The efficacy and safety of prone positioning in adults patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials
Introduction
Acute respiratory distress syndrome (ARDS) is one of the most common disorders requiring critical care. Despite numerous attempts to improve ventilation procedures, including protective ventilator strategies and recruitment maneuvers, the mortality rate associated with ARDS remains high, ranging between 27% and 45% (1,2).
Prone positioning ventilation has been used for four decades in patients with ARDS (3), and it can improve oxygenation (4-9), and drainage of secretions. Several mechanisms have been proposed to explain these effects, including improved ventilation perfusion mismatching (9-13), even distribution of the gravitational gradient in pleural pressure (14), and reduction in the lung stress and injury associated with mechanical ventilation (10,15). However, despite yielding significant improvements in oxygenation, prone positioning has no demonstrable impact on mortality rates based on research performed over the past few years (16-20).
A recent multicenter randomized trial by Guérin et al. demonstrated significant mortality rate reductions when using prone positioning for patients with severe ARDS (21). Subgroup analysis indicated that there are additional mortality rate reductions in patients with severe hypoxemia or other severe illnesses (16,22,23). It has also been suggested that ARDS patients should undergo prone positioning for longer durations (10,22-24). Furthermore, protective lung strategies may modulate the effects of prone positioning (10,24,25).
In the present meta-analysis, we aimed to evaluate the effects of prone positioning on mortality rates, particularly with respect to the duration and concurrent use of protective lung strategies.
Materials and methods
Literature search and study selection
We performed an extensive search of MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials, to identify randomized controlled trials (RCTs) pertaining to prone positioning during acute respiratory failure. The search employed the following medical subject headings (MeSH) and keywords. “ARDS” or “acute lung injury” and “prone position” or “prone positioning” and “mechanical ventilation” or “positive pressure ventilation” and “RCT” or “randomized clinical trial”. The detailed retrieval method was is included as a supplementary file. We included conference proceeding data from the Society of Critical Care Medicine, American Thoracic Society, and American College of Chest Physicians in addition to data from the three data bases. However, we were unable to identify conference proceedings that met the screening criteria. Two investigators independently searched the literature and evaluated the suitability of each study for inclusion. Inclusion was contingent upon reviewer consensus. Studies were considered if they employed a clinical, RCT design, and compared prone positioning with supine positioning during mechanical ventilation, for the management of adult patients (18 years or above) with ARDS.
Prone ventilation must have been applied either intermittently or continuously. Studies were excluded if they did not report mortality rates or evaluated only the effects of prone positioning on hemodynamics or respiratory mechanics. Eligible studies involving acute lung injury and ARDS were classified according to the definition of the 1994 American-European Consensus Conference (26). We categorized ARDS according to their PaO2/FiO2 ratio ≤300, according to the Berlin definition of ARDS (27).
We requested raw data for all included studies, to allow for analysis of subgroups of patients, however most authors did not respond and one author refused our request.
Data extraction and quality assessment
Two reviewers independently extracted data, on the year of publication, study design, study population, prone positioning details including interval of enrolment, application of techniques, and duration of prone positioning, ventilator settings, and clinical outcomes including mortality and complications such as ventilator-associated pneumonia (VAP), cardiac events, endotracheal tube dislocation, pneumothorax, pressure sores and loss of venous access. Disagreements were resolved by consensus between the two reviewers. The primary outcome measure under evaluation was the all-cause mortality rate. Associations of the mortality rate with the use of protective lung strategies, and prone positioning duration, were also evaluated. Protective lung strategies were considered as such if they included low tidal volumes and adequate positive end expiratory pressure (PEEP).
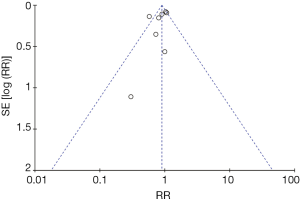
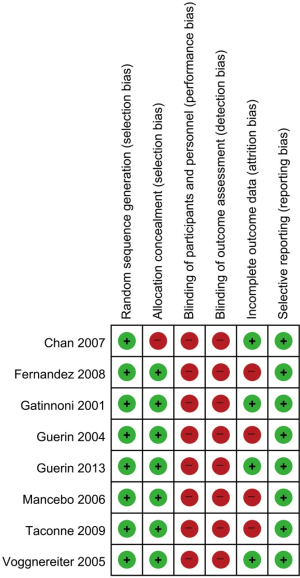
We assessed the methodological quality and risk of bias using a modified version of the Cochrane risk-of-bias instrument, which measures random sequence generation and allocation concealment (both selection biases), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias) (28). Two investigators independently evaluated the studies, extracted data on methods and outcomes, and assessed the risk of bias. Disagreements were resolved by consensus between the two reviewers. Because the prone position with ventilator was always shown and patient progress was explained to the family and patients in the intensive care unit, blinding of participants or outcome measure was not possible. Therefore, there were high selection and detection biases in all included studies. The study by Chan et al. (29) had high selection bias, because the randomized table was shown to the enrolled patients. We judged attrition bias by comparing the protocol and mortality outcomes in the included studies. The mortality data were not shown; Two of 344 in Taccone et al., 2 of 42 in Fernandez et al., 6 of 142 in Mancebo et al. Figure 1 depicts a funnel plot for publication bias.
Data analysis and statistics
We aggregated outcome data at the trial level and performed statistical calculations using the Review Manager software package (RevMan version 5.1; Nordic Cochrane Centre, Cochrane Collaboration, 2011). We reported continuous outcomes as mean differences (a measure of absolute change) and ratios of means (a measure of relative change), and we reported binary outcomes as risk ratios (RRs) (28). The primary outcome measure was the overall mortality at the longest available follow-up. For the primary outcome, we performed a z test of the interaction between the RR for mortality in the subgroup of patients for whom the prone position duration was >12 h and the RR in the subgroup for whom the prone position duration was ≤12 h (28). Furthermore, we evaluated the RR according to whether patients received protective lung ventilation. All statistical tests were two sided. We considered P<0.05 as statistically significant in all analyses and reported individual trial and summary results with 95% CIs (28). Furthermore, we assessed the between-study heterogeneity of each outcome using the I2 measure. We considered statistical heterogeneity to be low for I2=25-49%, moderate for I2=50-74%, and high for I2≥75% (28).
Results
Search results and study characteristics
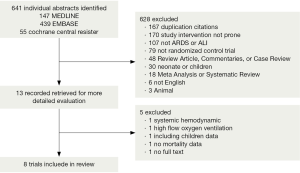
We identified 641 citations through our electronic bibliographic database searches. Thirteen records were retrieved for a more detailed evaluation, and eight of those trials (16-21,29,30) met the criteria for inclusion in our review (Figure 2). Studies on systemic hemodynamic applications of prone positioning during mechanical ventilation or analyses pertaining to high flow oxygen ventilation (31) were excluded. One study was not available in a full text format (32), and another did not contain mortality data (33) while a third study included data on children (34). The eight trials (Table 1) (16-21,29,30) included in this study comprised data from 2,168 patients (median 271 per trial, range 22-802). Reviewers reached complete agreement regarding the inclusion of all studies. The follow-up period of the included studies was 28-180 days.
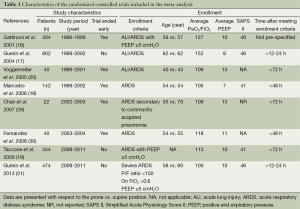
Full table
The baseline characteristics of the included studies are presented in Table 1. The baseline characteristics of the included studies are presented in Table 1. Four studies reported on the cause of ARDS (16-19). Those causes were pneumonia (58%), sepsis (18%), and aspiration (14%).
The treatment, outcome, and complications documented in the studies are presented in Table 2. Of the eight included RCTs, the 2013 study of Guérin et al. (21) was the most recent and five studies (16-21,29,30) were large. Five trials (19-21,29,30) mandated low-tidal-volume ventilation (6-8 mL/kg body weight) using lung protective ventilation. In four studies (18-21,29,30), the prone positioning duration exceeded 12 h. Outcome data on mortality, VAP, pressure sores, pneumothorax, dislocation of the endotracheal tube or loss of vascular access, and cardiac events, were pooled.
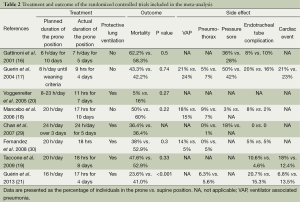
Full table
Methodological quality
The included trials had relatively high methodological quality (Figure 3). However, blinding, of participants and personnel, and pertaining to the outcome assessment, was not achieved in any study, because the type of positioning, and the outcomes of critical care, could not be concealed. One study (29) did not conceal allocation and another enrolled alternating patient. Four studies (17-19,30) had incomplete outcome data.
Outcome
Figure 4 shows the mortality rates of the included studies, all of which (16-21,29,30) provided mortality data. The mortality rates, for the prone and supine positions, were 41% (460/1,099) and 47% (487/1,042). This difference was statistically significant (RR, 0.90; 95% CI, 0.82-0.98, P=0.02). However, there was statistical heterogeneity among the trials that provided ICU mortality data (P=0.01, I2=61%). The RRs for mortality, in the individual RCTs, are presented in Figures 4 and 5.
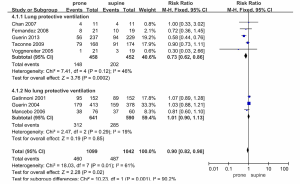
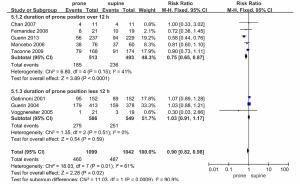
The results of subgroup analyses are summarized in Figures 4 and 5. The mortality rates in the five trials that included lung protective ventilation (19-21,29,30) were reduced in the prone position (RR 0.73, 95% CI 0.62-0.86, P=0.0002), and the heterogeneity of these trials was low (I2=46, P=0.12). All-cause mortality rates in the three trials not including those utilizing lung protective ventilation did not differ according to prone or supine positioning (RR 1.01, 95% CI 0.90-1.13, P=0.85) and had low heterogeneity (I2=19, P=0.29) (Figure 4).
In a further subgroup analysis (Figure 5), mortality was reduced when the daily duration of prone positioning exceeded 12 h (RR 0.75, 95% CI 0.65-0.87, P<0.0001), and heterogeneity between trials was low (I2=41, P=0.15). Mortality rates in trials with prone positioning durations of <12 h did not differ according to prone or supine positioning (RR 1.03, 95% CI 0.91-1.17, P=0.59) and they exhibited moderate heterogeneity (I2=61, P=0.01).
Adverse effects
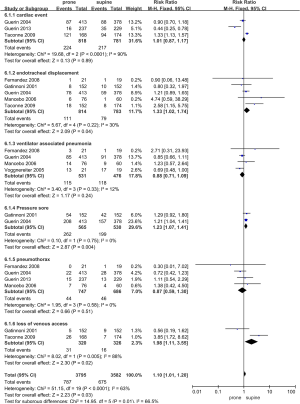
All the included RCTs reported data concerning complications related to prone positioning (Table 2 and Figure 6). Prone positioning was associated with a non-significant increase in the incidence of cardiac events (RR 1.01, 95% CI 0.87-1.17, I2=90%), ventilator associated pneumonia (RR 0.88, 95% CI 0.71-1.09, I2=12%), and pneumothorax (RR 0.87, 95% CI 0.59-1.30, I2=0%). The risks of pressure sores (RR 1.23, 95% CI 1.07-1.41, I2=0%) and endotracheal dislocation (RR 1.33, 95% CI 1.02-1.74, I2=30%) were increased during prone positioning. The incidence of venous access loss are also increased, but the associated heterogeneity of the data was high (RR 1.98, 95% CI 1.11-3.55, I2=88%).
Discussion
The main finding of our meta-analysis was that prone positioning during treatment with mechanical ventilation in patients with ARDS tends to reduce mortality rates, especially when used in conjunction with lung protective strategies and greater prone positioning durations. However, this effect was not statistically significant due to the high heterogeneity of the studies included in the meta-analysis. The well designed RCT of Guérin et al. (21) included a relatively high number of enrolled patients and showed large differences in the mortality rates associated with prone or supine positioning during the mechanical ventilation of patients with ARDS compared with other studies. Therefore, there was not at significant difference in the mortality rates of ARDS patients according to the prone or supine position in the meta-analysis of the remaining studies excluding the study by Guérin et al. (21). Additionally, the meta-analysis of the other studies excluding the study by Guérin et al. (21) revealed very low heterogeneity (I2=0%). This difference may be due to the subjects in the Guerin study having more severe ARDS compared with the subjects in the other studies. Furthermore, the lung protective strategy, longer prone position, and development of treatment for critical care may also explain this observation. This result suggests that further large-scale, RCTs on prone positioning in severe ARDS patients treated with lung protective strategies and greater prone position durations are needed.
Differences in hypoxia and illness severity represent patient specific factors that have been evaluated by recent meta-analyses (22,35-38). These studies focused on accounting for heterogeneity by disease related factors, and the degree of hypoxia as well as suggesting reasons for failure of the demonstrable mortality benefit in clinical trials. However, our study focused on a protective ventilator strategy (low tidal volume and adequate PEEP), which represents a modifiable treatment related factor. Lung protective strategies were used worldwide after studying low tidal volume ventilation in the ARDS network in 2000. Therefore, the time of study design or subject enrollment may result in differences in lung protective ventilation and prone positioning duration. The mortality rates in the five trials that included lung protective ventilation (19,20,29,30) were reduced in the context of prone positioning, but all-cause mortality in the three trials not including lung protective ventilation differ according to prone or supine positioning. This result could be explained by the association between prone positioning and a decreased risk of lung injury as a result of stress and strain forces. Patients with severe ARDS have the greatest risk of incurring lung injury from shear and strain forces due to the low ratio of well aerated lung tissues to poorly aerated or non-aerated lung tissues. When a patient is placed in the prone position, the lung has greater homogeneity and the stress and strain forces are decreased (10,15,22,39). This lung-protective effect of prone ventilation appears to be highly relevant in patients with severe hypoxemia (40). In severely hypoxemic patients, the lung-protective strategy of lowering the delivered tidal volumes may provide an additive benefit when combined with prone ventilation (41).
The most recent trials targeting alveolar recruitment and prevention of atelectrauma have advocated for the application of a considerably higher PEEP for any given FiO2 requirement (42-44) as part of an open lung protective approach. A high PEEP strategy is supported by a previous patient level meta-analysis that demonstrated reduced mortality rates among patients with moderate or severe ARDS (45). However, even the most recent study by Guérin et al. used the same low PEEP strategy as that used in the ARDS Network ALVEOLI trial (21).
A question facing clinicians intending to use this intervention concerns the optimal duration of prone positioning. In our study, the mortality rates were reduced when the daily duration of prone positioning was >12 h. Trials using shorter duration prone ventilation have been published less recently, whereas all trials employing a longer duration of prone ventilation were published after 2005. The recent study by Guérin et al. (21) maintained patients in the prone position for an average of 17±3 h/day. This duration is comparable to that used in the most recent trials on prone positioning, but that timeframe is much longer than that used in earlier trials. Several previous studies have suggested that the duration should be considered when assessing the effects of prone positioning, because alveolar recruitment in the prone position is a time dependent event (45). However, the time course of alveolar recruitment during prone positioning is not consistent and in fact differs markedly among patients (46).
Our study demonstrated that patients in the prone position group were at an increased risk of pressure ulcers and dislodgement of endotracheal and tracheostomy tubes. However, no significant differences were observed in the occurrence of other life threatening complications, including cardiac events or ventilator associated pneumonia. This result suggests that prone positioning is a relatively safe procedure if equipment and position changes are handled carefully. Following the outbreak of H1N1 (47), extracorporeal membrane oxygenation (ECMO) is frequently used in the treatment of refractory respiratory failure. ECMO is an important and advanced therapeutic strategy, but, its high invasiveness often leads to fatal complications including cerebral hemorrhage. The costs associated with the use of ECMO are also high. In contrast, prone positioning represents a relatively safe and inexpensive procedure.
A recent survey conducted in Germany suggests that there are more complications associated with prone positioning therapy than that suggested by RCTs. These complications include hemodynamic instability, cardiac arrhythmia, worsening gas exchange and inadequate sedation (48). Such complications, although infrequent, could be catastrophic in patients with acute respiratory failure. The prone position can appear unnatural, and altering the posture of an intubated patient requires both teamwork and skill. There is a risk of kinking and dislodgment, of not only the endotracheal and tracheostomy tubes but also the intravascular lines, body cavity drains, and feeding tubes. Electrocardiographic leads are repositioned on the back, such that suctioning can present a challenge; moreover, certain complications are unique to prone ventilation. Less-experienced centers may have greater difficulty managing life-threatening complications, but protocols and nursing care guidelines may mitigate this risk.
There were several limitations in the present analysis. First, the included trials were somewhat diverse, given the inclusion criteria employed, with variable ARDS severity, prone positioning durations, ventilation strategies, and associated treatments. We requested raw data for the included studies, to analyze subgroups of patients and assess the settings employed by each study. Unfortunately, we received either no response, or in one instance, a refusal to respond to our request. Second, it is likely that we did not include all of the relevant evidence, because we limited our analysis to articles in English. Third, the small number (<40) of available trials may have led to an underestimation of the heterogeneity, and reduced the precision of our pooled-effect estimates.
Conclusions
Our meta-analysis demonstrated that prone positioning tends to reduce the mortality rate associated with ARDS, especially when used in conjunction with lung-protective strategies and longer prone-positioning durations. Prone positioning for ARDS patients should be prioritized over other, riskier and/or more expensive procedures, because life-threatening adverse events are rare compared with those associated with invasive approaches. However, the heterogeneity of mortality in the included studies was high; accordingly, additional large, randomized controlled studies of severe ARDS cases (including studies incorporating lung-protective strategies and greater prone position durations) are required.
Acknowledgements
Disclosure: The authors declare no conflicts of interest.
References
- Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 2014;20:3-9. [PubMed]
- Milberg JA, Davis DR, Steinberg KP, et al. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA 1995;273:306-9. [PubMed]
- Bryan AC. Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil's advocate. Am Rev Respir Dis 1974;110:143-4. [PubMed]
- Blanch L, Mancebo J, Perez M, et al. Short-term effects of prone position in critically ill patients with acute respiratory distress syndrome. Intensive Care Med 1997;23:1033-9. [PubMed]
- Chatte G, Sab JM, Dubois JM, et al. Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med 1997;155:473-8. [PubMed]
- Jolliet P, Bulpa P, Chevrolet JC. Effects of the prone position on gas exchange and hemodynamics in severe acute respiratory distress syndrome. Crit Care Med 1998;26:1977-85. [PubMed]
- Langer M, Mascheroni D, Marcolin R, et al. The prone position in ARDS patients. A clinical study. Chest 1988;94:103-7. [PubMed]
- Mure M, Martling CR, Lindahl SG. Dramatic effect on oxygenation in patients with severe acute lung insufficiency treated in the prone position. Crit Care Med 1997;25:1539-44. [PubMed]
- Pappert D, Rossaint R, Slama K, et al. Influence of positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest 1994;106:1511-6. [PubMed]
- Gattinoni L, Taccone P, Carlesso E, et al. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med 2013;188:1286-93. [PubMed]
- Gattinoni L, Vagginelli F, Chiumello D, et al. Physiologic rationale for ventilator setting in acute lung injury/acute respiratory distress syndrome patients. Crit Care Med 2003;31:S300-4. [PubMed]
- Lamm WJ, Graham MM, Albert RK. Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med 1994;150:184-93. [PubMed]
- Mure M, Domino KB, Lindahl SG, et al. Regional ventilation-perfusion distribution is more uniform in the prone position. J Appl Physiol (1985) 2000;88:1076-83. [PubMed]
- Mutoh T, Guest RJ, Lamm WJ, et al. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis 1992;146:300-6. [PubMed]
- Mentzelopoulos SD, Roussos C, Zakynthinos SG. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J 2005;25:534-44. [PubMed]
- Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001;345:568-73. [PubMed]
- Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 2004;292:2379-87. [PubMed]
- Mancebo J. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;173:1233-9. [PubMed]
- Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009;302:1977-84. [PubMed]
- Voggenreiter G, Aufmkolk M, Stiletto RJ, et al. Prone positioning improves oxygenation in post-traumatic lung injury--a prospective randomized trial. J Trauma 2005;59:333-41; discussion 341-3. [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [PubMed]
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010;36:585-99. [PubMed]
- Abroug F, Ouanes-Besbes L, Dachraoui F, et al. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit Care 2011;15:R6. [PubMed]
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014;40:332-41. [PubMed]
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev 2013;2:CD003844. [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Higgins JP, Altman DG, Giggins PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PubMed]
- Chan MC, Hsu JY, Liu HH, et al. Effects of prone position on inflammatory markers in patients with ARDS due to community-acquired pneumonia. J Formos Med Assoc 2007;106:708-16. [PubMed]
- Fernandez R, Trenchs X, Klamburg J, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med 2008;34:1487-91. [PubMed]
- Riera J, Pérez P, Cortés J, et al. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care 2013;58:589-96. [PubMed]
- Gaillard S, Guérin C. The prone position in acute respiratory distress syndrome. Reanimation Urgences 2001;10:27-34.
- Watanabe I, Fujihara H, Sato K, et al. Beneficial effect of a prone position for patients with hypoxemia after transthoracic esophagectomy. Crit Care Med 2002;30:1799-802. [PubMed]
- Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 2005;294:229-37. [PubMed]
- Ashley EA, Kardos A, Jack ES, et al. Angiotensin-converting enzyme genotype predicts cardiac and autonomic responses to prolonged exercise. J Am Coll Cardiol 2006;48:523-31. [PubMed]
- Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med 2008;36:603-9. [PubMed]
- Hu SL, He HL, Pan C, et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care 2014;18:R109. [PubMed]
- Lee JM, Bae W, Lee YJ, et al. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med 2014;42:1252-62. [PubMed]
- Reignier J. Prone position: can we move from better oxygenation to better survival? Crit Care Med 2005;33:453-5. [PubMed]
- Gattinoni L, Pesenti A. The concept of "baby lung". Intensive Care Med 2005;31:776-84. [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [PubMed]
- Briel M, Studer M, Glass TR, et al. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 2004;117:596-606. [PubMed]
- Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med 2003;31:S312-6. [PubMed]
- McAuley DF, Giles S, Fichter H, et al. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med 2002;28:414-8. [PubMed]
- Reutershan J, Schmitt A, Dietz K, et al. Alveolar recruitment during prone position: time matters. Clin Sci (Lond) 2006;110:655-63. [PubMed]
- Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for adult respiratory failure. Respir Care 2013;58:1038-52. [PubMed]
- Bein T, Ritzka M, Schmidt F, et al. Positioning therapy in intensive care medicine in Germany. Results of a national survey. Anaesthesist 2007;56:226-31. [PubMed]