Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3496
Peer-review started: January 11, 2017
First decision: February 9, 2017
Revised: February 21, 2017
Accepted: March 31, 2017
Article in press: March 31, 2017
Published online: May 21, 2017
To determine the prevalence and diagnostic value of autoantibodies in α-fetoprotein (AFP)-negative hepatocellular carcinoma (HCC).
Fifty-six serum samples from AFP-negative HCC cases, 86 from AFP-positive HCC cases, 168 from chronic liver disease cases, and 59 from normal human controls were included in this study. Autoantibodies to nucleophosmin (NPM)1, 14-3-3zeta and mouse double minute 2 homolog (MDM2) proteins in AFP-negative HCC serum were evaluated by enzyme-linked immunosorbent assay. Partially positive sera were further evaluated by western blotting. Immunohistochemistry was used to detect the expression of three tumor-associated antigens (TAAs) in AFP-negative HCC and normal control tissues.
The frequency of autoantibodies to the three TAAs in AFP-negative HCC sera was 21.4%, 19.6% and 19.6%, which was significantly higher than in the chronic liver disease cases and normal human controls (P < 0.01) as well as AFP-positive HCC cases. The sensitivity of the three autoantibodies for diagnosis of AFP-negative HCC ranged from 19.6% to 21.4%, and the specificity was approximately 95%. When the three autoantibodies were combined, the sensitivity reached 30.4% and the specificity reached 91.6%.
Autoantibodies to NPM1, 14-3-3zeta and MDM2 may be useful biomarkers for immunodiagnosis of AFP-negative HCC.
Core tip: We firstly and specifically investigated the diagnostic value of autoantibodies in α-fetoprotein (AFP)-negative hepatocellular carcinoma (HCC). We retrospectively evaluated the prevalence and diagnostic value of autoantibodies to nucleophosmin (NPM)1, 14-3-3zeta and mouse double minute 2 homolog (MDM2) proteins and their different combinations in 56 AFP-negative HCC patients by enzyme-linked immunosorbent assay and western blotting. Immunohistochemistry was used to detect the expression of three tumor-associated antigens (TAAs) in AFP-negative HCC. Our study demonstrated that autoantibodies to NPM1, 14-3-3zeta and MDM2 may be useful biomarkers for immunodiagnosis of AFP-negative HCC.
- Citation: Wang T, Liu M, Zheng SJ, Bian DD, Zhang JY, Yao J, Zheng QF, Shi AM, Li WH, Li L, Chen Y, Wang JH, Duan ZP, Dong L. Tumor-associated autoantibodies are useful biomarkers in immunodiagnosis of α-fetoprotein-negative hepatocellular carcinoma. World J Gastroenterol 2017; 23(19): 3496-3504
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3496
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related death worldwide. Nearly 50% of the new cases of liver cancer and related deaths occur in China[1]. Due to the lack of reliable methods for early diagnosis, most patients die within 1 year after diagnosis of HCC. Although ultrasound is used as an assistive tool for early detection of HCC, it is not sufficiently sensitive and is operator dependent. Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended as common screening tools for HCC because of the attendant radiation exposure and high cost[2,3]. So far, α-fetoprotein (AFP) is still the only widely used clinical serum biomarker; however, some studies have shown that the sensitivity and predictive value of AFP for the diagnosis of HCC is only 41%-65% and 12%, respectively, especially for early HCC and AFP-negative HCC. There are still approximately 40% of cases of HCC with normal AFP levels that cannot be detected early[4,5].
HCC can be diagnosed by significantly increased serum AFP levels and definitive imaging results. However, AFP-negative HCC cannot be diagnosed easily and depends largely on imaging results, which often leads to misdiagnosis[6]. Thus, many HCC patients cannot obtain timely diagnosis and treatment. In recent years, numerous studies have been performed to identify a diagnostic biomarker for HCC[7], however, all of the potential candidates have shown poor specificity and sensitivity, and there are few studies on AFP-negative HCC[8,9].
Recently, many studies have shown that the serum of cancer patients contains autoantibodies that react with a unique group of autologous cellular antigens known as tumor-associated antigens (TAAs)[10,11]. Unlike autoantibodies appearing in autoimmune diseases, TAA autoantibodies have been detected in a variety of tumors[12]. Some autoantibodies are present several months to years before the clinical diagnosis of tumor[13-15]. Furthermore, TAA autoantibodies may have greater advantages as immunodiagnostic markers, because their magnified signals can be easier to detect than TAAs themselves[12,16]. One drawback of this method is the lower sensitivity when a single or individual TAA is used in diagnosis of HCC. However, this drawback can be overcome by using a panel of carefully selected TAAs to improve the sensitivity and specificity[17,18]. Therefore, TAA autoantibodies seem to have great potential in early diagnosis of cancer.
Although many studies have been performed to determine the roles of autoantibodies to TAAs in immunodiagnosis of HCC, no previous study has specifically evaluated the diagnostic value of TAA autoantibodies in AFP-negative HCC. Our previous studies have shown that the level of autoantibodies to nucleophosmin (NPM)1, 14-3-3zeta autoantibody and mouse double minute 2 homolog (MDM2) are all significantly higher in the serum of patients with HCC than other chronic liver diseases (CLDs) and normal human controls (NHCs). They were detected 6-9 mo before clinical diagnosis, which suggested that they may be potential biomarkers for early stage HCC screening and diagnosis[8,14,19].
In the present study, we evaluated the diagnostic value of autoantibodies to NPM1, 14-3-3zeta and MDM2 and their different combinations in immunodiagnosis of AFP-negative HCC.
Sera from 56 patients with AFP-negative HCC, 86 with AFP-positive HCC and 168 with CLD, and from 59 NHC samples were obtained from outpatients or inpatients between January 2015 and January 2016 at Beijing You’an Hospital, Capital Medical University. The AFP levels of all patients were measured with a commercially available electrochemiluminescence immunoassay kit (reagents from Roche Ltd, Indianapolis, IN, United States). Samples with AFP < 20 ng/mL were defined as AFP-negative. The diagnosis of AFP-negative and AFP-positive HCC patients was based on ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) characteristics and biochemistry (AFP serology and liver function enzymes), according to the Primary Liver Cancer Treatment Protocols (2011 edition). No HCC patients received any surgical treatment, such as resection, ablation or transarterial chemoembolization, chemotherapy, radiotherapy or multikinase inhibitor (sorafenib). Patients with CLD were followed up for at least 12 mo to exclude individuals with autoimmune liver diseases. This study was approved by the Institutional Review Board of Capital Medical University, Beijing, China. All enrolled patients gave written consent.
NPM1 construct GFP-NPM WT (plasmid ID: 17578), 14-3-3zeta construct GST-14-3-3 WT (plasmid ID: 1944) and MDM2 construct pGEX-4T MDM2 WT (plasmid ID: 16237) were purchased from Addgene (Cambridge, MA, United States), and the first two were subcloned into the pET28a vector. The recombinant protein NPM1 expressed in Escherichia coli BL21 (DE3) was purified using nickel column chromatography. The recombinant protein 14-3-3zeta expressed in ArcticExpress (DE3) RP was purified using nickel column chromatography. The recombinant protein MDM2 expressed in ArcticExpress (DE3) RP was purified using SP-sepharose and DEAE Sephocel.
The recombinant proteins were examined in SDS-PAGE and the expected molecular size of expression products were determined using Coomassie blue staining. In addition, western blot analysis was used to confirm that the bands seen in SDS-PAGE were reactive with corresponding antibodies.
Three recombinant proteins were respectively diluted in phosphate-buffered saline (PBS) to a final concentration of 0.5 μg/mL for coating a 96-well microtiter plate (No. 3590; Corning, Corning, NY, United States) overnight at 4 °C. The antigen-coated wells were blocked with 10% fetal bovine serum (FBS) at 37 °C for 1 h. Human serum diluted 1:200 was incubated in the antigen-coated wells for 60 min. Horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Zhongshan Golden Bridge Biological Technology Co Ltd, Beijing, China) as a secondary antibody was diluted 1:10000 for coating (1 h) followed by washing with PBS containing 0.1% Tween 20 (PBST). The 3,3’,5,5’-Tetramethylbenzidine Liquid Substrate System (Solarbio Science & Technology Co Ltd, Beijing, China) was used as the detecting agent.
The optical density (OD) value of all wells was read at 450 nm, and the cut-off value for defining a positive reaction was designated as the mean OD value of the 59 normal sera plus three standard deviations (SDs). Each microtiter plate included 10 NHC samples representing a range of absorbance values above and below the mean of 59 NHC samples, and the average OD value of 10 NHC samples was used to normalize all OD values to the standard mean of the 59 NHC samples. Each sample was tested in triplicate.
The purified recombinant proteins of three TAAs were electrophoresed on 12% SDS-PAGE and subsequently transferred to a nitrocellulose membrane. After blocking in Tris-buffered saline with 5% nonfat milk and 0.1% Tween-20 for 1 h at ambient temperature, the membranes were cut into strips and incubated with patient sera diluted 1:200, polyclonal anti-NPM1 antibody diluted 1:1000, anti-14-3-3zeta antibody diluted 1:1000 or polyclonal anti-MDM2 antibody diluted 1:1000 separately, and finally incubated with HRP-conjugated goat anti-human IgG or HRP-conjugated goat anti-rabbit IgG diluted 1:10000 for 1 h. Positive signals were detected by the ECL kit (Thermo Scientific, Waltham, MA, United States).
The liver cancer tissue array slides with normal tissue controls (9 AFP-negative HCC tissues/10 normal tissues, including pathological diagnosis and clinical information) were purchased (Outdo Biotech Co Ltd, Shanghai, China) and used to detect the expression of the three antigen proteins. Tissue array slides were baked for 1 h and deparaffinized with xylene, and dehydrated with ethanol. Antigen retrieval was performed by microwave heating method in citrate antigen retrieval solution for 20 min. After incubation with acid methanol for 15 min, goat serum blocking solution was used to prevent nonspecific binding of antibodies. The tissue microarrays were incubated with polyclonal NPM1 antibody, polyclonal 14-3-3zeta antibody or polyclonal MDM2 antibody (1:100 dilution) for overnight at 4 °C. The HRP Detection System (HRP streptavidin label and polyvalent biotinylated link) and DAB Substrate Kit (Zhongshan Golden Bridge Biotechnology Co Ltd) were used as detecting reagents. The sections were counterstained with hematoxylin, dehydrated, and mounted. The slides were observed by light microscopy (Model BX51; Olympus, Tokyo, Japan).
A χ2 test with Yates’ correction was used to determine whether the frequency of autoantibodies to three TAAs in each cohort of patient sera was significantly higher than that in sera from normal individuals. Two significant levels (0.05 and 0.01) were used. Methods for calculating the sensitivity, specificity and accuracy were based on the methodology provided in Introduction to Epidemiology (6th edition, by Ray M. Merrill, published by Jones & Bartlett Learning Company, Burlington, 2012).
The baseline characteristics of patients in the HCC, CLD and NHC groups are summarized in Table 1. Most of the patients with AFP-negative HCC at an early stage had good liver function, lower Child-Pugh score and Barcelona Clinic Liver Cancer (BCLC) grade, and were without ascites, hepatic encephalopathy, vascular invasion or metastasis.
| Variable | AFP(-)HCC (n = 56) | AFP(+)HCC (n = 86) | CLD (n = 168) | NHC (n = 59) |
| Age, yr | 57 ± 9 | 56 ± 10 | 48 ± 14 | 39 ± 13 |
| Sex, male/female | 47/9 | 66/20 | 129/39 | 25/34 |
| AFP, ng/mL | 5.38 (3.4-8.2) | 795.1(116.1-11244.0) | 4.32 (2.5-10.4) | - |
| HBV/HCV/BC/NBNC | 36/4/6/10 | 75/5/1/5 | 103/39/3/23 | |
| ALT, U/L | 37.9 (26.2-53.6) | 40.5 (26.8-79.6) | 42.9 (24.5-135.1) | - |
| AST, U/L | 41.6 (29.8-66) | 63.9 (37.05-146.3) | 47.1 (27.8-92.6) | - |
| TBIL, μmol/L | 22.4 (15.9-33.7) | 28.4 (17.4-53.7) | 23.8 (14.8-49.1) | - |
| DBIL, μmol/L | 5.9 (3.8-10.6) | 7.3 (4.5-20.8) | 6 (3.6-18.6) | |
| ALB, g/L | 34.2 ± 5.9 | 34.8 ± 5.7 | 38.1(32.4-43) | - |
| CR, μmol/L | 63 (55.3-73.2) | 64 (52.2-74.3) | 63.85 (55.1-73.3) | - |
| INR | 1.09 (1-1.21) | 1.13 (1.03-1.24) | - | - |
| PT, s | 12.4 (11.4-13.8) | 12.7 (11.5-14.1) | - | - |
| Child-Pugh score | 6 (5-8) | 7 (6-9) | - | - |
| Child-Pugh grade, A/B/C | 6/1/49 | 62/19/5 | - | - |
| Meld score | 9 (8-11) | 10 (8-13) | - | - |
| BCLC grade, A/B/C/D | 24/16/13/3 | 21/17/42/6 | - | - |
| Tumor size, > 5 cm/< 5 cm | 19/37 | 42/44 | - | - |
| Tumor no., single/double/multiple | 4/18/34 | 31/7/48 | - | - |
| Vascular invasion, yes/no | 11/45 | 44/42 | - | - |
| Metastasis, yes/no | 5/51 | 11/75 | - | - |
| Encephalopathy, non-/1-2/3-4 | 53/3/0 | 1/4/81 | - | - |
| Ascites degree, non/low/medium/high | 24/25/2/5 | 31/40/2/13 | - | - |
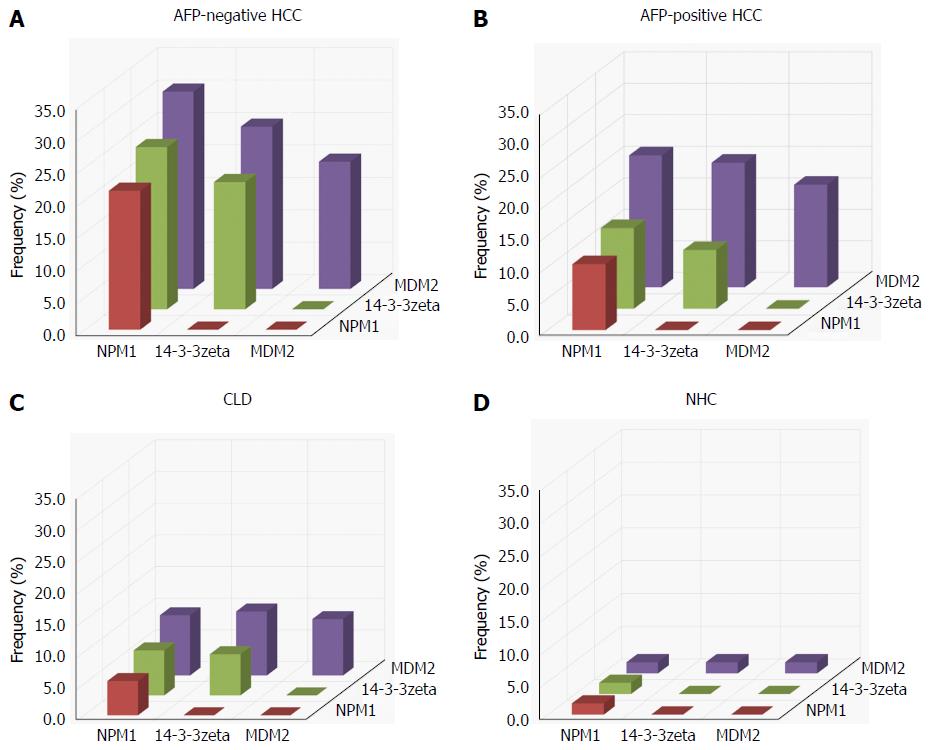
Three recombinant proteins were used as coating antigens in enzyme-linked immunosorbent assay (ELISA) to screen for autoantibodies against NPM1, 14-3-3zeta and MDM2 in sera from patients with HCC and CLD as well as NHCs. The prevalence of autoantibodies against NPM1, 14-3-3zeta and MDM2 was 21.4% (12/56), 19.6% (11/56) and 19.6% (11/56) in AFP-negative HCC, which was significantly higher than in CLD and NHCs (P < 0.01) and higher than in AFP-positive HCC, although not significantly (Table 2). These results were confirmed by western blot analysis. Representative HCC sera with a positive reaction to NPM1, 14-3-3zeta and MDM2 in ELISA also had strong reactivity in western blotting compared to CLD and normal human sera (Figure 1).
The presence or absence of co-expression of autoantibodies to any combination of two of the three TAAs in AFP-negative HCC, AFP-positive HCC, CLD and NHCs is shown in Figure 2. The frequency of AFP-negative HCC sera co-expressing antibodies to NPM1 and MDM2 was highest among all the combinations and significantly higher than in AFP-positive HCC, CLD and NHCs. Most of the normal human sera showed a low level of co-expression of antibodies to any combination of two of the three TAAs. Similar results were observed in AFP-positive HCC and CLD.
The sensitivity and specificity of diagnosis for AFP-negative HCC were 21.4% and 95.6% with NPM1 autoantibody, 19.6% and 95.2% with 14-3-3zeta autoantibody, and 19.6% and 93.0% with MDM2 autoantibody (Table 3). In a further analysis, with combined NPM1 with 14-3-3zeta autoantibodies, the sensitivity and specificity for immunodiagnosis of AFP-negative HCC reached 25% and 94.3%, respectively. When we combined 14-3-3zeta with MDM2 autoantibodies, the sensitivity and specificity reached 25% and 92.1%, respectively. When we combined NPM1 with MDM2 autoantibodies, the sensitivity and specificity reached 30.4% and 93.0%, respectively. Finally, when we combined the three antigens, the sensitivity was still 30.4% and specificity was maintained at 91.6%. This suggested that the three TAA autoantibodies had higher consistency in the diagnosis of AFP-negative HCC. The accuracy for this TAA array was 79.5%.
| Type of sera | Sensitivity | Specificity | Accuracy |
| NPM1 | 21.4% | 95.6% | 80.9% |
| 14-3-3zeta | 19.6% | 95.2% | 80.2% |
| MDM2 | 19.6% | 93.0% | 78.4% |
| NPM1 + 14-3-3zeta | 25.0% | 94.3% | 80.7% |
| NPM1 + MDM2 | 30.4% | 93.0% | 80.7% |
| 14-3-3zeta + MDM2 | 25.0% | 92.1% | 78.8% |
| NPM1 + 14-3-3zeta + MDM2 | 30.4% | 91.6% | 79.5% |
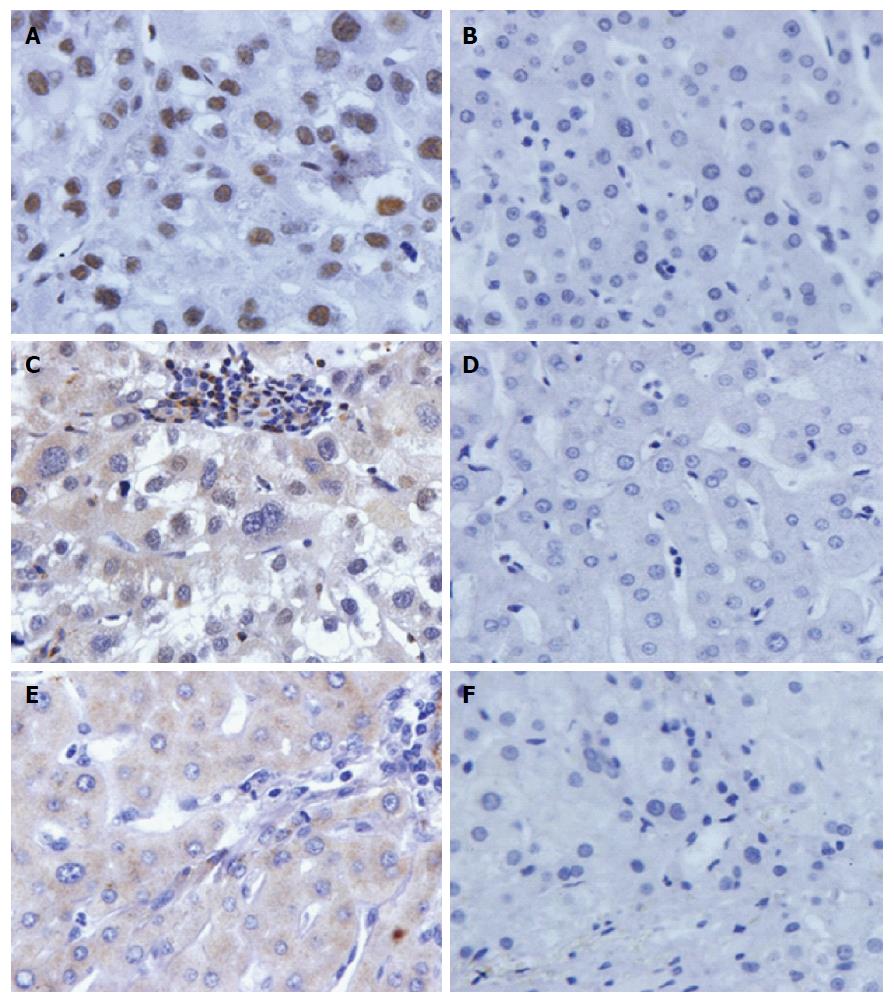
The expression profiles of three proteins in AFP-negative HCC tissues and normal liver tissues was examined by immunohistochemistry of tissue array slides. Tissue array slides were commercially available for this study, and included 9 AFP-negative HCC tissues and 10 normal hepatic tissues. The polyclonal NPM1 antibody, 14-3-3zeta antibody and MDM2 antibody were used as primary antibodies to detect the expression of the three proteins in liver cancer and normal hepatic tissues. The characteristics of patients and protein expression in AFP-negative HCC are shown in Table 4.
| Variable | Age, yr | Sex, male/female | Grade, I-II/III-IV | Frequency | ||
| NPM1 | 14-3-3zeta | MDM2 | ||||
| Liver cancer | 52 ± 9 | 8/1 | 6/3 | 9 (100) | 5 (55.6) | 3 (33.3) |
| Normal liver tissue | 58 ± 9 | 9/1 | - | 2 (20.0) | 1 (10.0) | 2 (20.0) |
All 9 HCC tissues and 2/10 normal hepatic tissues were positively stained in the NPM1 autoantibody group. Five of the 9 HCC tissues and 1/10 of the normal hepatic tissues were positively stained in the 14-3-3zeta autoantibody group, and 3/9 HCC tissues and 2/10 normal hepatic tissues were positively stained in the MDM2 autoantibody group. Due to the small sample size of tissues in this study, it was difficult to perform a statistical analysis. The expression of the three proteins in AFP-negative HCC tissues and normal hepatic tissues is shown in Figure 3.
In this study, we firstly and specifically evaluated the diagnostic value of three TAA autoantibodies and their different combinations in immunodiagnosis of AFP-negative HCC. The sensitivity of diagnosis for AFP-negative HCC was 19.6%-21.4% for the three TAA autoantibodies, and specificity was approximately 95%. When we combined two of the TAA autoantibodies, the diagnostic sensitivity for AFP-negative HCC was significantly increased. When we combined three of the autoantibodies, the sensitivity reached 30.4%, with a higher level of specificity.
Furthermore, we examined the expression level of three TAA proteins in AFP-negative HCC tissues. The three proteins were all overexpressed in HCC tissues, and 30%-100% of AFP-negative HCC liver tissues were positively stained with three TAA autoantibodies. Due to the small sample size of AFP-negative HCC tissues in this study, it was difficult to perform a further statistical analysis.
With immunological proteomics technology, a variety of TAAs and TAA autoantibodies have be detected in HCC, such as Imp-1[20], c-Myc[21] and CIP2A/p90[22]. NPM1 (also known as nucleolar phosphoprotein B23 or numatrin) is a member of the nucleoplasmin family, and has multiple functional roles, including in cell proliferation[23], DNA repair[24], tumorigenesis[25] and apoptosis[26]. A previous study has demonstrated that NPM1 is expressed highly in liver cancer cells and weakly in normal hepatocytes, which is closely related to tumor grade and poor prognosis; thus, it is possible that NPM1 can be a TAA biomarker for early HCC diagnosis[27].
The 14-3-3zeta protein is one of the 14-3-3 protein family members, which is a group of highly conserved acidic proteins encoded by different genes and which includes the β, γ, ε, ζ (zeta), η, σ, and τ isoforms in mammals[28]. Studies have shown that the 14-3-3zeta protein is overexpressed in a variety of tumor types, including HCC[29,30].
The MDM2 oncogene, biochemically known as E3 ubiquitin protein ligase, is deregulated in many human cancers and exerts oncogenic activity predominantly by binding to p53 and inhibiting p53 transactivation function as well as the p53 tumor suppressor, thus resulting in tumorigenesis[31]. Our previous studies[8,14,19] have shown that the levels of NPM1, anti-14-3-3zeta and anti-MDM2 autoantibodies were all significantly higher in the HCC patient sera, with a 16.7%-22.4% positive rate, which was confirmed in the present study. In addition, we specifically evaluated the diagnostic value of three TAA autoantibodies and their different combinations in immunodiagnosis of AFP-negative HCC.
Some researchers have tried to find serological biomarkers for diagnosis of AFP-negative HCC, but only a few have investigated the present antigenic proteins in serum or tissue of AFP-negative HCC patients. Zhang et al[32] found that the sensitivity of AFP-L3 and GP73 for diagnosis of AFP-negative HCC was 50.0% and 66.0%, respectively, and combination of AFP-L3 and GP73 improved diagnostic accuracy and sensitivity. Li et al[33] tested liver tissue glypican (GPC)3 (GPC3L) expression to evaluate the diagnostic value of GPC3 in patients with AFP-negative hepatitis-B-related HCC and 80.0% of HCC samples were positive for GPC3L expression. However, antigen detection is often late and liver biopsy is an invasive procedure. Therefore, TAA autoantibodies have unique diagnostic value due to their early appearance and magnified signals.
However, there were some limitations to our study. First, our sample size was small. In addition, we only chose three autoantibodies to evaluate in AFP-negative HCC, which is not enough to measure sensitivity. Finally, we lacked serum samples with corresponding tissue samples to address the relationship between TAA expression in HCC and serum antibody positivity.
In conclusion, our study demonstrated that autoantibodies to NPM1, 14-3-3zeta and MDM2 may be useful biomarkers for immunodiagnosis of AFP-negative HCC. More potential TAA autoantibodies could be identified and added to the panel of TAAs identified previously, to create an optimized TAA array, which would be useful to increase the sensitivity for diagnosis of AFP-negative HCC. In addition, the mechanism underlying the production of TAA autoantibodies in AFP-negative HCC remains to be investigated in serial serum samples.
The authors thank Dr. Jian-Ying Zhang (Border Biological Research Center Core Facilities at The University of Texas at El Paso) for his experimental guidance.
Many autoantibodies to tumor-associated antigens (TAAs) have been reported in hepatocellular carcinoma (HCC), and have been suggested to be useful tools for immunodiagnosis of HCC. However, no previous study has specifically evaluated the diagnostic value of TAA autoantibodies in α-fetoprotein (AFP)-negative HCC.
No previous study has specifically evaluated the diagnostic value of TAA autoantibodies in AFP-negative HCC.
This is believed to be the first study to evaluate specifically the diagnostic value of TAA autoantibodies in AFP-negative HCC.
This study demonstrated that autoantibodies to nucleophosmin 1, 14-3-3zeta and mouse double minute 2 homolog may be useful biomarkers for immunodiagnosis of AFP-negative HCC.
In this study, the authors determined the prevalence and diagnostic value of autoantibodies in AFP-negative HCC. Partially positive sera were further evaluated by western blotting. Immunohistochemistry was used to detect the expression of three TAAs in AFP-negative HCC and normal control tissues. The frequency of autoantibodies to three TAAs in AFP-negative HCC sera was significantly higher than in chronic liver diseases and normal human controls as well as AFP-positive HCC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dediulia T, Lo GH S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Lee JM, Yoon JH, Kim KW. Diagnosis of hepatocellular carcinoma: newer radiological tools. Semin Oncol. 2012;39:399-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, Gerken G, Schlaak JF. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Sutherland T, Watts J, Ryan M, Galvin A, Temple F, Vuong J, Little AF. Diffusion-weighted MRI for hepatocellular carcinoma screening in chronic liver disease: Direct comparison with ultrasound screening. J Med Imaging Radiat Oncol. 2017;61:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Meng X, Franklin DA, Dong J, Zhang Y. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2014;74:7161-7167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Liu M, Varela-Ramirez A, Li J, Dai L, Aguilera RJ, Zhang JY. Humoral autoimmune response to nucleophosmin in the immunodiagnosis of hepatocellular carcinoma. Oncol Rep. 2015;33:2245-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Dai L, Ren P, Liu M, Imai H, Tan EM, Zhang JY. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin Immunol. 2014;152:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Old LJ, Chen YT. New paths in human cancer serology. J Exp Med. 1998;187:1163-1167. [PubMed] [Cited in This Article: ] |
| 11. | Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009;276:6880-6904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen JH, Zheng CP, Wang SH, Guo HP, Li EM. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am J Gastroenterol. 2014;109:36-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Liu M, Liu X, Ren P, Li J, Chai Y, Zheng SJ, Chen Y, Duan ZP, Li N, Zhang JY. A cancer-related protein 14-3-3ζ is a potential tumor-associated antigen in immunodiagnosis of hepatocellular carcinoma. Tumour Biol. 2014;35:4247-4256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Werner S, Chen H, Tao S, Brenner H. Systematic review: serum autoantibodies in the early detection of gastric cancer. Int J Cancer. 2015;136:2243-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Lacombe J, Mangé A, Solassol J. Use of autoantibodies to detect the onset of breast cancer. J Immunol Res. 2014;2014:574981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136-143. [PubMed] [Cited in This Article: ] |
| 18. | Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EK. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J Hepatol. 2007;46:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Liu M, Zheng SJ, Chen Y, Li N, Ren PF, Dai LP, Duan ZP, Zhang JY. Autoantibody response to murine double minute 2 protein in immunodiagnosis of hepatocellular carcinoma. J Immunol Res. 2014;2014:906532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Hämmerle M, Gutschner T, Uckelmann H, Ozgur S, Fiskin E, Gross M, Skawran B, Geffers R, Longerich T, Breuhahn K. Posttranscriptional destabilization of the liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding protein 1 (IGF2BP1). Hepatology. 2013;58:1703-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Dang CV. MYC on the path to cancer. Cell. 2012;149:22-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1984] [Cited by in F6Publishing: 2305] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 22. | Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 489] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 23. | Lindström MS, Zhang Y. Ribosomal protein S9 is a novel B23/NPM-binding protein required for normal cell proliferation. J Biol Chem. 2008;283:15568-15576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Okuwaki M, Matsumoto K, Tsujimoto M, Nagata K. Function of nucleophosmin/B23, a nucleolar acidic protein, as a histone chaperone. FEBS Lett. 2001;506:272-276. [PubMed] [Cited in This Article: ] |
| 25. | Grisendi S, Bernardi R, Rossi M, Cheng K, Khandker L, Manova K, Pandolfi PP. Role of nucleophosmin in embryonic development and tumorigenesis. Nature. 2005;437:147-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 421] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 26. | Khandelwal N, Simpson J, Taylor G, Rafique S, Whitehouse A, Hiscox J, Stark LA. Nucleolar NF-κB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011;18:1889-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Liu X, Liu D, Qian D, Dai J, An Y, Jiang S, Stanley B, Yang J, Wang B, Liu X. Nucleophosmin (NPM1/B23) interacts with activating transcription factor 5 (ATF5) protein and promotes proteasome- and caspase-dependent ATF5 degradation in hepatocellular carcinoma cells. J Biol Chem. 2012;287:19599-19609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Gardino AK, Smerdon SJ, Yaffe MB. Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: a comparison of the X-ray crystal structures of all human 14-3-3 isoforms. Semin Cancer Biol. 2006;16:173-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Hatzipetros I, Gocze P, Koszegi T, Jaray A, Szereday L, Polgar B, Farkas N, Farkas B. Investigating the clinical potential for 14-3-3 zeta protein to serve as a biomarker for epithelial ovarian cancer. J Ovarian Res. 2013;6:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Fukai M, Kobayashi N, Ishikawa T, Wakayama K, Shimada S, Umemoto K, Ohtani S, Fujiyoshi M, Yamashita K, Shimamura T. 14-3-3ζ-Mediated Stimulation of Oxidative Phosphorylation Exacerbates Oxidative Damage Under Hypothermic Oxygenated Conditions in Human Renal Tubular Cells (HK-2). Transplant Proc. 2016;48:1288-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 826] [Cited by in F6Publishing: 870] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 32. | Zhang Z, Zhang Y, Wang Y, Xu L, Xu W. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther. 2016;9:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Li B, Liu H, Shang HW, Li P, Li N, Ding HG. Diagnostic value of glypican-3 in alpha fetoprotein negative hepatocellular carcinoma patients. Afr Health Sci. 2013;13:703-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |