Published online Aug 15, 2014. doi: 10.4239/wjd.v5.i4.511
Revised: March 6, 2014
Accepted: May 31, 2014
Published online: August 15, 2014
The Src homology 2B (SH2B) family members (SH2B1, SH2B2 and SH2B3) are adaptor signaling proteins containing characteristic SH2 and PH domains. SH2B1 (also called SH2-B and PSM) and SH2B2 (also called APS) are able to form homo- or hetero-dimers via their N-terminal dimerization domains. Their C-terminal SH2 domains bind to tyrosyl phosphorylated proteins, including Janus kinase 2 (JAK2), TrkA, insulin receptors, insulin-like growth factor-1 receptors, insulin receptor substrate-1 (IRS1), and IRS2. SH2B1 enhances leptin signaling by both stimulating JAK2 activity and assembling a JAK2/IRS1/2 signaling complex. SH2B1 promotes insulin signaling by both enhancing insulin receptor catalytic activity and protecting against dephosphorylation of IRS proteins. Accordingly, genetic deletion of SH2B1 results in severe leptin resistance, insulin resistance, hyperphagia, obesity, and type 2 diabetes in mice. Neuron-specific overexpression of SH2B1β transgenes protects against diet-induced obesity and insulin resistance. SH2B1 in pancreatic β cells promotes β cell expansion and insulin secretion to counteract insulin resistance in obesity. Moreover, numerous SH2B1 mutations are genetically linked to leptin resistance, insulin resistance, obesity, and type 2 diabetes in humans. Unlike SH2B1, SH2B2 and SH2B3 are not required for the maintenance of normal energy and glucose homeostasis. The metabolic function of the SH2B family is conserved from insects to humans.
Core tip: The Src homology 2B (SH2B) family members mediate cell signaling in response to a variety of hormones, cytokines, and growth factors. In the brain, SH2B1 enhances leptin signaling and leptin’s anti-obesity action. In peripheral tissues, SH2B1 cell-autonomously enhances insulin signaling. In pancreatic islets, SH2B1 is required for compensatory β cell expansion in response to insulin resistance and β cell stress. SH2B1-deficiency results in severe leptin resistance, energy imbalance, obesity, and type 2 diabetes. SH2B1 mutations are linked to leptin resistance, insulin resistance, obesity, and type 2 diabetes in humans. Thus, SH2B1 is a critical metabolic regulator in mammals.
- Citation: Rui L. SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J Diabetes 2014; 5(4): 511-526
- URL: https://www.wjgnet.com/1948-9358/full/v5/i4/511.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i4.511
The Src homology 2B (SH2B) family contains three members (SH2B1, SH2B2 and SH2B3) in mammals. All members contain a characteristic pleckstrin homology (PH) domain and SH2 domain. SH2B1 (also called SH2-B and PSM) was initially identified as a high affinity immunoglobin E receptor (Fc RI) binding protein in the yeast tribrid screen in 1995[1]. SH2B2 (also called APS) was identified as a c-Kit-binding protein by the yeast two-hybrid system in 1997[2]. SH2B3 (also called Lnk) was identified as a SH2 domain-containing, tyrosyl phosphorylated protein in rat lymph node lymphocytes in 1995[3]. The SH2B family is evolutionarily conserved from insects through humans. Unlike mammals, insects have only one SH2B gene (also called Lnk)[4,5]. Deletion of SH2B1, but not SH2B2 or SH2B3, results in obesity and metabolic diseases in mice, whereas deletion of either SH2B2 or SH2B3, but not SH2B1, impairs immune function[6-11]. Therefore, individual SH2B1 family members have distinct function in mammals. In this review, I will mainly discuss mammalian SH2B1 and SH2B2.
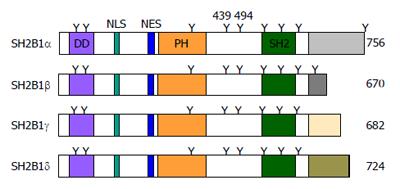
The SH2B1 gene generates four SH2B1 isoforms (α, β, γ, and δ) through mRNA alternative splicing[1,12-14]. All isoforms have an identical N-terminal region (amino acids 1-632), but differ at their C-termini after the SH2 domain (Figure 1).
SH2B1 structure: All four isoforms have identical dimerization (DD), PH, and SH2 domains (Figure 1). The DD domain mediates SH2B1 homodimerization or its heterodimerization with SH2B2[15-17]. The SH2 domain binds to the phospho-tyrosine motifs of its binding partners (e.g., JAK2 and insulin receptors)[12,18]. The function of the central PH domain remains unclear.
SH2B1 subcellular localization: SH2B1 is located mainly in the cytoplasm, but a subset shuttles between the cytoplasm and the nucleus[19]. SH2B1 contains a nuclear localization sequence (NLS) (KLK150KR) which is required for its nuclear translocation[20]. SH2B1 also contains a nuclear export sequence (NES) (GERWTHRFERL231RLSR) (Figure 1), and replacement of the conserved Leu231 and Leu233 with Ala increases its nuclear localization[19]. SH2 domain-defective SH2B1β(R555E) mutant is also excluded from the nucleus[20]. Therefore, the NLS, NES, and SH2 domain all are involved in the regulation of SH2B1 trafficking between the cytoplasmic and nuclear compartments. SH2B1 is also translocated to the plasma membrane[21]. A N-terminal polybasic region (S145KPKLKKRF), which overlaps the NLS, is required, but not sufficient, for SH2B1β translocation to the plasma membrane[22].
SH2B1 posttranslational modification: SH2B1α and SH2B1β contain nine Tyr residues, and SH2B1γ and SH2B1δ have eight (Figure 1). Tyr439 and Tyr494 are conserved in all four isoforms, and are able to be phosphorylated by JAK1 and JAK2[23]. Src tyrosine kinases also phosphorylate all four isoforms[24]. Additionally, insulin, insulin-like growth factor (IGF-1), and nerve growth factor (NGF) also stimulate tyrosine phosphorylation of SH2B1 via their cognate receptor tyrosine kinases[14,18,25].
SH2B1 contains numerous Ser and Thr residues. NGF stimulates SH2B1 phosphorylation on multiple Ser/Thr residues[21]. Mitogen-activated protein kinase (MAPK) directly phosphorylates Ser96[21], and protein kinase C phosphorylates both Ser161 and Ser165 residues[22,26]. However, the physiological consequence of SH2B1 phosphorylation remains unknown.
SH2B1 tissue distribution: SH2B1 is ubiquitously expressed in both peripheral tissues and the central nervous system, including adipose tissue, skeletal muscle, liver, pancreas, heart, spleen, hypothalamus, and other brain areas[27]. SH2B1 expression is regulated by neuronal, hormonal, and nutritional signals. The mRNA levels of hypothalamic SH2B1 are 20-fold higher in fed mice than in fasted mice[28]. The expression of hypothalamic SH2B1 in rats is downregulated by high fat diet (HFD) feeding[29]. Chronic overexpression of bovine growth hormone (GH) increases the levels of hepatic SH2B1 protein in GH transgenic mice[30]. The molecular steps, which control the activity of the SH2B1 promoter and the stability of SH2B1 mRNA and protein, remain completely unknown.
In cultured cells, SH2B1 acts as an adaptor to couple upstream activators to downstream effectors, to assemble a multiple-protein signaling complex, and/or to enhance the catalytic activity of its bound enzymes.
SH2B1 mediates/modulates leptin signaling: Leptin is an adipose hormone identified by Friedman and his colleagues using positional cloning[31]. Leptin deficiency results in morbid obesity in ob/ob mice[31], and recombinant leptin fully corrects obesity and metabolic disorders in ob/ob mice[32-34]. Leptin exerts its biological action by binding to and activating its long form receptors (called LEPRb)[35-38]. LEPRb binds to JAK2, a cytoplasmic tyrosine kinase which also mediates GH, prolactin, erythropoietin (EPO), and other cytokine signaling[39,40]. Leptin stimulates tyrosine phosphorylation and activation of JAK2 which activates multiple downstream signaling pathways, including the signal transducer and activator of transcription 3 (STAT3) and the PI 3-kinase pathways[39,40]. Both the STAT3 and the PI 3-kinase pathways are required for leptin’s anti-obesity action[39,40]. Impaired leptin signaling and action (leptin resistance) are believed to be the primary risk factor for obesity[39,40].
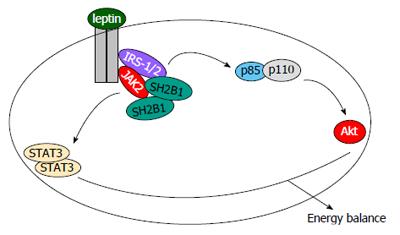
We reported that leptin stimulates activation of JAK2 which subsequently autophosphorylates on Tyr813[41]. SH2B1 binds via its SH2 domain to phospho-Tyr813[41]. This physical interaction markedly increases JAK2 catalytic activity, thus enhancing activation of leptin signaling pathways downstream of JAK2[41-43]. In agreement, leptin-stimulated activation of hypothalamic JAK2 is dramatically attenuated in SH2B1 knockout mice[10]. Leptin sensitivity has been well documented to be negatively regulated by protein tyrosine phosphatase 1B (PTP1B) and SOCS3[39,40]. Overexpression of SH2B1 reverses PTP1B-induced inhibition of leptin stimulation of tyrosine phosphorylation of STAT3[10]. Therefore, cellular leptin sensitivity is likely to be determined, at least in part, by the ability of endogenous SH2B1 to counteract negative regulators such as PTP1B and SOCS3.
Leptin stimulates tyrosine phosphorylation of insulin receptor substrate-1 (IRS1) and IRS2, and IRS proteins subsequently bind to the p85 regulatory submit of PI 3-kinase and activate the PI 3-kinase pathway[39,40,44]. Genetic deletion of IRS2 in LEPR-expressing cells results in leptin resistance and obesity in mice[45]. SH2B1 directly binds to both IRS1 and IRS2 in addition to JAK2[46]. In response to leptin, SH2B1 recruits IRS proteins to JAK2, thus allowing JAK2 to phosphorylate IRS proteins on tyrosine residues[46]. Accordingly, in SH2B1 knockout mice, leptin is unable to stimulate tyrosine phosphorylation of hypothalamic IRS2[10]. SH2B1 is likely to mediate leptin stimulation of the PI 3-kinase pathway by coupling JAK2 to IRS proteins (Figure 2).
SH2B1 C-terminal SH2 domain binds to phospho-Tyr813 in JAK2 as discussed above; in contrast, its N-terminal region binds to different sites on JAK2 in a tyrosine phosphorylation-independent manner[43]. Similarly, SH2B1 binds to phospho-tyrosine(s) of IRS1 or IRS2 via its SH2 domain, and binds to other sites on IRS proteins via its PH domain-containing regions in a tyrosine phosphorylation-independent fashion[46]. SH2B1 forms homodimers or oligomers via its N-terminal domains[15-17]. Each individual SH2B1 molecule is able to bind to JAK2 and/or IRS proteins; therefore, SH2B1 dimers or oligomers are predicted to assemble a large signaling complex containing multiple copies of JAK2 and IRS proteins (Figure 2). Physical proximity allows JAK2 to transphosphorylate and activate each other in this complex, contributing to SH2B1 stimulation of JAK2 activation and leptin signaling. Additionally, this highly-organized SH2B1/JAK2/IRS complex may also provide a permissive condition for JAK2 to efficiently phosphorylate IRS proteins and activate the PI 3-kinase pathway in response to leptin.
SH2B1 enhances insulin and IGF-1 signaling: SH2B1 was reported to bind to insulin receptors (IRs) via its SH2 domain[18]. Insulin stimulates the binding of SH2B1α to phospho-Tyr1158, Tyr1162 and/or Tyr1163 within the IR activation loop, and IRs subsequently tyrosyl phosphorylate SH2B1α[13,47]. Overexpression of SH2B1β markedly enhances the ability of insulin to stimulate tyrosine phosphorylation of IRS1 and IRS2[9]. In contrast, SH2B1β(R555E), which has a defective SH2 domain, acts as a dominant negative mutant to inhibit insulin signaling[9]. Moreover, deletion of SH2B1 impairs insulin signaling in the skeletal muscle, adipose tissue, and livers of SH2B1 knockout mice[9].
Mechanistically, SH2B1-IR interaction markedly increases IR catalytic activity and IR-mediated tyrosine phosphorylation of IRS proteins[48]. Replacement of IR Tyr1158 with Phe disrupts IR binding to SH2B1, and completely blocks the ability of SH2B1β to stimulate IR kinase activity[48]. SH2B1α similarly increases IR catalytic activity[49]. Additionally, SH2B1β directly binds to tyrosyl phosphorylated IRS1 and IRS2 and protects IRS proteins against dephosphoarylation, thus prolonging the ability of IRS proteins to activate their downstream pathways[48]. Accordingly, overexpression of SH2B1α delays dephosphorylation of IRS proteins in cells[50]. SH2B1 homodimers and oligomers are predicted to simultaneously bind to both IRs and IRS proteins and assemble a large, highly-organized signaling complex, thereby increasing insulin signaling specificity and efficiency.
SH2B1 also binds via its SH2 domain to IGF-1 receptors[14], and is predicted to promote IGF-1 signaling in a similar fashion.
SH2B1 enhances TrkA, TrkB and TrkC signaling: Amino acid sequence analysis reveals that like IRs, Trk family members (TrkA, TrkB and TrkC) contain potential SH2B1-binding site(s) within their activation loops. NGF stimulates both the binding of SH2B1 to NGF receptor TrkA and phosphorylation of SH2B1 on Tyr/Ser/Thr residues in PC12 cells[21,25]. NGF also stimulates the binding of TrkA to both SH2B1 and SH2B2 in primary neurons[51]. SH2B1-TrkA interaction is mediated by the SH2 domain of SH2B1 and phospho-Tyr679, -Tyr683 and/or -Tyr684 within TrkA activation loop[21,25,51]. Additionally, SH2B1α binds via its proline rich regions (amino acids 394-504 between the PH and SH2 domains) to Grb2, contributing to NGF-stimulated activation of the MAPK pathway[51]. Overexpression of SH2B1β also enhances NGF-stimulated activation of Akt in PC12 cells[52].
Brain-derived neurotrophic factor (BDNF) or neurotrophin-3 (NT-3) similarly stimulates the binding of SH2B1 to TrkB or TrkC, respectively, and they also stimulate tyrosine phosphorylation of SH2B1[51,53,54]. Unlike JAK2 and IRs, TrkB kinase activity is not enhanced by SH2B1[53].
SH2B1 regulates GH, prolactin, and EPO signaling: JAK2 binds to GH receptors and mediates GH signaling[55]. GH stimulates the binding of SH2B1 to JAK2 and robust tyrosine phosphorylation of SH2B1 by JAK2 in 3T3-F442A fibroblasts[12]. GH stimulates JAK2-mediated phosphorylation of SH2B1 on Tyr439 and Tyr494 residues[56]. Like leptin, GH stimulates phosphorylation of JAK2 on Tyr813 which binds to the SH2 domain of SH2B1[57]. SH2 domain-phospho-Tyr813 interaction markedly increases JAK2 activity, thus enhancing GH signaling (e.g., phosphorylation and activation of STAT5B)[42,43].
JAK2 also mediates prolactin signaling[58]. Like GH, prolactin stimulates tyrosine phosphorylation of SH2B1[59]. Overexpression of SH2B1β enhances prolactin signaling, including tyrosine phosphorylation of JAK2[59].
Unlike GH, EPO stimulates the binding of SH2B1 to EPO receptors rather than to JAK2[60]. SH2B1 constitutively binds to unphosphorylated EPO receptors under basal conditions, and EPO stimulates phosphorylation of EPO receptors on Tyr343 and Tyr401 which subsequently bind to the SH2 domain of SH2B1[60]. EPO rapidly stimulates phosphorylation of SH2B1 on Ser/Thr residues[60]. Knockdown of SH2B1 increases EPO-stimulated tyrosine phosphorylation of EPO receptors, JAK2, and ERK1/2, raising the possibility that SH2B1 may negatively regulate EPO signaling[60].
SH2B1 binds to JAK1, JAK2 and JAK3, but it only stimulates JAK2, but not JAK1 and JAK3, kinase activity[61]. Both JAK1 and JAK2 are able to phosphorylate SH2B1 on Tyr439 and Tyr494, but Tyr439/Tyr494 phosphorylation does not affect the ability of SH2B1 to stimulate JAK2[23]. The JAK family members mediate cell signaling and action in response to numerous hormones and cytokines in addition to GH, prolactin, and EPO, so it is conceivable that SH2B1 may mediate or modulate cellular responses to these hormones and cytokines through interacting with JAK family members.
SH2B1 regulates additional receptor tyrosine kinase signaling: SH2B1 binds via its SH2 domain to tyrosyl phosphorylated platelet-derived growth factor (PDGF) receptors in response to PDGF-BB stimulation[62]. PDGF-BB stimulates phosphorylation of SH2B1 on Tyr/Ser/Thr residues[62]. PDGF-BB is able to stimulate tyrosine phosphorylation of all four isoforms of SH2B1[14]. PDGF receptors directly phosphorylate SH2B1 on Tyr439 residue[23].
Glial cell line-derived neurotrophic factor (GDNF) stimulates the binding of SH2B1β to GDNF receptor RET through SH2B1β SH2 domain and RET phospho-Tyr981 motifs[63,64]. This interaction increases RET kinase activity, RET autophosphorylation, and RET-mediated tyrosine phosphorylation of STAT3[64].
SH2B1 directly interacts with fibroblast growth factor receptor 3 (FGFR3) and is tyrosyl phosphorylated by FGFR3[65]. The SH2 domain of SH2B1 binds to phospho-Tyr724 and phospho-Tyr760 of FGFR3, and the interaction increases the ability of FGFR3 to phosphorylate and activate STAT5[65].
In cultured cells, SH2B1 has been demonstrated to regulate multiple cellular processes, including migration, proliferation, and differentiation.
SH2B1 regulates actin cytoskeletal reorganization and cell motility: SH2B1 is able to regulate cell morphology, adhesion, and motility through modifying actin cytoskeletal reorganization in cultured cells. SH2B1β is detected in membrane ruffles, filopodia, and focal adhesions[26,59], and is colocalizated with filamentous actin (F-actin) in membrane ruffles[66]. SH2B1β binds via both its N-terminal (amino acids 150-200) and C-terminal regions (amino acids 615-670) to F-actin, and promotes actin filament cross-link[59]. SH2B1 directly binds via its amino acids 200-260 to the actin-binding protein filamin A[67]. Additionally, SH2B1 binds via its amino acids 85-106 to Rac, a critical regulator of actin cytoskeletal reorganization[68].
SH2B1 mediates GH regulation of cell adhesion and migration. GH increases the cycling of SH2B1 into and out of focal adhesions[26], and promotes SH2B1 colocalization with F-actin in membrane ruffles[66]. Overexpression of SH2B1β, but not SH2 domain-defective SH2B1β(R555E), enhances the ability of GH to stimulate both membrane ruffles in 3T3-F442A fibroblasts[59,66] and macrophage migration[56]. In fact, SH2B1β(R555E) blocks GH-induced lamellipodia dynamics in 3T3-F442A cells[68]. Both the N-terminal region (amino acids 85-106) and the SH2 domain of SH2B1β are required for GH stimulation of cell motility[68]. Additionally, SH2B1β mutants lacking Tyr439 and Tyr494 phosphorylation sites are unable to enhance GH-stimulated membrane ruffling in 3T3-F442A fibroblasts[23] and GH-stimulated motility of RAW264.7 macrophages[56]. SH2B1-Rac interaction is involved in mediating GH-promoted actin cytoskeletal reorganization and cell motility[68].
Overexpression of SH2B1β similarly enhances prolactin-stimulated membrane ruffling[59]. SH2B1 directly binds to filamin A, which appears to mediate prolactin stimulation of membrane ruffling and cell motility[67].
SH2B1 promotes neuronal survival and neuronal differentiation: Overexpression of SH2B1β markedly enhances the ability of NGF to stimulate neurite outgrowth in PC12 cells[25], and both SH2B1α and SH2B2 are able to promote NGF/TrkA-induced neuronal differentiation[51]. In contrast, overexpression of SH2 domain-defective SH2B1(R555E) blocks NGF-induced neuronal differentiation of PC12 cells[25]. SH2B1β mutants lacking either the NES or the NLS also are unable to enhance NGF-induced neuronal differentiation[19,20]. Overexpression of a N-terminal (amino acids 1-499) truncated SH2B1 mutant, which lacks both NES and NLS, induces axon degeneration in NGF-treated primary sympathetic neurons[51]. Moreover, neutralization of endogenous SH2B1 with anti-SH2B1 antibody decreases the survival of primary sympathetic neurons[51]. These observations suggest that the SH2 domain, NES, and NLS all are required for SH2B1 to mediate NGF stimulation of neuronal differentiation and survival.
SH2B1β also enhances GDNF/RET-induced neuronal differentiation of PC12 cells[63,64]. However, the molecular mechanisms, by which SH2B1 promotes neuronal survival, differentiation, and neurite outgrowth, remain largely unknown.
SH2B1 promotes mitogenesis and transformation: All four SH2B1 isoforms are able to increase the mitogenic response to epidermal growth factor, IGF-1, and PDGF-BB[14,69]. SH2B1 increases the ability of RET to promote transformation of NIH 3T3 cells[64]. SH2B1 is abnormally expressed in non-small cell lung cancer (NSCLC) tissues and NSCLC cell lines[70]. SH2B1 overexpression is associated with increased tumor grade, tumor size, lymph node metastasis in NSCLC patients[70].
We reported that genetic deletion of SH2B1 results in severe obesity and type 2 diabetes in mice[9,10].
Central SH2B1 regulates energy balance and body weight: We disrupt the SH2B1 gene to generate SH2B1 knockout (KO) mice by DNA homologous recombination[9]. Exons 1-6, which encode the N-terminal region of all four isoforms of SH2B1, are replaced by a neo cassette[9]. SH2B1-null mice are hyperphagic and morbidly obese[10]. Both SH2B1 KO males and females gain more body weights than wild type (WT) littermates after 7 wk of age[10,71]. White adipose tissue mass and fat content are much higher in SH2B1 KO mice in either C57BL/6 or 129Sv/C57BL mixed congenic background, and the size of individual white adipocytes is also larger in SH2B1 KO mice[10].
SH2B1 KO mice are extremely hyperphagic, causing obesity[10]. Surprisingly, energy expenditure, as estimated by O2 consumption and CO2 production, is also higher in SH2B1 KO mice than in WT littermates[10]. Accordingly, in the pair-feeding paradigm in which each individually-housed mouse is fed the identical amount of food daily, SH2B1 KO mice gain less body weights and become leaner than WT littermates[10].
Food intake is controlled largely by the brain, particularly the hypothalamus[72], so we generate SH2B1 transgenic (Tg) mice in which a rat SH2B1β transgene is expressed specifically in neurons under the control of neuron-specific enolase promoter[27]. SH2B1β Tg mice are crossed with SH2B1 KO mice to generate TgKO mice which lack endogenous SH2B1 in all cell types but express recombinant SH2B1β specifically in neurons[27]. Neuron-specific restoration of SH2B1β into SH2B1 KO mice fully rescues the hyperphagic and obese phenotypes in TgKO mice[27]. Energy expenditure, which is abnormally higher in SH2B1 KO mice, is normal in TgKO mice[27]. Furthermore, SH2B1β Tg mice, which contain homozygous SH2B1β transgenes and overexpress recombinant SH2B1β in the brain, resist HFD-induced obesity[27]. These observations indicate that central SH2B1 is a key regulator of energy balance and body weight. Multiple brain areas and neural circuits are involved in the control of energy metabolism and body weight[72]; however, SH2B1 target neural circuits remain unknown.
Surprisingly, Ohtsuka et al[11] reported that disruption of SH2B1 did not cause obesity, insulin resistance, and glucose tolerance; however, their subsequent studies show that their SH2B1 KO mice indeed display insulin resistance and glucose intolerance as we observed in our SH2B1 KO mice[9,73]. Since SH2B1 KO mice have high energy expenditure[10], a slight disturbance of food intake is expected to lead to reduction in body weight. Thus, variations in house conditions and other environmental factors may contribute to body weight discrepancy between these studies.
SH2B1 KO mice have relatively normal somatic growth, indicating that SH2B1 is not required for GH stimulation of body growth[9,11,71]. Nonetheless, it is still possible that SH2B1 may modulate GH regulation of metabolism and/or other physiological processes.
Central SH2B1 regulates glucose and lipid metabolism: Obesity is the primary risk factor for insulin resistance and type 2 diabetes[39]. As expected, obese SH2B1 KO mice develop hyperglycemia, hyperinsulinemia, glucose intolerance, and insulin resistance[9,71]. Insulin signaling is impaired in the skeletal muscle, adipose tissue, and livers of SH2B1 KO mice[9]. SH2B1 KO male mice display frank type 2 diabetes after 7 mo of age[9]. Moreover, SH2B1 haploinsuffiency predisposes to HFD-induced insulin resistance[9]. SH2B1 KO mice develop the symptoms of metabolic syndrome, including hyperlipidemia, hepatic steatosis, and lipid accumulation in skeletal muscle[10]. Moreover, neuron-specific restoration of SH2B1β reveres obesity, type 2 diabetes, and metabolic syndrome in TgKO mice[27]. These observations indicate that central SH2B1 is absolutely required for the maintenance of normal glucose and lipid homeostasis in mice.
Central insulin and leptin are able to regulate systemic glucose and lipid metabolism independently of their action on energy balance and body weight[39,40,74-77]. SH2B1 positively regulates both leptin and insulin signaling, so central SH2B1 may regulate peripheral glucose and lipid metabolism independently of its action on energy balance and body weight.
Central SH2B1 positively regulates hypothalamic leptin sensitivity: Central SH2B1 controls food intake and body weight at least in part by enhancing leptin sensitivity in the brain. SH2B1 cell-autonomously enhances leptin signaling by promoting JAK2 activity and activation of pathways downstream of JAK2[41]. SH2B1 also mediates leptin-stimulated activation of the PI 3-kinase pathway by binding to IRS1/2 and recruiting IRS proteins to JAK2[46]. SH2B1 KO mice display severe hyperleptinemia, a hallmark of leptin resistance[10]. Hyperleptinemia develops prior to the onset of obesity, suggesting that leptin resistance is a causal factor for obesity progression in SH2B1 KO mice[10]. In agreement, exogenous leptin is unable to suppress food intake and weight gain in SH2B1 KO mice, and has reduced ability to stimulate phosphorylation of hypothalamic JAK2, STAT3 and IRS2 in these mice[10]. Furthermore, neuron-specific expression of recombinant SH2B1β in SH2B1-null mice reverses hyperleptinemia, leptin resistance, hyperphagia, and obesity in TgKO mice[27]. However, neuron-specific expression of SH2B1β(R555E) is unable to rescue leptin resistant, hyperphagic, and obese phenotypes in SH2B1-null mice[78], suggesting that the SH2 domain of SH2B1 is required for its anti-obesity action. Like SH2B1 KO mice, SH2B1β(R555E) transgenic mice develop obesity, insulin resistance, hyperglycemia, and glucose intolerance[78], suggesting that SH2B1β(R555E) blocks the action of endogenous SH2B1 as a dominant negative mutant.
Orexigenic agouti-related protein (AgRP) neurons and anorexigenic proopiomelanocortin (POMC) neurons in the arcuate nucleus are key leptin targets[39]. Leptin suppresses the expression of AgRP and neuropeptide Y (NPY) but stimulates POMC expression[72]. The expression of hypothalamic AgRP and NPY is higher in SH2B1 KO mice[10], and neuron-specific expression of SH2B1β in SH2B1 KO mice normalizes AgRP and NPY expression[27]. By contrast, the expression of hypothalamic POMC is normal in SH2B1 KO mice[10]. Since SH2B1 KO mice develop severe hyperleptinemia[10], leptin-stimulated expression of POMC may still be impaired in SH2B1-null mice.
Leptin promotes energy expenditure[39]; therefore, increased energy expenditure in SH2B1 KO mice cannot be explained by leptin resistance. It is likely that central SH2B1 regulates energy metabolism by an additional leptin-independent mechanism. SH2B1 is able to mediate or modulate cell signaling in response to multiple factors as described above. These pathways may be involved in central regulation of energy balance and body weight. For instance, SH2B1 enhances BDNF signaling[51,54]. Central administration of BDNF suppresses food intake and weight gain; conversely, haploinsufficiency of BDNF or TrkB leads to hyperphagia and obesity in mice[79-83]. Mutations in either BDNF or TrkB are associated with obesity in humans[82,84]. Therefore, neuronal SH2B1 may regulate energy metabolism and body weight by enhancing TrkB signaling in addition to LEPRb signaling in the brain.
SH2B1 is expressed in both central and peripheral tissues[27], and peripheral SH2B1 also regulates nutrient metabolism.
Peripheral SH2B1 regulates insulin sensitivity and glucose metabolism: TgKO mice, which lack endogenous SH2B1 in all tissues but express SH2B1β transgenes in the brain, have relatively normal blood glucose, plasma insulin, and glucose tolerance[27]. These observations suggest that peripheral SH2B1 is not required for the maintenance of insulin sensitivity and glucose metabolism in mice fed a normal chow diet. We feed TgKO mice a HFD for 16 wk to induce metabolic stress. TgKO mice develop more severe HFD-induced hyperglycemia, hyperinsulinemia, insulin resistance, and glucose intolerance, even though they have similar body weight and fat content as WT mice[48]. Insulin signaling in skeletal muscle, adipose tissue, and the liver is impaired to a greater extent in HFD-fed TgKO mice[48], and these mice display more severe hepatic steatosis[85]. Thus, peripheral SH2B1 promotes insulin signaling and glucose and lipid metabolism under obesity conditions.
SH2B1 in pancreaticβcells promotesβcell expansion and insulin secretion: Pancreatic β cells express high levels of several SH2B1 isoforms[86]. To examine the role of β cell SH2B1, we generate pancreas-specific SH2B1 KO (PKO) mice, using the Pdx1-cre/loxp system[86]. PKO mice have normal body weight, blood glucose, insulin sensitivity, and glucose intolerance; however, they develop more severe HFD-induced glucose intolerance[86]. Pancreatic insulin content, β cell mass, and glucose-stimulated insulin secretion are significantly lower in PKO than control mice fed a HFD, and PKO islets have more apoptotic cells and less mitotic cells[86]. These observations indicate that SH2B1 in β cells is required for HFD-induced compensatory β cell expansion to counteract insulin resistance in obesity.
SH2B1 appears to directly promote β cell expansion by both promoting proliferation and inhibiting apoptosis[86]. In a rat INS-1 832/13 β cell line, silencing of SH2B1 decreases, whereas overexpression of SH2B1β increases, β cell toxin streptozotocin (STZ)-induced apoptosis[86]. In line with these findings, PKO mice are more susceptible to STZ-induced β cell destruction, insulin deficiency, and glucose intolerance[86]. Mechanistically, SH2B1 directly enhances insulin and IGF-1 signaling in β cells[86], and both insulin and IGF-1 potently increase β cell survival and proliferation[87-91]. Therefore, β cell SH2B1 cell-autonomously promotes β cell survival, proliferation, and expansion under stress conditions at least in part by enhancing insulin and IGF-1 signaling in β cells.
Hepatic SH2B1 regulates liver triacylglycerol content and very low-density lipoprotein secretion: SH2B1 is also highly expressed in the liver[27], so we generate hepatocyte-specific SH2B1 KO (HKO) mice using the albumin-cre/loxP system[85]. Surprisingly, somatic growth, body weight, insulin sensitivity, and glucose metabolism are similar between HKO and control mice fed either a normal chow diet or a HFD[85]. Adult-onset deletion of SH2B1 in the liver also does not alter insulin sensitivity and glucose metabolism in mice fed a HFD[85]. These data indicate that hepatic SH2B1 is dispensable for the maintenance of systemic insulin sensitivity and glucose metabolism in mice. However, adult-onset deletion of liver SH2B1 decreases liver triacylglycerol content in mice fed a HFD[85], suggesting that hepatic SH2B1 regulates hepatocyte lipid metabolism. Liver-specific deletion of SH2B1 alone does not alter very low-density lipoprotein (VLDL) secretion; however, deletion of liver SH2B1 in SH2B2 knockout mice decreases VLDL secretion[85]. These observations suggest that liver SH2B1 and SH2B2 act redundantly to promote VLDL secretion.
SH2B1 is highly expressed in testes and ovaries, and systemic deletion of SH2B1 severely impairs fertility in both male and female mice[11]. Ovary size and follicle number are lower in SH2B1 KO females; similarly, testis size and sperm number are also lower in SH2B1 KO males[11]. SH2B1 deficiency impairs both follicle-stimulating hormone and IGF-1 signal transduction in ovaries, which may contribute to impaired fertility in SH2B1 KO mice[11].
SH2B1 rs7498665, the first human SH2B1 single nucleotide polymorphism (SNP), was reported in 2007[92]. It is associated with hyperleptinemia, increased body weight, increased total fat, and increased waist circumference in a United Kingdom white female cohort[92].
Human SH2B1 is a candidate obesity gene: In 2009, two groups independently reported that SH2B1 rs7498665 is genetically linked to human obesity in genome-wide association studies (GWAS) on large populations[93,94]. Since then, SH2B1 rs7498665 has been reported to be associated with human obesity in Swedish adults[95], Belgian adults[96], children of European ancestry[97], Chinese women[98], Hong Kong Chinese[99], Japanese adults[100], the MONIKA/KORA cohort[101], a Mexican cohort[102], and a African-American cohort[103]. SH2B1 rs7498665 risk allele is associated with increased visceral adiposity in Japanese[104] and German[105]. SH2B1 rs7498665 is also associated with increased fat intake in Dutch females[106].
Several additional SH2B1 SNPs have been described since 2009. In GWAS, SH2B1 rs7359397 is associated with obesity in 249796 adult individuals of European ancestry[107] and in Danish adults[108]. SH2B1 rs4788102 is associated with obesity in Chinese girls[109] and in Japanese populations[100]. SH2B1 rs4788099 is associated with increased body mass index (BMI) in individuals of European ancestry[110], and is linked to more servings of dairy products[111]. SH2B1 rs8055982 is associated with severe obesity in children of European ancestry[97].
Aside from SH2B1 SNPs, chromosomal 16p11.2 deletion is associated with severe obesity in European cohorts[112-115]. The deleted region contains the SH2B1 gene. In contrast, chromosomal 16p11.2 duplication is associated with underweight in humans[116].
Several SH2B1 non-synonymous variants have been identified. SH2B1 rs7498665 risk allele encodes a non-synonymous substitution of Thr484Ala[92]. However, Thr484Ala substitution alone is not sufficient to cause obesity[117], raising the possibility that other unidentified SH2B1 mutations, which co-segregate with rs7498665, may increase risk for obesity. Several SH2B1 missense mutations (P90H, T175N, P322S and F344LfsX20) were reported to be genetically linked to obesity and insulin resistance in mixed European descents[118]. F344LfsX20A mutation causes a frameshift, resulting in production of a C-terminally-truncated SH2B1 variant lacking the entire SH2 domain[118]. A separate study reported that SH2B1 g.9483(C/T) missense mutation, but not Thr175Asp non-synonymous variant (rs147094247), is linked to obesity[119]. SH2B1 g.9483(C/T) mutation results in generation of non-synonymous SH2B1β(Thr656Ile) and SH2B1γ(Pro674Ser) variants[119]. Four additional rare non-synonymous variants (G131S, V209I, L293R, M465T, and W649G) have been identified in Chinese populations[120]. V209I and M465T variants are detected in obese children, whereas G131S, L293R and W649G variants are observed in lean children[120].
None of the above human SH2B1 variants has been verified in animal models to be a causal factor for obesity or obesity-associated metabolic syndrome. We reported that neuron-specific expression of SH2 domain-defective SH2B1β(R555E), which is functionally similar to F344LfsX20A variant, is sufficient to cause obesity and insulin resistance in mice[78]. These findings raise the possibility that F344LfsX20A non-synonymous variant may be a causal factor for obesity in humans.
SH2B1 mutations increase risk for type 2 Diabetes in humans: Obesity is the primary risk factor for insulin resistance and type 2 diabetes[39], so SH2B1 risk alleles are expected to be associated with type 2 diabetes in humans. SH2B1 rs7498665 is associated with type 2 diabetes in both United Kingdom[92] and French populations[121]. Heterozygous carriers of a P90H, T175N, P322S, or F344LfsX20 non-synonymous variant develop severe early-onset obesity as well as insulin resistance and type 2 diabetes[118].
We reported that peripheral SH2B1 regulates insulin sensitivity and glucose metabolism independently of its action on body weight in mice[48]. SH2B1 in pancreatic β cells directly promotes β cell expansion and insulin secretion in mice[86]. Hepatic SH2B1 regulates liver lipid levels and VLDL secretion[85]. In agreement, SH2B1 rs4788102 is associated with type 2 diabetes after adjustment for BMI in Japanese[100]. SH2B1 rs7498665 is associated with increased risk for type 2 diabetes independently of BMI in middle aged Danes[122]. SH2B1 rs7359397 is associated with insulin resistance after adjustment of BMI in Sweden men at 71 years of age[123]. Thus, SH2B1 also regulates nutrient metabolism by a body weight-independent mechanism.
SH2B1 may regulate multiple physiological processes in humans: Chromosomal 16p11.2 deletion, which results in loss of SH2B1, is associated with cognitive deficits, developmental delays[112-115], and autism[115]. Chromosomal 16p11.2 deletion is also linked to abnormal renal and enteric development in humans[124]. SH2B1 rs4788102 (G/A) is associated with increased circulating triacylglycerol levels in the Northern Swedish Population Health Study cohort[125], and is linked to myocardial infarction[126]. SH2B1 rs7498665 G allele is linked to increased bone mineral density in Japanese women[127]. However, none of these potential functions have been verified in animal models.
We reported that insulin stimulates the binding of Drosophila SH2B (also called Lnk) to Chico, a homologue of mammalian IRS proteins[5]. Almudi et al[128] showed that Drosophila SH2B binds to both Chico and insulin receptors in Drosophila cells. SH2B-deficient flies display defects in insulin/IGF signaling, developmental delay, small size, and female sterility[4,5]. Like SH2B1 null mice, SH2B-deficient flies accumulate abnormally-high levels of lipids in their fat bodies[4,5,129].
Interestingly, loss of SH2B increases resistance to oxidative stress as well as lifespan in flies, suggesting that SH2B may regulate aging and longevity[5,129]. However, SH2B1-null mice have a shorter lifespan compared with WT littermates[5]. Obesity and obesity-associated diseases may contribute to early death of SH2B1-null mice. Thus, the role of mammalian SH2B family members in aging remains unclear.
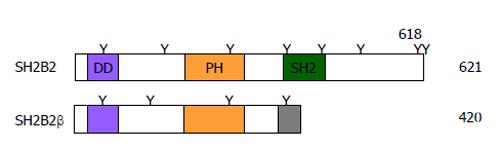
SH2B2 was originally identified in 1997[2], and the amino acids of its SH2 and PH domains are 78% and 63% identical to that of SH2B1, respectively.
Crystal structure analysis reveals that the N-terminal region of SH2B2 mediates its homodimerization via a Phe zipper[15]. The C-terminal SH2 domain is also able to form a dimer[130]. SH2B2 dimerization is predicted to induce and/or stabilize dimerization of its binding proteins, including JAK2, insulin receptors, or IGF-1 receptors, thus promoting activation of these kinases[15,130].
The SH2B2 gene also generates an additional C-terminally-truncated isoform (named SH2B2β) through alternative mRNA splicing[131]. SH2B2β contains N-terminal DD and PH domains but lacks C-terminal SH2 domain (Figure 3). SH2B2β binds to both SH2B1 and SH2B2 via its DD domain and acts as an endogenous dominant negative variant to inhibit SH2B1 and SH2B2 signaling[131].
Like SH2B1, SH2B2 binds via its SH2 domain to phospho-Tyr1158 in the activation loop of insulin receptors[130,132,133]. Insulin stimulates phosphorylation of SH2B2 on Tyr618 residue in adipocytes[132-134]. Insulin stimulates tyrosine phosphorylation of SH2B2 to a higher level than that of SH2B1[50]. IGF-1 and IGF-II also stimulate tyrosine phosphorylation of SH2B2[135]. Additionally, insulin also stimulates Akt-mediated phosphorylation of SH2B2 on Ser588 residue[136].
The role of SH2B2 in insulin action is complex. SH2B2 overexpression prolongs insulin-stimulated tyrosine phosphorylation of insulin receptors and IRS proteins[50]. Phospho-Tyr618 binds to the tyrosine kinase-binding domain of c-Cbl and promotes c-Cbl phosphorylation by insulin receptors[134,137,138]. Accordingly, knockdown of SH2B2 inhibits insulin-stimulated tyrosine phosphorylation of c-Cbl[139]. SH2B2 also enhances insulin-stimulated phosphorylation of Cbl-b on Tyr665 and Tyr709 residues[140]. SH2B2 directly binds to SHIP2 and increases SHIP2 activity, and SHIP2 in turn negatively regulates insulin-stimulated tyrosine phosphorylation of SH2B2 and its interaction with c-Cbl[141]. Furthermore, SH2B2 mediates insulin-stimulated plasma membrane translocation of both c-Cbl and Cbl-b in adipocytes[140]. SH2B2 also binds to CAP[134,139] and mediates the activation of the CAP/Cbl/Crk/C3G/TC10 pathway in adipocytes[142]. The SH2B2/CAP/Cbl/Crk/C3G/TC10 pathway is believed to be required for insulin stimulation of GLUT4 trafficking and glucose uptake in adipocytes[142]; consistently, overexpression of SH2B2(Y618F) inhibits insulin-stimulated GLUT4 trafficking[134]. However, SH2B2 also promotes c-Cbl-mediated ubiquitination and internalization of insulin receptors, thus inhibiting insulin signaling[138,143]. Additionally, SH2B2 binds to Asb6, a SOCS family member that may negatively regulate insulin signaling[144].
Deletion of SH2B2 increases insulin sensitivity in mice[6]. We reported that deletion of SH2B2 does not affect HFD-induced insulin resistance and glucose intolerance in SH2B2 KO mice in either 129Sv/C57BL mixed or C57BL congenic background[71]. Deletion of SH2B2 in SH2B1 KO mice also does not further exacerbate obesity and insulin resistance in SH2B1 and SH2B2 double KO mice relative to SH2B1 KO mice[71]. The metabolic function of SH2B2 remains unclear.
Like SH2B1, SH2B2 binds via its SH2 domain to JAK1, JAK2 and JAK3, and is tyrosyl phosphorylated by these kinases[61,145]. SH2B2 binds via both its SH2 domain and non-SH2 domain regions to JAK2, and its SH2 domain binds to phospho-Tyr813 of JAK2[16,146]. Unlike SH2B1, SH2B2 is unable to activate, or only slightly activates, JAK2[61,146]. Multiple cytokines, including interferon-γ, EPO, leukemia inhibitor factor, granulocyte-macrophage colony stimulating factor, interleukin-5 (IL-5) and IL-3, stimulate tyrosine phosphorylation of SH2B2, presumably through JAK family members[135,145,147]. Stem cell factor stimulates the binding of SH2B2 via its SH2 domain to phospho-Tyr568 and -Tyr936 of c-Kit and subsequent tyrosine phosphorylation of SH2B2[2,148]. SH2B2 binds via its SH2 domain to phospho-Tyr343 of EPO receptors[145] , and it also binds via its phospho-Tyr618 motif to c-Cbl and recruits c-Cbl E3 ligase to EPO receptors, thereby inhibiting the JAK2/STAT5 pathway in hematopoietic cell lines[145]. SH2B2 is colocalized with B cell antigen receptors (BCRs) and negatively regulates BCR signaling, and it is tyrosyl phosphorylated in response to BCR activation[2,149,150].
SH2B1 and SH2B2 play different roles in regulating immune cell function. Deletion of SH2B1 does not affect the development of T and B lymphocytes and mast cells in mice[11]. In contrast, SH2B2-deficient mast cells display augmented degradulation after cross-linking FcRI[151]. SH2B2 is expressed in B cells but not in T cells[150]. Overexpression of SH2B2 in lymphocytes impairs BCR-induced B cell proliferation and reduces B-1 and B-2 cell number in SH2B2 transgenic mice[150]. Conversely, SH2B2 KO mice have increased B-1 cell number, and SH2B2-deficient B cells display enhanced response to trinitrophenol-Ficoll, a thymus-independent type 2 antigen[149]. SH2B2 appears to be a negative regulator of a subset of immune cells.
Like SH2B1, SH2B2 binds via its SH2 domain to phospho-Tyr679, -Tyr683 and/or -Tyr684 of TrkA in response to NGF[51]. BDNF and NT-3 also stimulate the binding of SH2B2 to TrkB and TrkC, respectively[51]. NGF, BDNF and NT-3 stimulate tyrosine phosphoryation of SH2B2[51]. SH2B2 promotes NGF-induced neuronal differentiation of PC12 cells[51].
PDGF-BB stimulates the binding of SH2B2 via its SH2 domain to phospho-Tyr1021 of PDGFRβ, and SH2B2 in turn inhibits PDGF-stimulated phosphorylation of PLC-γ by competing for phospho-Tyr1021 site with PLC-γ[135]. Additionally, PDGF-BB stimulates phosphorylation of SH2B2 on Tyr618 which binds to c-Cbl, which recruits c-Cbl E3 ligase to PDGFR complex to negatively regulate PDGFR signaling and PDGFR-promoted mitogenesis[135].
Study of the SH2B family is in its early stages, and many important questions remain unaddressed. Central SH2B1 is required for the maintenance of normal energy balance, body weight, and nutrient metabolism; however, SH2B1 target neurons and neural circuits are unknown. It is unclear whether and how central SH2B1 regulates nutrient mobilization, utilization, and metabolism by a body weight-independent mechanism, and whether and how SH2B1 regulates neuronal activity by a leptin- and insulin-independent mechanism. Numerous SH2B1 mutations are associated with obesity and type 2 diabetes in humans; however, it is unclear whether these mutations are causal factors for the diseases. Does central SH2B1 regulate higher brain function independently of its action on body weight and metabolism? Do posttranslational modifications affect SH2B1 function? Do SH2B2 and SH2B3 play a role in nutrient metabolism? Can we treat obesity and type 2 diabetes by targeting SH2B family members?
P- Reviewer: Datta M, Piperi C S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Osborne MA, Dalton S, Kochan JP. The yeast tribrid system--genetic detection of trans-phosphorylated ITAM-SH2-interactions. Biotechnology (N Y). 1995;13:1474-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Yokouchi M, Suzuki R, Masuhara M, Komiya S, Inoue A, Yoshimura A. Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene. 1997;15:7-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Huang X, Li Y, Tanaka K, Moore KG, Hayashi JI. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1995;92:11618-11622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Werz C, Köhler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet. 2009;5:e1000596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Song W, Ren D, Li W, Jiang L, Cho KW, Huang P, Fan C, Song Y, Liu Y, Rui L. SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 2010;11:427-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Minami A, Iseki M, Kishi K, Wang M, Ogura M, Furukawa N, Hayashi S, Yamada M, Obata T, Takeshita Y. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes. 2003;52:2657-2665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Takaki S, Sauer K, Iritani BM, Chien S, Ebihara Y, Tsuji K, Takatsu K, Perlmutter RM. Control of B cell production by the adaptor protein lnk. Definition Of a conserved family of signal-modulating proteins. Immunity. 2000;13:599-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;195:1599-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Duan C, Yang H, White MF, Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol. 2004;24:7435-7443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metabolism. 2005;2:95-104. [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A. SH2-B is required for both male and female reproduction. Mol Cell Biol. 2002;22:3066-3077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C. Identification of SH2-Bbeta as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol. 1997;17:6633-6644. [PubMed] [Cited in This Article: ] |
| 13. | Nelms K, O’Neill TJ, Li S, Hubbard SR, Gustafson TA, Paul WE. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm Genome. 1999;10:1160-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Yousaf N, Deng Y, Kang Y, Riedel H. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J Biol Chem. 2001;276:40940-40948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Dhe-Paganon S, Werner ED, Nishi M, Hansen L, Chi Y-I, Shoelson SE. A phenylalanine zipper mediates APS dimerization. Nat Struct Mol Biol. 2004;11:968-974. [Cited in This Article: ] |
| 16. | Nishi M, Werner ED, Oh BC, Frantz JD, Dhe-Paganon S, Hansen L, Lee J, Shoelson SE. Kinase activation through dimerization by human SH2-B. Mol Cell Biol. 2005;25:2607-2621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Qian X, Ginty DD. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol Cell Biol. 2001;21:1613-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Riedel H, Wang J, Hansen H, Yousaf N. PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J Biochem. 1997;122:1105-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Chen L, Carter-Su C. Adapter protein SH2-B beta undergoes nucleocytoplasmic shuttling: implications for nerve growth factor induction of neuronal differentiation. Mol Cell Biol. 2004;24:3633-3647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Maures TJ, Chen L, Carter-Su C. Nucleocytoplasmic shuttling of the adapter protein SH2B1beta (SH2-Bbeta) is required for nerve growth factor (NGF)-dependent neurite outgrowth and enhancement of expression of a subset of NGF-responsive genes. Mol Endocrinol. 2009;23:1077-1091. [PubMed] [Cited in This Article: ] |
| 21. | Rui L, Herrington J, Carter-Su C. SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J Biol Chem. 1999;274:26485-26492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Maures TJ, Su HW, Argetsinger LS, Grinstein S, Carter-Su C. Phosphorylation controls a dual-function polybasic nuclear localization sequence in the adapter protein SH2B1β to regulate its cellular function and distribution. J Cell Sci. 2011;124:1542-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | O’Brien KB, Argetsinger LS, Diakonova M, Carter-Su C. YXXL motifs in SH2-Bbeta are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling. J Biol Chem. 2003;278:11970-11978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zhang M, Deng Y, Riedel H. PSM/SH2B1 splice variants: critical role in src catalytic activation and the resulting STAT3s-mediated mitogenic response. J Cell Biochem. 2008;104:105-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Rui L, Herrington J, Carter-Su C. SH2-B is required for nerve growth factor-induced neuronal differentiation. J Biol Chem. 1999;274:10590-10594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Lanning NJ, Su HW, Argetsinger LS, Carter-Su C. Identification of SH2B1β as a focal adhesion protein that regulates focal adhesion size and number. J Cell Sci. 2011;124:3095-3105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Yoganathan P, Karunakaran S, Ho MM, Clee SM. Nutritional regulation of genome-wide association obesity genes in a tissue-dependent manner. Nutr Metab (Lond). 2012;9:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Gutierrez-Aguilar R, Kim DH, Woods SC, Seeley RJ. Expression of new loci associated with obesity in diet-induced obese rats: from genetics to physiology. Obesity (Silver Spring). 2012;20:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Miquet JG, Sotelo AI, Bartke A, Turyn D. Increased SH2-B{beta} content and membrane association in transgenic mice overexpressing GH. J Endocrinol. 2005;185:301-306. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9119] [Cited by in F6Publishing: 8622] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 32. | Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2213] [Cited by in F6Publishing: 2162] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 33. | Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3184] [Cited by in F6Publishing: 3014] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 34. | Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2882] [Cited by in F6Publishing: 3014] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 35. | Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1573] [Cited by in F6Publishing: 1534] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 36. | Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 816] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 37. | Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1604] [Cited by in F6Publishing: 1562] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 38. | Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2526] [Cited by in F6Publishing: 2378] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 39. | Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247-E1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 40. | Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7:207-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 41. | Li Z, Zhou Y, Carter-Su C, Myers MG, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol. 2007;21:2270-2281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Rui L, Carter-Su C. Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA. 1999;96:7172-7177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Rui L, Gunter DR, Herrington J, Carter-Su C. Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-bbeta. Mol Cell Biol. 2000;20:3168-3177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413-E422. [PubMed] [Cited in This Article: ] |
| 45. | Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, Jones JC, Depinho RA, Myers MG, White MF. IRS2 signaling in LepR-b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab. 2012;15:703-712. [PubMed] [Cited in This Article: ] |
| 46. | Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem. 2004;279:43684-43691. [PubMed] [Cited in This Article: ] |
| 47. | Kotani K, Wilden P, Pillay TS. SH2-Balpha is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J. 1998;335:103-109. [PubMed] [Cited in This Article: ] |
| 48. | Morris DL, Cho KW, Zhou Y, Rui L. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Diabetes. 2009;58:2039-2047. [PubMed] [Cited in This Article: ] |
| 49. | Zhang M, Deng Y, Tandon R, Bai C, Riedel H. Essential role of PSM/SH2-B variants in insulin receptor catalytic activation and the resulting cellular responses. J Cell Biochem. 2008;103:162-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Ahmed Z, Pillay TS. Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem J. 2003;371:405-412. [PubMed] [Cited in This Article: ] |
| 51. | Qian X, Riccio A, Zhang Y, Ginty DD. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 1998;21:1017-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 185] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Wang X, Chen L, Maures TJ, Herrington J, Carter-Su C. SH2-B is a positive regulator of nerve growth factor-mediated activation of the Akt/Forkhead pathway in PC12 cells. J Biol Chem. 2004;279:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Suzuki K, Mizutani M, Hitomi Y, Kizaki T, Ohno H, Ishida H, Haga S, Koizumi S. Association of SH2-B to phosphorylated tyrosine residues in the activation loop of TrkB. Res Commun Mol Pathol Pharmacol. 2002;111:27-39. [PubMed] [Cited in This Article: ] |
| 54. | Shih CH, Chen CJ, Chen L. New function of the adaptor protein SH2B1 in brain-derived neurotrophic factor-induced neurite outgrowth. PLoS One. 2013;8:e79619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237-244. [PubMed] [Cited in This Article: ] |
| 56. | Su HW, Lanning NJ, Morris DL, Argetsinger LS, Lumeng CN, Carter-Su C. Phosphorylation of the adaptor protein SH2B1β regulates its ability to enhance growth hormone-dependent macrophage motility. J Cell Sci. 2013;126:1733-1743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O’Shea JJ, Carter-Su C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol Cell Biol. 2004;24:4557-4570. [PubMed] [Cited in This Article: ] |
| 58. | Goffin V, Hoang DT, Bogorad RL, Nevalainen MT. Prolactin regulation of the prostate gland: a female player in a male game. Nat Rev Urol. 2011;8:597-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Rider L, Tao J, Snyder S, Brinley B, Lu J, Diakonova M. Adapter protein SH2B1beta cross-links actin filaments and regulates actin cytoskeleton. Mol Endocrinol. 2009;23:1065-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Javadi M, Hofstätter E, Stickle N, Beattie BK, Jaster R, Carter-Su C, Barber DL. The SH2B1 adaptor protein associates with a proximal region of the erythropoietin receptor. J Biol Chem. 2012;287:26223-26234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | O’Brien KB, O’Shea JJ, Carter-Su C. SH2-B family members differentially regulate JAK family tyrosine kinases. J Biol Chem. 2002;277:8673-8681. [PubMed] [Cited in This Article: ] |
| 62. | Rui L, Carter-Su C. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bbeta with PDGF receptor and phosphorylation of SH2-Bbeta. J Biol Chem. 1998;273:21239-21245. [PubMed] [Cited in This Article: ] |
| 63. | Zhang Y, Zhu W, Wang YG, Liu XJ, Jiao L, Liu X, Zhang ZH, Lu CL, He C. Interaction of SH2-Bbeta with RET is involved in signaling of GDNF-induced neurite outgrowth. J Cell Sci. 2006;119:1666-1676. [PubMed] [Cited in This Article: ] |
| 64. | Donatello S, Fiorino A, Degl’Innocenti D, Alberti L, Miranda C, Gorla L, Bongarzone I, Rizzetti MG, Pierotti MA, Borrello MG. SH2B1beta adaptor is a key enhancer of RET tyrosine kinase signaling. Oncogene. 2007;26:6546-6559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Kong M, Wang CS, Donoghue DJ. Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. A role in STAT5 activation. J Biol Chem. 2002;277:15962-15970. [PubMed] [Cited in This Article: ] |
| 66. | Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C. SH2-B is required for growth hormone-induced actin reorganization. J Biol Chem. 2000;275:13126-13133. [PubMed] [Cited in This Article: ] |
| 67. | Rider L, Diakonova M. Adapter protein SH2B1beta binds filamin A to regulate prolactin-dependent cytoskeletal reorganization and cell motility. Mol Endocrinol. 2011;25:1231-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Diakonova M, Gunter DR, Herrington J, Carter-Su C. SH2-Bbeta is a Rac-binding protein that regulates cell motility. J Biol Chem. 2002;277:10669-10677. [PubMed] [Cited in This Article: ] |
| 69. | Riedel H, Yousaf N, Zhao Y, Dai H, Deng Y, Wang J. PSM, a mediator of PDGF-BB-, IGF-I-, and insulin-stimulated mitogenesis. Oncogene. 2000;19:39-50. [PubMed] [Cited in This Article: ] |
| 70. | Zhang H, Duan CJ, Chen W, Wang SQ, Zhang SK, Dong S, Cheng YD, Zhang CF. Clinical significance of SH2B1 adaptor protein expression in non-small cell lung cancer. Asian Pac J Cancer Prev. 2012;13:2355-2362. [PubMed] [Cited in This Article: ] |
| 71. | Li M, Ren D, Iseki M, Takaki S, Rui L. Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology. 2006;147:2163-2170. [PubMed] [Cited in This Article: ] |
| 72. | Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord. 2013;14:387-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Yoshiga D, Sato N, Torisu T, Mori H, Yoshida R, Nakamura S, Takaesu G, Kobayashi T, Yoshimura A. Adaptor protein SH2-B linking receptor-tyrosine kinase and Akt promotes adipocyte differentiation by regulating peroxisome proliferator-activated receptor gamma messenger ribonucleic acid levels. Mol Endocrinol. 2007;21:1120-1131. [PubMed] [Cited in This Article: ] |
| 74. | Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943-947. [PubMed] [Cited in This Article: ] |
| 75. | Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376-1382. [PubMed] [Cited in This Article: ] |
| 76. | Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026-1031. [PubMed] [Cited in This Article: ] |
| 77. | Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182-3189. [PubMed] [Cited in This Article: ] |
| 78. | Morris DL, Cho KW, Rui L. Critical role of the Src homology 2 (SH2) domain of neuronal SH2B1 in the regulation of body weight and glucose homeostasis in mice. Endocrinology. 2010;151:3643-3651. [PubMed] [Cited in This Article: ] |
| 79. | Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229-238. [PubMed] [Cited in This Article: ] |
| 80. | Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290-1300. [PubMed] [Cited in This Article: ] |
| 81. | Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736-742. [PubMed] [Cited in This Article: ] |
| 82. | Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187-1189. [PubMed] [Cited in This Article: ] |
| 83. | Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748-1757. [PubMed] [Cited in This Article: ] |
| 84. | Vanevski F, Xu B. Molecular and neural bases underlying roles of BDNF in the control of body weight. Front Neurosci. 2013;7:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Sheng L, Liu Y, Jiang L, Chen Z, Zhou Y, Cho KW, Rui L. Hepatic SH2B1 and SH2B2 regulate liver lipid metabolism and VLDL secretion in mice. PLoS One. 2013;8:e83269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Chen Z, Morris DL, Jiang L, Liu Y, Rui L. SH2B1 in β-cells regulates glucose metabolism by promoting β-cell survival and islet expansion. Diabetes. 2014;63:585-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Arumugam R, Horowitz E, Lu D, Collier JJ, Ronnebaum S, Fleenor D, Freemark M. The interplay of prolactin and the glucocorticoids in the regulation of beta-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy. Endocrinology. 2008;149:5401-5414. [PubMed] [Cited in This Article: ] |
| 88. | Bai L, Meredith G, Tuch BE. Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J Endocrinol. 2005;186:343-352. [PubMed] [Cited in This Article: ] |
| 89. | Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet. 2002;31:111-115. [PubMed] [Cited in This Article: ] |
| 90. | Xu GG, Rothenberg PL. Insulin receptor signaling in the beta-cell influences insulin gene expression and insulin content: evidence for autocrine beta-cell regulation. Diabetes. 1998;47:1243-1252. [PubMed] [Cited in This Article: ] |
| 91. | Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest. 2002;110:1011-1019. [PubMed] [Cited in This Article: ] |
| 92. | Jamshidi Y, Snieder H, Ge D, Spector TD, O’Dell SD. The SH2B gene is associated with serum leptin and body fat in normal female twins. Obesity (Silver Spring). 2007;15:5-9. [PubMed] [Cited in This Article: ] |
| 93. | Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1302] [Cited by in F6Publishing: 1287] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 94. | Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18-24. [PubMed] [Cited in This Article: ] |
| 95. | Renström F, Payne F, Nordström A, Brito EC, Rolandsson O, Hallmans G, Barroso I, Nordström P, Franks PW. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18:1489-1496. [PubMed] [Cited in This Article: ] |
| 96. | Beckers S, Zegers D, Van Gaal LF, Van Hul W. Replication of the SH2B1 rs7498665 association with obesity in a Belgian study population. Obes Facts. 2011;4:473-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | González JR, Estévez MN, Giralt PS, Cáceres A, Pérez LM, González-Carpio M, Ballester F, Sunyer J, Rodríguez-López R. Genetic risk profiles for a childhood with severely overweight. Pediatr Obes. 2014;9:272-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Shi J, Long J, Gao YT, Lu W, Cai Q, Wen W, Zheng Y, Yu K, Xiang YB, Hu FB. Evaluation of genetic susceptibility loci for obesity in Chinese women. Am J Epidemiol. 2010;172:244-254. [PubMed] [Cited in This Article: ] |
| 99. | Ng MC, Tam CH, So WY, Ho JS, Chan AW, Lee HM, Wang Y, Lam VK, Chan JC, Ma RC. Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab. 2010;95:2418-2425. [PubMed] [Cited in This Article: ] |
| 100. | Takeuchi F, Yamamoto K, Katsuya T, Nabika T, Sugiyama T, Fujioka A, Isono M, Ohnaka K, Fujisawa T, Nakashima E. Association of genetic variants for susceptibility to obesity with type 2 diabetes in Japanese individuals. Diabetologia. 2011;54:1350-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Holzapfel C, Grallert H, Huth C, Wahl S, Fischer B, Döring A, Rückert IM, Hinney A, Hebebrand J, Wichmann HE. Genes and lifestyle factors in obesity: results from 12,462 subjects from MONICA/KORA. Int J Obes (Lond). 2010;34:1538-1545. [PubMed] [Cited in This Article: ] |
| 102. | León-Mimila P, Villamil-Ramírez H, Villalobos-Comparán M, Villarreal-Molina T, Romero-Hidalgo S, López-Contreras B, Gutiérrez-Vidal R, Vega-Badillo J, Jacobo-Albavera L, Posadas-Romeros C. Contribution of common genetic variants to obesity and obesity-related traits in mexican children and adults. PLoS One. 2013;8:e70640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 103. | Hester JM, Wing MR, Li J, Palmer ND, Xu J, Hicks PJ, Roh BH, Norris JM, Wagenknecht LE, Langefeld CD. Implication of European-derived adiposity loci in African Americans. Int J Obes (Lond). 2012;36:465-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 104. | Hotta K, Kitamoto T, Kitamoto A, Mizusawa S, Matsuo T, Nakata Y, Hyogo H, Ochi H, Kamohara S, Miyatake N. Computed tomography analysis of the association between the SH2B1 rs7498665 single-nucleotide polymorphism and visceral fat area. J Hum Genet. 2011;56:716-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Haupt A, Thamer C, Heni M, Machicao F, Machann J, Schick F, Stefan N, Fritsche A, Häring HU, Staiger H. Novel obesity risk loci do not determine distribution of body fat depots: a whole-body MRI/MRS study. Obesity (Silver Spring). 2010;18:1212-1217. [PubMed] [Cited in This Article: ] |
| 106. | Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, van Vliet-Ostaptchouk JV, Wijmenga C, van der Schouw YT. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90:951-959. [PubMed] [Cited in This Article: ] |
| 107. | Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Magi R. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2202] [Cited by in F6Publishing: 2138] [Article Influence: 152.7] [Reference Citation Analysis (0)] |
| 108. | Paternoster L, Evans DM, Nohr EA, Holst C, Gaborieau V, Brennan P, Gjesing AP, Grarup N, Witte DR, Jørgensen T. Genome-wide population-based association study of extremely overweight young adults--the GOYA study. PLoS One. 2011;6:e24303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 109. | Xi B, Shen Y, Reilly KH, Zhao X, Cheng H, Hou D, Wang X, Mi J. Sex-dependent associations of genetic variants identified by GWAS with indices of adiposity and obesity risk in a Chinese children population. Clin Endocrinol (Oxf). 2013;79:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Guo Y, Lanktree MB, Taylor KC, Hakonarson H, Lange LA, Keating BJ. Gene-centric meta-analyses of 108 912 individuals confirm known body mass index loci and reveal three novel signals. Hum Mol Genet. 2013;22:184-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 111. | McCaffery JM, Papandonatos GD, Peter I, Huggins GS, Raynor HA, Delahanty LM, Cheskin LJ, Balasubramanyam A, Wagenknecht LE, Wing RR. Obesity susceptibility loci and dietary intake in the Look AHEAD Trial. Am J Clin Nutr. 2012;95:1477-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 112. | Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O’Rahilly S. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 113. | Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, Falchi M, Chen F, Andrieux J, Lobbens S. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 345] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 114. | Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, Bader PI, Hamati A, Reitnauer PJ, Smith R. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 115. | Tabet AC, Pilorge M, Delorme R, Amsellem F, Pinard JM, Leboyer M, Verloes A, Benzacken B, Betancur C. Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother. Eur J Hum Genet. 2012;20:540-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, Martinet D, Shen Y, Valsesia A, Beckmann ND. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 117. | Volckmar AL, Pütter C, Song JY, Graniger J, Knoll N, Wolters B, Hebebrand J, Scherag A, Reinehr T, Hinney A. Analyses of non-synonymous obesity risk alleles in SH2B1 (rs7498665) and APOB48R (rs180743) in obese children and adolescents undergoing a 1-year lifestyle intervention. Exp Clin Endocrinol Diabetes. 2013;121:334-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, Cline JM, Saeed S, Dale A, Cheetham T. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012;122:4732-4736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Volckmar AL, Bolze F, Jarick I, Knoll N, Scherag A, Reinehr T, Illig T, Grallert H, Wichmann HE, Wiegand S. Mutation screen in the GWAS derived obesity gene SH2B1 including functional analyses of detected variants. BMC Med Genomics. 2012;5:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Zheng Z, Hong L, Huang X, Yang P, Li J, Ding Y, Yao RE, Geng J, Shen Y, Shen Y. Screening for coding variants in FTO and SH2B1 genes in Chinese patients with obesity. PLoS One. 2013;8:e67039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Robiou-du-Pont S, Bonnefond A, Yengo L, Vaillant E, Lobbens S, Durand E, Weill J, Lantieri O, Balkau B, Charpentier G. Contribution of 24 obesity-associated genetic variants to insulin resistance, pancreatic beta-cell function and type 2 diabetes risk in the French population. Int J Obes (Lond). 2013;37:980-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Sandholt CH, Vestmar MA, Bille DS, Borglykke A, Almind K, Hansen L, Sandbæk A, Lauritzen T, Witte D, Jørgensen T. Studies of metabolic phenotypic correlates of 15 obesity associated gene variants. PLoS One. 2011;6:e23531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Fall T, Arnlöv J, Berne C, Ingelsson E. The role of obesity-related genetic loci in insulin sensitivity. Diabet Med. 2012;29:e62-e66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Sampson MG, Coughlin CR, Kaplan P, Conlin LK, Meyers KE, Zackai EH, Spinner NB, Copelovitch L. Evidence for a recurrent microdeletion at chromosome 16p11.2 associated with congenital anomalies of the kidney and urinary tract (CAKUT) and Hirschsprung disease. Am J Med Genet A. 2010;152A:2618-2622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 125. | Västermark Å, Jacobsson JA, Johansson Å, Fredriksson R, Gyllensten U, Schiöth HB. Polymorphisms in sh2b1 and spns1 loci are associated with triglyceride levels in a healthy population in northern Sweden. J Genet. 2012;91:237-240. [PubMed] [Cited in This Article: ] |
| 126. | Prudente S, Morini E, Larmon J, Andreozzi F, Di Pietro N, Nigro A, Gervino EV, Mannino GC, Bacci S, Hauser TH. The SH2B1 obesity locus is associated with myocardial infarction in diabetic patients and with NO synthase activity in endothelial cells. Atherosclerosis. 2011;219:667-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Yamada Y, Ando F, Shimokata H. Association of genetic variants of MAOA and SH2B1 with bone mineral density in community-dwelling Japanese women. Mol Med Rep. 2008;1:269-274. [PubMed] [Cited in This Article: ] |
| 128. | Almudi I, Poernbacher I, Hafen E, Stocker H. The Lnk/SH2B adaptor provides a fail-safe mechanism to establish the Insulin receptor-Chico interaction. Cell Commun Signal. 2013;11:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Slack C, Werz C, Wieser D, Alic N, Foley A, Stocker H, Withers DJ, Thornton JM, Hafen E, Partridge L. Regulation of lifespan, metabolism, and stress responses by the Drosophila SH2B protein, Lnk. PLoS Genet. 2010;6:e1000881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 130. | Hu J, Liu J, Ghirlando R, Saltiel AR, Hubbard SR. Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Mol Cell. 2003;12:1379-1389. [PubMed] [Cited in This Article: ] |
| 131. | Li M, Li Z, Morris DL, Rui L. Identification of SH2B2beta as an inhibitor for SH2B1- and SH2B2alpha-promoted Janus kinase-2 activation and insulin signaling. Endocrinology. 2007;148:1615-1621. [PubMed] [Cited in This Article: ] |
| 132. | Moodie SA, Alleman-Sposeto J, Gustafson TA. Identification of the APS protein as a novel insulin receptor substrate. J Biol Chem. 1999;274:11186-11193. [PubMed] [Cited in This Article: ] |
| 133. | Ahmed Z, Smith BJ, Kotani K, Wilden P, Pillay TS. APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem J. 1999;341:665-668. [PubMed] [Cited in This Article: ] |
| 134. | Liu J, Kimura A, Baumann CA, Saltiel AR. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol Cell Biol. 2002;22:3599-3609. [PubMed] [Cited in This Article: ] |
| 135. | Yokouchi M, Wakioka T, Sakamoto H, Yasukawa H, Ohtsuka S, Sasaki A, Ohtsubo M, Valius M, Inoue A, Komiya S. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene. 1999;18:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 136. | Katsanakis KD, Pillay TS. Cross-talk between the Two Divergent Insulin Signaling Pathways Is Revealed by the Protein Kinase B (Akt)-mediated Phosphorylation of Adapter Protein APS on Serine 588. J Biol Chem. 2005;280:37827-37832. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 137. | Hu J, Hubbard SR. Structural Characterization of a Novel Cbl Phosphotyrosine Recognition Motif in the APS Family of Adapter Proteins. J Biol Chem. 2005;280:18943-18949. [Cited in This Article: ] |
| 138. | Ahmed Z, Smith BJ, Pillay TS. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 2000;475:31-34. [PubMed] [Cited in This Article: ] |
| 139. | Ahn MY, Katsanakis KD, Bheda F, Pillay TS. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J Biol Chem. 2004;279:21526-21532. [PubMed] [Cited in This Article: ] |
| 140. | Liu J, DeYoung SM, Hwang JB, O’Leary EE, Saltiel AR. The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J Biol Chem. 2003;278:36754-36762. [PubMed] [Cited in This Article: ] |
| 141. | Onnockx S, De Schutter J, Blockmans M, Xie J, Jacobs C, Vanderwinden JM, Erneux C, Pirson I. The association between the SH2-containing inositol polyphosphate 5-Phosphatase 2 (SHIP2) and the adaptor protein APS has an impact on biochemical properties of both partners. J Cell Physiol. 2008;214:260-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 142. | Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 143. | Kishi K, Mawatari K, Sakai-Wakamatsu K, Yuasa T, Wang M, Ogura-Sawa M, Nakaya Y, Hatakeyama S, Ebina Y. APS-mediated ubiquitination of the insulin receptor enhances its internalization, but does not induce its degradation. Endocr J. 2007;54:77-88. [PubMed] [Cited in This Article: ] |
| 144. | Wilcox A, Katsanakis KD, Bheda F, Pillay TS. Asb6, an adipocyte-specific ankyrin and SOCS box protein, interacts with APS to enable recruitment of elongins B and C to the insulin receptor signaling complex. J Biol Chem. 2004;279:38881-38888. [PubMed] [Cited in This Article: ] |
| 145. | Wakioka T, Sasaki A, Mitsui K, Yokouchi M, Inoue A, Komiya S, Yoshimura A. APS, an adaptor protein containing Pleckstrin homology (PH) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia. 1999;13:760-767. [PubMed] [Cited in This Article: ] |
| 146. | Kurzer JH, Saharinen P, Silvennoinen O, Carter-Su C. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol Cell Biol. 2006;26:6381-6394. [PubMed] [Cited in This Article: ] |
| 147. | Iseki M, Takaki S, Takatsu K. Molecular cloning of the mouse APS as a member of the Lnk family adaptor proteins. Biochem Biophys Res Commun. 2000;272:45-54. [PubMed] [Cited in This Article: ] |
| 148. | Wollberg P, Lennartsson J, Gottfridsson E, Yoshimura A, Rönnstrand L. The adapter protein APS associates with the multifunctional docking sites Tyr-568 and Tyr-936 in c-Kit. Biochem J. 2003;370:1033-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Iseki M, Kubo C, Kwon SM, Yamaguchi A, Kataoka Y, Yoshida N, Takatsu K, Takaki S. Increased numbers of B-1 cells and enhanced responses against TI-2 antigen in mice lacking APS, an adaptor molecule containing PH and SH2 domains. Mol Cell Biol. 2004;24:2243-2250. [PubMed] [Cited in This Article: ] |
| 150. | Iseki M, Kubo-Akashi C, Kwon S-M, Yamaguchi A, Takatsu K, Takaki S. APS, an adaptor molecule containing PH and SH2 domains, has a negative regulatory role in B cell proliferation. Biochem Biophys Res Commun. 2005;330:1005-1013. [Cited in This Article: ] |
| 151. | Kubo-Akashi C, Iseki M, Kwon SM, Takizawa H, Takatsu K, Takaki S. Roles of a conserved family of adaptor proteins, Lnk, SH2-B, and APS, for mast cell development, growth, and functions: APS-deficiency causes augmented degranulation and reduced actin assembly. Biochem Biophys Res Commun. 2004;315:356-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |