Kuwano M. Increased expression of an ATP-binding cassette superfamily transporter, multidrug resistance protein 2, in human colorectal carcinomas. Clin Cancer Res. 2000;6:2401-2407. [PubMed] [Cited in This Article: ] |
| 47. | Gradilone A, Pulcinelli FM, Lotti LV, Trifirò E, Martino S, Gandini O, Gianni W, Frati L, Aglianò AM, Gazzaniga P. Celecoxib upregulates multidrug resistance proteins in colon cancer: lack of synergy with standard chemotherapy. Curr Cancer Drug Targets. 2008;8:414-420. [PubMed] [Cited in This Article: ] |
| 48. | Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 466] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 49. | Wu Y, Zhang Y, Zhang W, Sun C, Wu J, Tang J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf B Biointerfaces. 2016;138:60-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Xie N, Mou L, Yuan J, Liu W, Deng T, Li Z, Jing Y, Hu Z. Modulating drug resistance by targeting BCRP/ABCG2 using retrovirus-mediated RNA interference. PLoS One. 2014;9:e103463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Kowalski P, Surowiak P, Lage H. Reversal of different drug-resistant phenotypes by an autocatalytic multitarget multiribozyme directed against the transcripts of the ABC transporters MDR1/P-gp, MRP2, and BCRP. Mol Ther. 2005;11:508-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Lo YL, Liu Y. Reversing multidrug resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS One. 2014;9:e90180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Lo YL, Liu Y, Tsai JC. Overcoming multidrug resistance using liposomal epirubicin and antisense oligonucleotides targeting pump and nonpump resistances in vitro and in vivo. Biomed Pharmacother. 2013;67:261-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946-2025. [PubMed] [Cited in This Article: ] |
| 55. | Li XQ, Wang L, Lei Y, Hu T, Zhang FL, Cho CH, To KK. Reversal of P-gp and BCRP-mediated MDR by tariquidar derivatives. Eur J Med Chem. 2015;101:560-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1:93-105. [PubMed] [Cited in This Article: ] |
| 57. | Crowley E, McDevitt CA, Callaghan R. Generating inhibitors of P-glycoprotein: where to, now? Methods Mol Biol. 2010;596:405-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Benson AB 3rd, Trump DL, Koeller JM, Egorin MI, Olman EA, Witte RS, Davis TE, Tormey DC. Phase I study of vinblastine and verapamil given by concurrent iv infusion. Cancer Treat Rep. 1985;69:795-799. [PubMed] [Cited in This Article: ] |
| 59. | Yahanda AM, Alder KM, Fisher GA, Brophy NA, Halsey J, Hardy RI, Gosland MP, Lum BL, Sikic BI. Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1992;10:1624-1634. [PubMed] [Cited in This Article: ] |
| 60. | Warner E, Hedley D, Andrulis I, Myers R, Trudeau M, Warr D, Pritchard KI, Blackstein M, Goss PE, Franssen E. Phase II study of dexverapamil plus anthracycline in patients with metastatic breast cancer who have progressed on the same anthracycline regimen. Clin Cancer Res. 1998;4:1451-1457. [PubMed] [Cited in This Article: ] |
| 61. | van der Holt B, Löwenberg B, Burnett AK, Knauf WU, Shepherd J, Piccaluga PP, Ossenkoppele GJ, Verhoef GE, Ferrant A, Crump M. The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood. 2005;106:2646-2654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Pusztai L, Wagner P, Ibrahim N, Rivera E, Theriault R, Booser D, Symmans FW, Wong F, Blumenschein G, Fleming DR. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104:682-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 63. | Zhang W, Han Y, Lim SL, Lim LY. Dietary regulation of P-gp function and expression. Expert Opin Drug Metab Toxicol. 2009;5:789-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Wu CP, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:609-620. [PubMed] [Cited in This Article: ] |
| 65. | Li Y, Revalde JL, Reid G, Paxton JW. Interactions of dietary phytochemicals with ABC transporters: possible implications for drug disposition and multidrug resistance in cancer. Drug Metab Rev. 2010;42:590-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Hu T, To KK, Wang L, Zhang L, Lu L, Shen J, Chan RL, Li M, Yeung JH, Cho CH. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine. 2014;21:1264-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 67. | Zhang Y, Zhang YK, Wang YJ, Vispute SG, Jain S, Chen Y, Li J, Youssef DT, El Sayed KA, Chen ZS. Esters of the marine-derived triterpene sipholenol A reverse P-GP-mediated drug resistance. Mar Drugs. 2015;13:2267-2286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Neerati P, Sudhakar YA, Kanwar JR. Curcumin Regulates Colon Cancer by Inhibiting P-Glycoprotein in In-situ Cancerous Colon Perfusion Rat Model. J Cancer Sci Ther. 2013;5:313-319. [PubMed] [Cited in This Article: ] |
| 69. | Nabekura T. Overcoming multidrug resistance in human cancer cells by natural compounds. Toxins (Basel). 2010;2:1207-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Lewis AD, Forrester LM, Hayes JD, Wareing CJ, Carmichael J, Harris AL, Mooghen M, Wolf CR. Glutathione S-transferase isoenzymes in human tumours and tumour derived cell lines. Br J Cancer. 1989;60:327-331. [PubMed] [Cited in This Article: ] |
| 71. | Hao XY, Widersten M, Ridderström M, Hellman U, Mannervik B. Co-variation of glutathione transferase expression and cytostatic drug resistance in HeLa cells: establishment of class Mu glutathione transferase M3-3 as the dominating isoenzyme. Biochem J. 1994;297:59-67. [PubMed] [Cited in This Article: ] |
| 72. | Drake FH, Zimmerman JP, McCabe FL, Bartus HF, Per SR, Sullivan DM, Ross WE, Mattern MR, Johnson RK, Crooke ST. Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem. 1987;262:16739-16747. [PubMed] [Cited in This Article: ] |
| 73. | Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 365] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 74. | Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541-546. [PubMed] [Cited in This Article: ] |
| 75. | Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1995] [Cited by in F6Publishing: 1994] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 76. | Tóthová E, Fricova M, Stecová N, Kafková A, Elbertová A. High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Neoplasma. 2002;49:141-144. [PubMed] [Cited in This Article: ] |
| 77. | Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2013;2:e23510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57-70. [PubMed] [Cited in This Article: ] |
| 79. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7556] [Cited by in F6Publishing: 8639] [Article Influence: 508.2] [Reference Citation Analysis (0)] |
| 80. | Solary E, Dubrez L, Eymin B. The role of apoptosis in the pathogenesis and treatment of diseases. Eur Respir J. 1996;9:1293-1305. [PubMed] [Cited in This Article: ] |
| 81. | Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941-2953. [PubMed] [Cited in This Article: ] |
| 82. | Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2430] [Cited by in F6Publishing: 2658] [Article Influence: 156.4] [Reference Citation Analysis (0)] |
| 83. | Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1319] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 84. | Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 85. | Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 602] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 86. | Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013;65:1162-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 87. | Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2692] [Cited by in F6Publishing: 2884] [Article Influence: 169.6] [Reference Citation Analysis (0)] |
| 88. | Chapman RS, Whetton AD, Chresta CM, Dive C. Characterization of drug resistance mediated via the suppression of apoptosis by Abelson protein tyrosine kinase. Mol Pharmacol. 1995;48:334-343. [PubMed] [Cited in This Article: ] |
| 89. | Chapman RS, Whetton AD, Dive C. The suppression of drug-induced apoptosis by activation of v-ABL protein tyrosine kinase. Cancer Res. 1994;54:5131-5137. [PubMed] [Cited in This Article: ] |
| 90. | Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 91. | Hu T, Wang L, Zhang L, Lu L, Shen J, Chan RL, Li M, Wu WK, To KK, Cho CH. Sensitivity of apoptosis-resistant colon cancer cells to tanshinones is mediated by autophagic cell death and p53-independent cytotoxicity. Phytomedicine. 2015;22:536-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 92. | Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989-992. [PubMed] [Cited in This Article: ] |
| 93. | Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 825] [Cited by in F6Publishing: 890] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 94. | Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31-53. [PubMed] [Cited in This Article: ] |
| 95. | Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2716] [Cited by in F6Publishing: 2620] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 96. | Cadwell C, Zambetti GP. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene. 2001;277:15-30. [PubMed] [Cited in This Article: ] |
| 97. | Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1484] [Cited by in F6Publishing: 1527] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 98. | Samuel T, Weber HO, Funk JO. Linking DNA damage to cell cycle checkpoints. Cell Cycle. 2002;1:162-168. [PubMed] [Cited in This Article: ] |
| 99. | Attardi LD, DePinho RA. Conquering the complexity of p53. Nat Genet. 2004;36:7-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Hemann MT, Lowe SW. The p53-Bcl-2 connection. Cell Death Differ. 2006;13:1256-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 101. | Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855-4878. [PubMed] [Cited in This Article: ] |
| 102. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [Cited in This Article: ] |
| 103. | Liu Y, Bodmer WF. Analysis of P53 mutations and their expression in 56 colorectal cancer cell lines. Proc Natl Acad Sci USA. 2006;103:976-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 104. | Reles A, Wen WH, Schmider A, Gee C, Runnebaum IB, Kilian U, Jones LA, El-Naggar A, Minguillon C, Schönborn I. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001;7:2984-2997. [PubMed] [Cited in This Article: ] |
| 105. | Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116:4077-4085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 1011] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 106. | Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957-967. [PubMed] [Cited in This Article: ] |
| 107. | O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285-4300. [PubMed] [Cited in This Article: ] |
| 108. | Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD, Longley DB, Johnston PG. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158-2167. [PubMed] [Cited in This Article: ] |
| 109. | Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 861] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 110. | Benhattar J, Cerottini JP, Saraga E, Metthez G, Givel JC. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer. 1996;69:190-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 111. | Fichtner I, Slisow W, Gill J, Becker M, Elbe B, Hillebrand T, Bibby M. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur J Cancer. 2004;40:298-307. [PubMed] [Cited in This Article: ] |
| 112. | Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12:1691-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 113. | Okamoto K, Zaanan A, Kawakami H, Huang S, Sinicrope FA. Reversal of Mutant KRAS-Mediated Apoptosis Resistance by Concurrent Noxa/Bik Induction and Bcl-2/Bcl-xL Antagonism in Colon Cancer Cells. Mol Cancer Res. 2015;13:659-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Gariboldi MB, Taiana E, Bonzi MC, Craparotta I, Giovannardi S, Mancini M, Monti E. The BH3-mimetic obatoclax reduces HIF-1α levels and HIF-1 transcriptional activity and sensitizes hypoxic colon adenocarcinoma cells to 5-fluorouracil. Cancer Lett. 2015;364:156-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Traini R, Ben-Josef G, Pastrana DV, Moskatel E, Sharma AK, Antignani A, Fitzgerald DJ. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther. 2010;9:2007-2015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Maamer-Azzabi A, Ndozangue-Touriguine O, Bréard J. Metastatic SW620 colon cancer cells are primed for death when detached and can be sensitized to anoikis by the BH3-mimetic ABT-737. Cell Death Dis. 2013;4:e801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3767] [Cited by in F6Publishing: 3625] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 118. | Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311-2319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 694] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 119. | Lefranc F, Facchini V, Kiss R. Proautophagic drugs: a novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 120. | Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 722] [Cited by in F6Publishing: 738] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 121. | Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336-3346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 430] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 122. | Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2004;101:18030-18035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 466] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 123. | Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 372] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 124. | Mukhtar E, Adhami VM, Khan N, Mukhtar H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr Drug Targets. 2012;13:1831-1841. [PubMed] [Cited in This Article: ] |
| 125. | Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012;45:466-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 126. | Hu T, Cho CH. Potential Applications of Tanshinones in Gastrointestinal and Hepatic Diseases. J Biomol Res Ther. 2013;2:110. [DOI] [Cited in This Article: ] |
| 127. | Wang L, Hu T, Shen J, Zhang L, Chan RL, Lu L, Li M, Cho CH, Wu WK. Dihydrotanshinone I induced apoptosis and autophagy through caspase dependent pathway in colon cancer. Phytomedicine. 2015;22:1079-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 128. | Mahyar-Roemer M, Katsen A, Mestres P, Roemer K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer. 2001;94:615-622. [PubMed] [Cited in This Article: ] |
| 129. | Wang L, Yeung JH, Hu T, Lee WY, Lu L, Zhang L, Shen J, Chan RL, Wu WK, Cho CH. Dihydrotanshinone induces p53-independent but ROS-dependent apoptosis in colon cancer cells. Life Sci. 2013;93:344-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 130. | Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9:95-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 131. | Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 2006;25:231-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 132. | Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12:273-286. [PubMed] [Cited in This Article: ] |
| 133. | Rowinsky EK, Smith L, Wang YM, Chaturvedi P, Villalona M, Campbell E, Aylesworth C, Eckhardt SG, Hammond L, Kraynak M. Phase I and pharmacokinetic study of paclitaxel in combination with biricodar, a novel agent that reverses multidrug resistance conferred by overexpression of both MDR1 and MRP. J Clin Oncol. 1998;16:2964-2976. [PubMed] [Cited in This Article: ] |
| 134. | Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 2006;580:1103-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 135. | O’Connor R, O’Leary M, Ballot J, Collins CD, Kinsella P, Mager DE, Arnold RD, O’Driscoll L, Larkin A, Kennedy S. A phase I clinical and pharmacokinetic study of the multi-drug resistance protein-1 (MRP-1) inhibitor sulindac, in combination with epirubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2007;59:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV, Limtrakul P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother Pharmacol. 2006;57:376-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 137. | Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J. 2005;272:4725-4740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 138. | van Zanden JJ, de Mul A, Wortelboer HM, Usta M, van Bladeren PJ, Rietjens IM, Cnubben NH. Reversal of in vitro cellular MRP1 and MRP2 mediated vincristine resistance by the flavonoid myricetin. Biochem Pharmacol. 2005;69:1657-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 139. | Dharmapuri G, Doneti R, Philip GH, Kalle AM. Celecoxib sensitizes imatinib-resistant K562 cells to imatinib by inhibiting MRP1-5, ABCA2 and ABCG2 transporters via Wnt and Ras signaling pathways. Leuk Res. 2015;39:696-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 140. | Zhang H, Patel A, Ma SL, Li XJ, Zhang YK, Yang PQ, Kathawala RJ, Wang YJ, Anreddy N, Fu LW. In vitro, in vivo and ex vivo characterization of ibrutinib: a potent inhibitor of the efflux function of the transporter MRP1. Br J Pharmacol. 2014;171:5845-5857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Cihalova D, Ceckova M, Kucera R, Klimes J, Staud F. Dinaciclib, a cyclin-dependent kinase inhibitor, is a substrate of human ABCB1 and ABCG2 and an inhibitor of human ABCC1 in vitro. Biochem Pharmacol. 2015;98:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 143. | Ahmed-Belkacem A, Pozza A, Muñoz-Martínez F, Bates SE, Castanys S, Gamarro F, Di Pietro A, Pérez-Victoria JM. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005;65:4852-4860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 144. | Morita H, Koyama K, Sugimoto Y, Kobayashi J. Antimitotic activity and reversal of breast cancer resistance protein-mediated drug resistance by stilbenoids from Bletilla striata. Bioorg Med Chem Lett. 2005;15:1051-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 146. | Ahmed-Belkacem A, Macalou S, Borrelli F, Capasso R, Fattorusso E, Taglialatela-Scafati O, Di Pietro A. Nonprenylated rotenoids, a new class of potent breast cancer resistance protein inhibitors. J Med Chem. 2007;50:1933-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 147. | Li CY, Huang WF, Wang QL, Wang F, Cai E, Hu B, Du JC, Wang J, Chen R, Cai XJ. Crocetin induces cytotoxicity in colon cancer cells via p53-independent mechanisms. Asian Pac J Cancer Prev. 2012;13:3757-3761. [PubMed] [Cited in This Article: ] |
| 148. | Lian F, Li Y, Bhuiyan M, Sarkar FH. p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer. 1999;33:125-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 149. | Besbes S, Pocard M, Mirshahi M, Billard C. The first MCL-1-selective BH3 mimetics have therapeutic potential for chronic lymphocytic leukemia. Crit Rev Oncol Hematol. 2016;100:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 150. | Ke EE, Wu YL. Afatinib in the first-line treatment of epidermal-growth-factor-receptor mutation-positive non-small cell lung cancer: a review of the clinical evidence. Ther Adv Respir Dis. 2016;10:256-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Lo Nigro C, Ricci V, Vivenza D, Monteverde M, Strola G, Lucio F, Tonissi F, Miraglio E, Granetto C, Fortunato M. Evaluation of antibody-dependent cell-mediated cytotoxicity activity and cetuximab response in KRAS wild-type metastatic colorectal cancer patients. World J Gastrointest Oncol. 2016;8:222-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 152. | Ou SH, Soo RA. Dacomitinib in lung cancer: a “lost generation” EGFR tyrosine-kinase inhibitor from a bygone era? Drug Des Devel Ther. 2015;9:5641-5653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 153. | Kaseb AO, Morris JS, Iwasaki M, Al-Shamsi HO, Raghav KP, Girard L, Cheung S, Nguyen V, Elsayes KM, Xiao L. Phase II trial of bevacizumab and erlotinib as a second-line therapy for advanced hepatocellular carcinoma. Onco Targets Ther. 2016;9:773-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | Bogdanowicz BS, Hoch MA, Hartranft ME. Flipped script for gefitinib: A reapproved tyrosine kinase inhibitor for first-line treatment of epidermal growth factor receptor mutation positive metastatic nonsmall cell lung cancer. J Oncol Pharm Pract. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 155. | Saloura V, Cohen EE, Licitra L, Billan S, Dinis J, Lisby S, Gauler TC. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2014;73:1227-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 156. | Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015;125:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |