Published online Dec 10, 2015. doi: 10.4239/wjd.v6.i17.1323
Peer-review started: August 21, 2015
First decision: September 30, 2015
Revised: October 1, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: December 10, 2015
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease targeting the pancreatic beta-cells and rendering the person hypoinsulinemic and hyperglycemic. Despite exogenous insulin therapy, individuals with T1DM will invariably develop long-term complications such as blindness, kidney failure and cardiovascular disease. Though often overlooked, skeletal muscle is also adversely affected in T1DM, with both physical and metabolic derangements reported. As the largest metabolic organ in the body, impairments to skeletal muscle health in T1DM would impact insulin sensitivity, glucose/lipid disposal and basal metabolic rate and thus affect the ability of persons with T1DM to manage their disease. In this review, we discuss the impact of T1DM on skeletal muscle health with a particular focus on the proposed mechanisms involved. We then identify and discuss established and potential adjuvant therapies which, in association with insulin therapy, would improve the health of skeletal muscle in those with T1DM and thereby improve disease management- ultimately delaying the onset and severity of other long-term diabetic complications.
Core tip: Skeletal muscle is adversely affected in type 1 diabetes mellitus and strategies to maintain/improve muscle health will positively impact disease management and delay diabetic complications.
- Citation: Coleman SK, Rebalka IA, D’Souza DM, Hawke TJ. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J Diabetes 2015; 6(17): 1323-1336
- URL: https://www.wjgnet.com/1948-9358/full/v6/i17/1323.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i17.1323
Type 1 diabetes mellitus (T1DM) is a chronic disease characterized by the autoimmune destruction of the pancreatic beta cells. Without the insulin produced by these cells, the body is no longer able to manage blood glucose, leading to hyperglycemia. Even in the case of tightly regulated insulin therapy, it is extremely difficult to maintain blood glucose levels within an acceptable range[1]. Complications, such as blindness (retinopathy), kidney failure (nephropathy), peripheral nerve damage (neuropathy), cardiovascular disease and impairments to muscle health (myopathy), invariably arise as a direct/indirect result of the inability to manage blood glucose.
In healthy individuals, insulin is typically released postprandially and is responsible for promoting an influx of glucose into adipose, hepatic and skeletal muscle cells for storage or metabolism. Of these insulin-sensitive cells, skeletal muscle is the largest of these organs by mass in the body[2,3] and thus plays a prominent role in glucose homeostasis. Skeletal muscle is also capable of uptaking large amounts of glucose in a non-insulin mediated manner[4] such as is seen during muscle contraction. Not surprisingly then, if the health of skeletal muscle is sub-optimal, management of blood glucose will also be sub-optimal. Despite the vital role played by skeletal muscle in whole body metabolic control and blood glucose management, our understanding of changes to the health of this organ system in both acute and long-term T1DM is still in its infancy. Much of our current knowledge is derived from rodent models with uncontrolled hyperglycemia for a period of weeks or months. The resultant impairments to skeletal muscle health, referred to as “diabetic myopathy” manifests as impaired muscle growth and strength[5-8], altered metabolic capacity[5-7] and reduced regenerative and stem cell capacities[9-15]. Though human studies investigating diabetic myopathy are sparse, the results to date suggest consistency in the observations with rodent models[6]. Specifically, reductions in muscle mass, fiber size, work capacity and maximal force production[6] are seen in persons with T1DM.
In this review, we will introduce some of the key factors impacting skeletal muscle health in those with T1DM and then discuss established and possible therapeutic strategies focused on improving skeletal muscle health as a means of improving skeletal muscle health with the ultimate goal of attenuating the development of other diabetic complications.
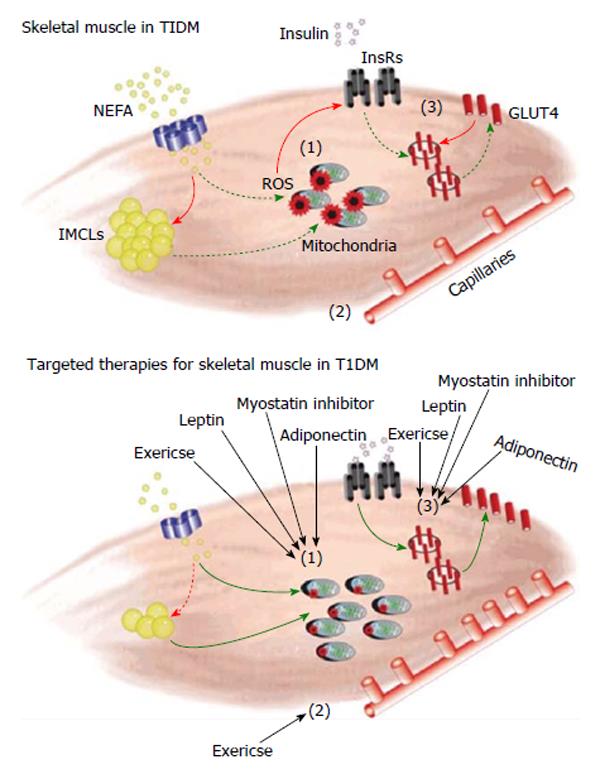
In a state such as T1DM, excessive accumulation of glucose in the blood incites excessive stress on the entire body. Specifically within the muscle, damaging metabolites, such as reactive oxygen species (ROS), wreak havoc within the tissue causing damage to cellular structures with resultant functional impairments. The oxidative capacity of T1DM skeletal muscle is altered when compared to healthy, non-diabetic muscle. In the Ins2Akita+/- model of T1DM, glycolytic fibers exhibit atrophy, as demonstrated through a decreased proportion of type IIB/X fibers, as well as a decrease in type IIA and IIB/X fiber area[5]. Studies in human T1DM populations also displayed alterations in fiber type variability through an increased proportion of fast glycolytic fibers, and an increased amount of glycolytic enzyme activity[16,17]. Correspondingly, changes in the normal fiber type distribution are accompanied by changes in fuel oxidation and metabolic capacity of the muscle. Due to the reduced ability of skeletal muscle to access carbohydrates in times of inadequate/low insulin, diabetic skeletal muscle must promote the use of other fuel sources. Skeletal muscle of individuals with T1DM is associated with the excessive deposition of intramyocellular lipids (IMCL)[5,18]. This high level of IMCLs is noted in the muscle following food consumption, and very low levels in the fasted state, as this fuel source is heavily relied upon. Muscle from the streptozotocin (STZ) T1DM mouse model also demonstrates increased acetyl CoA/CoA ratio, hypothesized to be due to increased fatty acid oxidation[19], as well as increased fat utilization and mobilization[20], as the muscle tries to deal with the increased fat content. Along with these changes in the skeletal muscle of both the Ins2Akita+/- and STZ models, there is an upregulation of CD36, a fatty acid transporter[5,21-23]. The alloxan-induced T1DM model similarly demonstrates an increase in free fatty acid levels in cardiac and skeletal muscle tissues[24]. It is believed that as the levels of IMCL deposition increase, lipotoxicity ensues[25], enhancing stress to the tissue. Despite a heavier reliance on triglycerides, diabetic myopathy is accompanied with decreased activity of lipid metabolism enzymes citrate synthase[5,26,27], β-hydroxybutyrate[5], and 3-hydroxybutyrate dehydrogenase[26]. The trend of increased IMCL persists in human populations of T1DM, and is correlated with the degree of insulin resistance observed in these subjects[28]. Contrarily, the Ins2Akita+/- mouse model does not show the same increase in intramuscular triglyceride content[5,29] seen in the (disease duration-matched) STZ model, and does not demonstrate a decrease in citrate synthase or β-hydroxybutyrate activity[5]. It is worth noting, however, in the case of the STZ-induced diabetic model, that STZ itself has been implicated in the generation of oxidative stress within muscle cells, even in the absence of hyperglycemia[30]. Thus the STZ model could be held to represent a much more severe model of T1DM due to the elevated levels of oxidative stress than may be seen in diabetes alone.
Studies have shown that hyperglycemia and T1DM specifically display elevated markers of oxidative stress in the skeletal muscle[31,32], resulting in insulin resistance[33]. Accumulation of damaging ROS in skeletal muscle has been linked with a loss of protein mass[34] and disrupted protein turnover[35]. This oxidative stress has an effect on transcription of glucose transporters which contributes to the development of insulin resistance[32]. Specifically in STZ rats, oxidative stress was seen to upregulate atrogin-1 and MuRF-1, markers of muscle atrophy, and downregulate MyoD, Myogenin and JunD, genes required for normal muscle growth and repair[15]. Though there is clear evidence that accumulation of IMCL deposits causes dysfunctional fatty acid oxidation, generation of ROS, and stress on the muscle, future studies are needed in other diabetic models to more fully elucidate the contribution(s) of these stressors to diabetic myopathy development and progression.
An intricate network of vasculature supplying the skeletal muscle with adequate blood supply is required for optimal muscle performance. In T1DM, however, there is dysfunction of the capillary network and endothelial cells. Hyperglycemia has been found to alter the capillary bed, reducing capillary diffusing capacity and disrupting hemodynamic regulation to skeletal muscle[36,37]. T1DM mice demonstrate both a decrease in capillary-to-fiber ratio[5,38] and dysregulated angiogenesis[38]. Moreover, thickening of the basement membrane of skeletal muscle blood vessels in T1DM rats has been found to be positively related to their level of dysglycemia[39-41]. Thickening of the basement membrane in skeletal muscle capillaries is also greater in patients experiencing worsening retinopathy, a serious complication of T1DM[42]. Furthermore, studies show that peripheral microvascular dysfunction could also be seen as an indicator of atherosclerotic damage in individuals with T1DM[43]. In the case of ApoE-/- STZ mice, a T1DM rodent model which mimics macrovascular complications, mice which were returned to normoglycemia exhibited expansion of the vasa vasorum microvascular network[44]. This expansion was directly correlated with attenuation of atherogenesis[44]. Overall, early attenuation of vascular dysfunction within the skeletal muscle would help prevent further long-term complications.
Brownlee[31], in his unifying theory of diabetic complications, has suggested that a large part of cardiovascular disease risk in those with diabetes is due to insulin resistance. Though insulin resistance is more commonly associated with the development of type 2 diabetes, individuals with T1DM also demonstrate insulin resistance[29,45,46]. In fact, insulin resistance has been observed in T1DM youth[45] and long-duration type 1 diabetics[47,48], and occurs independent of glycemic control[49]. Impairment of glucose transporters[50] and glucose transport following exercise[51] have been observed in insulin resistant T1DM, further enhancing the diabetic phenotype. Insulin resistance in T1DM has been linked directly with skeletal muscle pathology[52] through increased IMCL deposition and dysregulation of fatty acid oxidation[53].
Interestingly, exposure to a long-acting human insulin analogue, insulin detemir, has been shown to result in more significant insulin resistance, oxidative stress, skeletal muscle ectopic fat accumulation and mitochondrial impairments compared to hyperglycemia alone[54]. These results indicate that insulin resistance may in fact be a response to insulin treatment as opposed to hyperglycemia. Therapeutic strategies targeting an improvement in peripheral insulin sensitivity would reduce exogenous insulin needs, preventing insulin resistance and thus delaying the onset of diabetic complications[55].
In response to T1DM, skeletal muscle is negatively impacted, as is evident by increased metabolic stress, vascular impairments and insulin resistance (Figure 1). With all of these decrements, muscle is not able to respond optimally to stressors or combat the elevated glycemic and lipid loads frequently experienced in T1DM. It is believed that maintaining or improving skeletal muscle health in T1DM can contribute significantly to delaying diabetic complications. For example, improving muscle metabolic health would reduce oxidative stress, and increasing insulin sensitivity would have the combined effect of improving glycemic control and reducing exogenous insulin needs. In the following section we propose a variety of skeletal muscle-centric therapeutic strategies as a means to both improve the overall health of those with diabetes mellitus and reduce the complications associated with this disease state.
Exercise therapy is now being regarded as an important component in the management of T1DM due to its resultant improvements towards attenuation of microvascular complications and improvements of insulin sensitivity[56]. In a variety of metabolic disorders (independent of T1DM) exercise is associated with improvements in glucose and lipid metabolism[57-59], enhanced glucose transport[60], increased insulin sensitivity[61,62], reductions in daily insulin requirement, and a decreased risk of related co-morbidities[63,64]. Accordingly, it is predicted that improvements in skeletal muscle health, by way of exercise, would promote a greater state of well-being in individuals with T1DM.
Due to the onset of myopathy with T1DM disease advancement[6], as well as the presence of disease onset during the critical growth period, it is not surprising that the physical fitness of T1DM children is often observed to be reduced when compared to their healthy age-matched counterparts[6,65]. This disparity has been attributed, in part, to the inverse association between glycemic control and skeletal muscle function, resulting in reduced aerobic fitness. As mentioned, T1DM individuals commonly experience both functional and growth impairments[5-8]. A decrease in cardiorespiratory fitness has similarly been observed in T1DM adolescents and adults with poor glycemic control[66,67]. Based on these data, the implementation of an exercise training program would be considered an effective therapeutic strategy to improve muscle health and delay the onset and progression of diabetic complications.
A primary clinical measure to define the risk for complications development in those with T1DM is glycosylated haemoglobin (HbA1c). Changes to long term glycemic control (measured by HbA1c) are a contributing factor to disease progression, and it has been shown that hyperglycemia is prone to induce an assortment of co-morbidities that further perpetuate the disease state[68] including muscle morphology and function[69-71]. Many studies investigating the therapeutic benefit of exercise on the overall health of those with T1DM have relied on HbA1c as a primary outcome measure. Indeed, while exercise has been shown to increase glucose uptake and improve insulin sensitivity, information on changes to HbA1c remains largely controversial. Studies assessing the impact of either aerobic and/or strength training protocols in T1DM rodents and humans fail to establish a consensus on whether or not increasing physical activity improves glycemic control. For instance, a number of studies have reported a decrease in HbA1c levels following a period of aerobic training[72,73], while others report no difference in HbA1c following a period of comparable training volume[74-76]. Similarly, investigations incorporating strength training protocols have reported no effect on HbA1c levels[77,78], while others indicate beneficial effects incorporating both strength and aerobic exercise[79]. Nevertheless, longitudinal data suggests that improvements in glycemic control are still observed despite minimal improvements in HbA1c levels following aerobic training[80,81]. Discrepancies in HbA1c improvements amongst the studies reported are thought to be a result of variations in insulin dosage (reducing dosages as a means to prevent exercise-induced hypoglycaemia) and carbohydrate uptake, which override any quantifiable changes in glucose disposal. Although increased fitness may not dramatically improve glycemic control, physical activity is still encouraged for all T1DM individuals due to the additional skeletal muscle health benefits incurred, including the attenuation in microvascular complications, improved insulin sensitivity, reductions in inflammation, and enhanced muscle growth and repair. For a thorough review on exercise and T1DM, see[56].
As noted previously, the progression of T1DM promotes the onset of various microvascular complications. These complications not only promote a worsened disease state, but may also interfere with the individual’s physical capacity[82]. It is critical to address the role of vascular complications in the skeletal muscle in T1DM, as maladaptive changes to the diabetic muscle often precede the advancement of other complications[6,69,83]. The effect of exercise therapy on skeletal muscle vasculature is largely positive, with many studies reporting increases in angiogenesis-related genes[38], and enhanced vascular function[84,85]. In humans, an inverse correlation exists between physical activity and the development of macro-and micro-vascular complications in long-standing T1DM[86], however specific adaptions in skeletal muscle vasculature following exercise training remain largely unknown.
Elevations in markers of inflammatory and oxidative stress have also been identified in T1DM patients[87-89]. Inflammation is known to negatively impact skeletal muscle health, as observed by the positive correlation between inflammatory factors and muscle wasting[90,91]. Skeletal muscle from T1DM mice show an increased expression of inflammatory-related factors[92,93]. Exercise does elicit anti-inflammatory effects[94,95], which are dependent on exercise type, duration, intensity, endurance capacity and muscle morphology[96-98]. Recently, diabetic rats demonstrated reductions in inflammatory cytokine levels [i.e., interleukin 1B (IL-1B), IL-4, etc.] following exercise intervention[99]. Furthermore, T1DM children subjected to an acute bout of exercise demonstrated dysregulation in the expression of inflammatory and oxidative stress variables[100], thereby providing evidence for the importance of exercise training in the reduction of inflammation associated with T1DM disease progression. While exercise reduces pro-inflammatory cytokines, it has also been found to promote the expression of anti-inflammatory cytokines that enhance muscle health. For instance, STZ rats subjected to a 5-wk resistance exercise training regimen displayed an increase in IL-15, an anabolic cytokine that is known to induce hypertrophy in skeletal muscle[101,102], while hindering apoptosis[103]. The cytokine IL-6, while primarily believed to be pro-inflammatory in nature, is also known to exert beneficial effects on skeletal muscle following training. Specifically, increased IL-6 production promoted greater glucose uptake during exercise[104] and an up-regulation of additional anti-inflammatory cytokines[105]. These data, while not explicitly investigated within the context of T1DM, suggests a protective role of IL-6 release from skeletal muscle following exercise. While these studies implicate exercise in the support of muscle health via attenuation of the inflammatory state associated with T1DM development, future work using human data is needed to further delineate the role of exercise training in the regulation of chronic inflammation in T1DM.
Overall physical capacity is negatively affected by the presence of T1DM, particularly in those with long-standing disease, and thus it is predicted that any form of activity (endurance, resistance, etc.) will benefit the individual by maintaining and/or enhancing skeletal muscle health and the benefits therein. The literature to date makes a clear case that exercise training can positively affect the skeletal muscle of those with T1DM through its influence on skeletal muscle endothelial cell function, inflammation and insulin sensitivity. What remains to be clearly elucidated is the impact of exercise training on the modulation of long-term glycemic control; a measure hampered by subject variability in insulin dosage, intensity of exercise training, and degree of disease advancement between studies.
Myostatin (GDF-8), primarily synthesized by skeletal muscle and a negative regulator of muscle growth, was originally discovered in 1997 when a mutation in the myostatin gene was shown to be responsible for phenotypically hypermuscular cattle[106]. In the case of myostatin deficiency, muscle growth was observed to reach 2-3 times that of typical muscle size[106]. Instances of loss-of-function myostatin mutation have been observed in human populations to the same effect[107].
Myostatin levels have been measured in the STZ-diabetic mouse, and consistently show elevated protein[108] and gene expression[109,110]. Human populations of T2DM also demonstrate increased levels of myostatin[111-113]. This increase of myostatin in T1DM is consistent with the decreased muscle mass and myopathic phenotype observed. In a study of food deprivation, a state similar to that as found in uncontrolled T1DM, increased expression of myostatin was found to contribute to the observed muscle atrophy[114].
Methods of inhibiting or knocking down elements of the myostatin pathway have been, and are currently being investigated in a variety of disease states. Naturally, myostatin inhibition therapy via MYO-029[115], PF-06252616[116] and ACE-031[117], amongst others, was originally investigated in patient populations with genetic muscular diseases and muscle wasting disorders (e.g., cancer cachexia). More recently, blockade of the myostatin pathway has been linked to improvements of metabolic pathologies in animal studies. For instance, high-fat diet fed mice with myostatin reduction therapy did not gain weight as wildtype counterparts did[118-120] and myostatin inhibition is seen to prevent diabetes development in a model of lipodystrophy[121]. Furthermore, in the case of T1DM specifically, STZ animals treated with follistatin, a known inhibitor of myostatin, demonstrate improvements in the regenerative capacity of skeletal muscle[14].
In the case of other metabolic diseases, increased myostatin expression has been implicated in the development of insulin resistance[122] and reduction or inhibition of myostatin has been seen to improve insulin sensitivity[119,123-126]. It is clear that myostatin plays a role in glycemic control of skeletal muscle. Models examining mutated myostatin or myostatin inhibition coincide with significantly elevated levels of GLUT4[127,128] and GLUT1[128], resulting in increased glucose uptake[127]. This evidence demonstrates how myostatin plays an important role in increasing glucose disposal both dependent and independent of insulin. Reductions in circulating myostatin in T1DM may therefore aid in both reducing exogenous insulin needs and preventing the insulin resistance which may develop as a result.
Increased levels of myostatin may contribute to the elevated oxidative stress noted in diabetic myopathy. Myostatin is thought to operate both through[129] and independent[130] of the nuclear factor κB pathway to produce ROS, leading to muscle atrophy. In STZ-induced T1DM, myostatin was shown to contribute to oxidative stress leading to DNA damage[131]. Since myostatin contributes to oxidative stress, it is possible that in the case of myostatin inhibition, decreased oxidative stress (ROS production) could lead to functional problems as have been reported in rodents without myostatin[132]. It is important to remember however that in T1DM the fulcrum is already shifted towards increased ROS levels. Thus, reductions in myostatin could serve to restore balance resulting in healthier muscle and the associated benefits therein.
Myostatin inhibition has more recently been linked to the “browning” of white adipose tissue[133-136]. One study has postulated this effect is mediated through the 5' AMP-activated protein kinase (AMPK)-PGC1α-Fndc5 pathway originating in skeletal muscle[137]. While this is an indirect positive effect of myostatin inhibition (i.e., not specifically related to skeletal muscle), it would also provide benefits in reducing the diabetic condition. Gunawardana et al[138] have shown that a transplant of brown adipose tissue into STZ-diabetic mice resulted in normalization of glucose and attenuation of the diabetic state. This effect is thought to occur through recovery of subcutaneous white adipose tissue, resulting in the normalization of adipokines leptin, adiponectin and insulin-like growth factor-1 (IGF-1).
Although downregulation of myostatin shows promise in the treatment of T1DM via decreasing oxidative stress, upregulating glucose transporters, preventing insulin resistance and browning white adipose tissue, there are still many areas left to be explored. Production of ROS is a delicate balance, and a drastic decrease in ROS levels can cause harm to an organism as well. Further, Wang et al[139] explored a soluble myostatin receptor to downregulate the effects of myostatin in conjunction with STZ diabetes, and saw worsened hyperglycemia. Authors of this study observed severely low insulin levels and significantly elevated glucocorticoid levels, common to the STZ rodent model[139]. The lack of effect of myostatin reduction therapy may be the result of the rise in glucocorticoids (resulting in elevated blood glucose) or the absence of circulating insulin. Since the inhibition of myostatin may have its greatest metabolic effects via increasing insulin sensitivity, the lack of insulin seen in the STZ model may have been detrimental to any potential blood glucose lowering capacity of myostatin inhibition[139]. Overall, there is certainly enough compelling evidence to further investigate myostatin inhibition strategies as an adjuvant therapeutic strategy for T1DM.
Leptin, a hormone predominantly produced by adipose tissue, has been heavily implicated in metabolism. First unwittingly examined in the 1950s, the leptin knockout mouse (ob/ob mouse) demonstrated excessive hyperphagia and in turn, excessive weight gain[140]. The discovery of leptin itself in 1994 led to the understanding of leptin as an important hormone with regard to appetite control[141], and has further been implicated in reproductive health[142], bone metabolism[143], the immune response[144], and importantly in regulating fat metabolism, insulin resistance and overall metabolism. The identification of leptin brought about an understanding that adipose tissue was an endocrine organ. Currently, more than 19 different adipocyte-derived cell-signaling proteins, termed adipokines, have been identified[145]. Adipokines include inflammatory mediators, angiogenic proteins, and metabolic regulators. With the global rise in obesity, the relationship between adipose tissue and its systemic effects has attracted much interest. Adipokines are thought to influence multiple processes, including glucose and fatty acid metabolism, and insulin sensitivity.
It has been noted that children and adults with poorly controlled T1DM demonstrate low levels of leptin regardless of gender[23,146]. Leptin levels can be normalized via insulin treatment in T1DM children[146], but not in adults[23]. Furthermore, poorly managed diabetes has been associated with an increase in the soluble leptin receptor, leading to leptin resistance[147]. This same trend is seen in STZ diabetic rodents, in which the induction of T1DM caused a decrease in circulating leptin, which was reversed by insulin therapy[148,149].
Leptin therapy has been found to attenuate many of the effects of T1DM, most notably restoring euglycemia[150-153]. Considering the restoration of euglycemia coupled with leptin’s ties to appetite control, leptin treated STZ diabetic rodents demonstrate diminished hyperphagia[154]. While Fujikawa et al[155] have hypothesized that the improvements observed in T1DM via leptin treatment occur via CNS-dependent mechanisms, and Unger’s group has targeted leptins ability to decrease plasma glucagon levels[152,156-158], there is growing evidence that leptin therapy provides benefits through skeletal muscle as well. Leptin treatment has been found to increase insulin sensitivity and glucose uptake in skeletal muscle specifically[159-161]. Yu et al[162] demonstrate that hyperleptinemia leads to euglycemia independent of insulin. This causes an upregulation of IGF-1 and pIGF-1 receptor, which further leads to increases in skeletal muscle IRS-1, P13K and ERK phosphorylation[162]. Specifically in the soleus muscle, leptin was implicated to act in an insulin-like fashion, leading to increases in a variety of muscle metabolic factors including glucose uptake, glycogen synthesis, lactate formation and glucose oxidation[163].
Leptin has also been demonstrated to play a role in both regulating fatty acid oxidation and preventing insulin resistance in skeletal muscle. Skeletal muscle of STZ diabetic animals treated with leptin exhibit evidence of restored glucose uptake, but also enhanced skeletal muscle markers of fatty acid utilization and oxidation, notably independent of differences in food consumption[164]. Leptin has also been seen to direct lipids towards the muscle to be burned rather than stored[165], as well as increase fatty acid oxidation in the skeletal muscle[166]. These metabolic benefits are thought to occur through the activation of AMPK and the inhibition of acetyl-CoA carboxylase[167]. Insulin resistance in T1DM has also been found to be reversed through leptin therapy[168]. Interestingly, however, this was thought to occur in a method independent of skeletal muscle[168]. Kusakabe et al[169] found that leptin treated STZ mice fed high fat diet to induce insulin resistance demonstrated enhanced insulin sensitivity. This was again seen by Lin et al[170], although was attributed to neurological changes. Although leptin’s role in diminishing insulin resistance is clear, further work is necessary to elucidate the mechanism of its action in this role.
As leptin appears to mimic many of the effects of insulin, leptin may indeed be used as an adjuvant therapy to insulin[152,171]. When leptin and insulin were given in conjunction to STZ rodents, much smaller doses of insulin were required to achieve normoglycemia than would be required with each treatment alone[172]. Metreleptin, a leptin analogue, is currently under clinical trials (NCT01268644) in conjunction with insulin therapy in order to investigate the effectiveness of this combination seen in the literature. Considering both the prevalent development of insulin resistance and the difficulty in maintaining normoglycemia in T1DM patients, even in the presence of insulin therapy, this adjuvant therapy warrants further investigation in the human T1DM population.
Adiponectin, first characterized in 1995[173], is an insulin-sensitizing adipokine; capable of increasing both insulin-mediated uptake of glucose and β-oxidation of lipids[174-177]. Individuals with T2DM exhibit significantly lower levels of circulating adiponectin than healthy, non-diabetic individuals[178]. With adiponectin behaving as an insulin sensitizing factor, it is not surprising that this deficiency in adiponectin closely correlates with an individuals’ degree of insulin resistance[179]. Systemic injection of adiponectin has been shown to decrease resting blood glucose levels and attenuate insulin resistance[174,175,180]. Furthermore, stimulation of adiponectin production in an animal model of T2DM improves skeletal muscle insulin sensitivity[181]. Paradoxically, when compared to healthy non-diabetic subjects, adiponectin is present in elevated levels in individuals with T1DM, regardless of their level of glycemic control[28,182,183] and these elevations are positively correlated with duration of T1DM[184,185].
The presence of metabolic syndrome in patients with T1DM has previously been associated with insulin resistance[186]. Interestingly, T1DM patients with metabolic syndrome present with significantly lower levels of serum adiponectin than T1DM patients that do not present with metabolic syndrome[186]. Similar to the relationship between insulin sensitivity and adiponectin in non-diabetic individuals, levels of adiponectin are positively correlated with insulin sensitivity in T1DM[184]. Insulin sensitivity in T1DM individuals, however, is lower than in non-diabetic subjects at any given level of circulating adiponectin[184]. The preservation of the positive relationship between adiponectin and insulin sensitivity in T1DM coupled with the overall decrease in insulin sensitivity in T1DM individuals suggests a modification in the homeostatic regulation of adiponectin in the T1DM state[184].
Upon binding to adiponectin receptors in the pancreatic beta cells, adiponectin increases insulin gene expression and secretion[187]. The presence of insulin, on the other hand, has been shown to downregulate adiponectin gene expression[188]. In this light, it is possible that the overabundance of adiponectin in the T1DM state is a compensatory mechanism; an attempt at upregulating insulin production. As previously mentioned, however, despite higher levels of adiponectin being associated with insulin sensitivity, individuals with T1DM still have a lower insulin sensitivity than non-diabetic individuals[184].
Adult T1DM human and rodent muscle has been observed to have higher levels of intramyocellular lipids (IMCL) than muscle of healthy, non-diabetic subjects[5,28,189]. This accretion of IMCLs has been associated with insulin resistance in T1DM[189]. Interestingly, previous reports indicate no differences in IMCL content between T1DM and non-diabetic children[190], potentially indicating that, similar to circulating levels of adiponectin, IMCL content is affected by, and positively associated with T1DM disease duration. Furthermore, Krause et al[191] found a positive correlation between intramyocellular adiponectin expression and IMCL density in non-diabetic mice; elevated levels of adiponectin were detected in muscle fibers displaying a greater IMCL density, while adiponectin was virtually undetectable in muscle fibers with a low IMCL content. In the T1DM disease state, however, it is possible that this positive relationship may be a compensatory mechanism to remove lipid from circulation, and further investigation into this relationship in the diabetic state must be conducted. In 2007, Behre[192] proposed that adiponectin may in fact be a defense mechanism of the body in response to starvation (as can be compared to overt T1DM), resulting in increased fatty acid oxidation and glucose uptake via activation of AMPK and PPAR-α.
Overall, a great deal of research must still be conducted to elucidate the role of adiponectin in both overall health and skeletal muscle health in T1DM. While adiponectin levels are elevated in the T1DM state, adiponectin appears to act in a compensatory mechanism to improve insulin sensitivity in the absence of insulin. As insulin resistance develops in T1DM individuals that develop metabolic syndrome, adiponectin levels demonstrate a decline. Evidence suggests that it may be beneficial to supplement adiponectin in the T1DM disease state in order to boost insulin production and increase insulin sensitivity in order to prevent this insulin resistance.
The presence of insulin resistance, altered lipid metabolism, impaired vascularization and oxidative stresses are clear indicators of the presence of pathology in T1DM skeletal muscle. Exercise training, myostatin, leptin and adiponectin have been identified as potential therapeutic avenues to investigate with regard to improving skeletal muscle health (Figure 1). It is our hypothesis that, by improving skeletal muscle health in T1DM, the muscle will be better able to contribute to the reduction of diabetic symptoms. This would, in turn, lead to systemic benefits and delayed diabetic complications, increasing the quality and quantity of life of individuals with T1DM.
P- Reviewer: Gorgey AS, Grau JM S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Iscoe KE, Campbell JE, Jamnik V, Perkins BA, Riddell MC. Efficacy of continuous real-time blood glucose monitoring during and after prolonged high-intensity cycling exercise: spinning with a continuous glucose monitoring system. Diabetes Technol Ther. 2006;8:627-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Katz LD, Glickman MG, Rapoport S, Ferrannini E, DeFronzo RA. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983;32:675-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 164] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248:E353-E362. [PubMed] [Cited in This Article: ] |
| 4. | Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769-E774. [PubMed] [Cited in This Article: ] |
| 5. | Krause MP, Riddell MC, Gordon CS, Imam SA, Cafarelli E, Hawke TJ. Diabetic myopathy differs between Ins2Akita+/- and streptozotocin-induced Type 1 diabetic models. J Appl Physiol (1985). 2009;106:1650-1659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Krause MP, Riddell MC, Hawke TJ. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr Diabetes. 2011;12:345-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Gordon CS, Serino AS, Krause MP, Campbell JE, Cafarelli E, Adegoke OA, Hawke TJ, Riddell MC. Impaired growth and force production in skeletal muscles of young partially pancreatectomized rats: a model of adolescent type 1 diabetic myopathy? PLoS One. 2010;5:e14032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Fricke O, Seewi O, Semler O, Tutlewski B, Stabrey A, Schoenau E. The influence of auxology and long-term glycemic control on muscle function in children and adolescents with type 1 diabetes mellitus. J Musculoskelet Neuronal Interact. 2008;8:188-195. [PubMed] [Cited in This Article: ] |
| 9. | Gulati AK, Swamy MS. Regeneration of skeletal muscle in streptozotocin-induced diabetic rats. Anat Rec. 1991;229:298-304. [PubMed] [Cited in This Article: ] |
| 10. | Jerković R, Bosnar A, Jurisić-Erzen D, Azman J, Starcević-Klasan G, Peharec S, Coklo M. The effects of long-term experimental diabetes mellitus type I on skeletal muscle regeneration capacity. Coll Antropol. 2009;33:1115-1119. [PubMed] [Cited in This Article: ] |
| 11. | Vignaud A, Ramond F, Hourdé C, Keller A, Butler-Browne G, Ferry A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology. 2007;74:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Krause MP, Moradi J, Nissar AA, Riddell MC, Hawke TJ. Inhibition of plasminogen activator inhibitor-1 restores skeletal muscle regeneration in untreated type 1 diabetic mice. Diabetes. 2011;60:1964-1972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Krause MP, Al-Sajee D, D’Souza DM, Rebalka IA, Moradi J, Riddell MC, Hawke TJ. Impaired macrophage and satellite cell infiltration occurs in a muscle-specific fashion following injury in diabetic skeletal muscle. PLoS One. 2013;8:e70971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Jeong J, Conboy MJ, Conboy IM. Pharmacological inhibition of myostatin/TGF-β receptor/pSmad3 signaling rescues muscle regenerative responses in mouse model of type 1 diabetes. Acta Pharmacol Sin. 2013;34:1052-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Aragno M, Mastrocola R, Catalano MG, Brignardello E, Danni O, Boccuzzi G. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes. 2004;53:1082-1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Fritzsche K, Blüher M, Schering S, Buchwalow IB, Kern M, Linke A, Oberbach A, Adams V, Punkt K. Metabolic profile and nitric oxide synthase expression of skeletal muscle fibers are altered in patients with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2008;116:606-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Crowther GJ, Milstein JM, Jubrias SA, Kushmerick MJ, Gronka RK, Conley KE. Altered energetic properties in skeletal muscle of men with well-controlled insulin-dependent (type 1) diabetes. Am J Physiol Endocrinol Metab. 2003;284:E655-E662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Chao TT, Ianuzzo CD, Armstrong RB, Albright JT, Anapolle SE. Ultrastructural alterations in skeletal muscle fibers of streptozotocin-diabetic rats. Cell Tissue Res. 1976;168:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Goodman MN, Berger M, Ruderman NB. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974;23:881-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 108] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Stearns SB, Tepperman HM, Tepperman J. Studies on the utilization and mobilization of lipid in skeletal muscles from streptozotocin-diabetic and control rats. J Lipid Res. 1979;20:654-662. [PubMed] [Cited in This Article: ] |
| 21. | Bonen A, Benton CR, Campbell SE, Chabowski A, Clarke DC, Han XX, Glatz JF, Luiken JJ. Plasmalemmal fatty acid transport is regulated in heart and skeletal muscle by contraction, insulin and leptin, and in obesity and diabetes. Acta Physiol Scand. 2003;178:347-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Chen M, Yang YK, Loux TJ, Georgeson KE, Harmon CM. The role of hyperglycemia in FAT/CD36 expression and function. Pediatr Surg Int. 2006;22:647-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Attia N, Caprio S, Jones TW, Heptulla R, Holcombe J, Silver D, Sherwin RS, Tamborlane WV. Changes in free insulin-like growth factor-1 and leptin concentrations during acute metabolic decompensation in insulin withdrawn patients with type 1 diabetes. J Clin Endocrinol Metab. 1999;84:2324-2328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Garland PB, Randle PJ. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964;93:678-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 250] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 354] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 26. | Fewell JG, Moerland TS. Responses of mouse fast and slow skeletal muscle to streptozotocin diabetes: myosin isoenzymes and phosphorous metabolites. Mol Cell Biochem. 1995;148:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Noble EG, Ianuzzo CD. Influence of training on skeletal muscle enzymatic adaptations in normal and diabetic rats. Am J Physiol. 1985;249:E360-E365. [PubMed] [Cited in This Article: ] |
| 28. | Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De Cobelli F, Battezzati A, Secchi A, Del Maschio A, Luzi L. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1174-E1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 29. | Hong EG, Jung DY, Ko HJ, Zhang Z, Ma Z, Jun JY, Kim JH, Sumner AD, Vary TC, Gardner TW. Nonobese, insulin-deficient Ins2Akita mice develop type 2 diabetes phenotypes including insulin resistance and cardiac remodeling. Am J Physiol Endocrinol Metab. 2007;293:E1687-E1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Johnston AP, Campbell JE, Found JG, Riddell MC, Hawke TJ. Streptozotocin induces G2 arrest in skeletal muscle myoblasts and impairs muscle growth in vivo. Am J Physiol Cell Physiol. 2007;292:C1033-C1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3494] [Cited by in F6Publishing: 3367] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 32. | Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7:1553-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, Giacca A, Fantus IG. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab. 2003;285:E744-E753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12:871-880. [PubMed] [Cited in This Article: ] |
| 35. | Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Sexton WL, Poole DC, Mathieu-Costello O. Microcirculatory structure-function relationships in skeletal muscle of diabetic rats. Am J Physiol. 1994;266:H1502-H1511. [PubMed] [Cited in This Article: ] |
| 37. | Kindig CA, Sexton WL, Fedde MR, Poole DC. Skeletal muscle microcirculatory structure and hemodynamics in diabetes. Respir Physiol. 1998;111:163-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Kivelä R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006;20:1570-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Raskin P, Pietri AO, Unger R, Shannon WA. The effect of diabetic control on the width of skeletal-muscle capillary basement membrane in patients with Type I diabetes mellitus. N Engl J Med. 1983;309:1546-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Sosenko JM, Miettinen OS, Williamson JR, Gabbay KH. Muscle capillary basement-membrane thickness and long-term glycemia in type I diabetes mellitus. N Engl J Med. 1984;311:694-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Rosenstock J, Challis P, Strowig S, Raskin P. Improved diabetes control reduces skeletal muscle capillary basement membrane width in insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1988;4:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Rosenstock J, Friberg T, Raskin P. Effect of glycemic control on microvascular complications in patients with type I diabetes mellitus. Am J Med. 1986;81:1012-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Rossi M, Matteucci E, Pesce M, Consani C, Franzoni F, Santoro G, Giampietro O. Peripheral microvascular dysfunction as an independent predictor of atherosclerotic damage in type 1 diabetes patients: a preliminary study. Clin Hemorheol Microcirc. 2013;54:381-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Veerman KJ, Venegas-Pino DE, Shi Y, Khan MI, Gerstein HC, Werstuck GH. Hyperglycaemia is associated with impaired vasa vasorum neovascularization and accelerated atherosclerosis in apolipoprotein-E deficient mice. Atherosclerosis. 2013;227:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 46. | Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 47. | Yki-Järvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315:224-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 241] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982;31:795-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 264] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 50. | Kahn BB, Rosen AS, Bak JF, Andersen PH, Damsbo P, Lund S, Pedersen O. Expression of GLUT1 and GLUT4 glucose transporters in skeletal muscle of humans with insulin-dependent diabetes mellitus: regulatory effects of metabolic factors. J Clin Endocrinol Metab. 1992;74:1101-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Peltoniemi P, Yki-Järvinen H, Oikonen V, Oksanen A, Takala TO, Rönnemaa T, Erkinjuntti M, Knuuti MJ, Nuutila P. Resistance to exercise-induced increase in glucose uptake during hyperinsulinemia in insulin-resistant skeletal muscle of patients with type 1 diabetes. Diabetes. 2001;50:1371-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Nuutila P, Knuuti J, Ruotsalainen U, Koivisto VA, Eronen E, Teräs M, Bergman J, Haaparanta M, Voipio-Pulkki LM, Viikari J. Insulin resistance is localized to skeletal but not heart muscle in type 1 diabetes. Am J Physiol. 1993;264:E756-E762. [PubMed] [Cited in This Article: ] |
| 53. | Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130-E1141. [PubMed] [Cited in This Article: ] |
| 54. | Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem. 2009;284:27090-27100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia. 2004;47:1661-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 56. | Galassetti P, Riddell MC. Exercise and type 1 diabetes (T1DM). Compr Physiol. 2013;3:1309-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 713] [Cited by in F6Publishing: 683] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 58. | Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997;273:E1039-E1051. [PubMed] [Cited in This Article: ] |
| 59. | Henriksen EJ, Halseth AE. Adaptive responses of GLUT-4 and citrate synthase in fast-twitch muscle of voluntary running rats. Am J Physiol. 1995;268:R130-R134. [PubMed] [Cited in This Article: ] |
| 60. | Rodnick KJ, Henriksen EJ, James DE, Holloszy JO. Exercise training, glucose transporters, and glucose transport in rat skeletal muscles. Am J Physiol. 1992;262:C9-14. [PubMed] [Cited in This Article: ] |
| 61. | James DE, Kraegen EW, Chisholm DJ. Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Invest. 1985;76:657-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 99] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Berger M, Kemmer FW, Becker K, Herberg L, Schwenen M, Gjinavci A, Berchtold P. Effect of physical training on glucose tolerance and on glucose metabolism of skeletal muscle in anaesthetized normal rats. Diabetologia. 1979;16:179-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 69] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Rachmiel M, Buccino J, Daneman D. Exercise and type 1 diabetes mellitus in youth; review and recommendations. Pediatr Endocrinol Rev. 2007;5:656-665. [PubMed] [Cited in This Article: ] |
| 64. | Aouadi R, Khalifa R, Aouidet A, Ben Mansour A, Ben Rayana M, Mdini F, Bahri S, Stratton G. Aerobic training programs and glycemic control in diabetic children in relation to exercise frequency. J Sports Med Phys Fitness. 2011;51:393-400. [PubMed] [Cited in This Article: ] |
| 65. | Nguyen T, Obeid J, Walker RG, Krause MP, Hawke TJ, McAssey K, Vandermeulen J, Timmons BW. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr Diabetes. 2015;16:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Komatsu WR, Gabbay MA, Castro ML, Saraiva GL, Chacra AR, de Barros Neto TL, Dib SA. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr Diabetes. 2005;6:145-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Baldi JC, Cassuto NA, Foxx-Lupo WT, Wheatley CM, Snyder EM. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med Sci Sports Exerc. 2010;42:1454-1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6145] [Cited by in F6Publishing: 5938] [Article Influence: 258.2] [Reference Citation Analysis (0)] |
| 69. | Reske-Nielsen E, Harmsen A, Vorre P. Ultrastructure of muscle biopsies in recent, short-term and long-term juvenile diabetes. Acta Neurol Scand. 1977;55:345-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Almeida S, Riddell MC, Cafarelli E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve. 2008;37:231-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Andersen H, Schmitz O, Nielsen S. Decreased isometric muscle strength after acute hyperglycaemia in Type 1 diabetic patients. Diabet Med. 2005;22:1401-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Campaigne BN, Gilliam TB, Spencer ML, Lampman RM, Schork MA. Effects of a physical activity program on metabolic control and cardiovascular fitness in children with insulin-dependent diabetes mellitus. Diabetes Care. 1984;7:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Dahl-Jørgensen K, Meen HD, Hanssen KF, Aagenaes O. The effect of exercise on diabetic control and hemoglobin A1 (HbA1) in children. Acta Paediatr Scand Suppl. 1980;283:53-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Landt KW, Campaigne BN, James FW, Sperling MA. Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care. 1985;8:461-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Wallberg-Henriksson H. Exercise and diabetes mellitus. Exerc Sport Sci Rev. 1992;20:339-368. [PubMed] [Cited in This Article: ] |
| 76. | Zinman B, Zuniga-Guajardo S, Kelly D. Comparison of the acute and long-term effects of exercise on glucose control in type I diabetes. Diabetes Care. 1984;7:515-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Ramalho AC, de Lourdes Lima M, Nunes F, Cambuí Z, Barbosa C, Andrade A, Viana A, Martins M, Abrantes V, Aragão C. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res Clin Pract. 2006;72:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Heyman E, Toutain C, Delamarche P, Berthon P, Briard D, Youssef H, Dekerdanet M, Gratas-Delamarche A. Exercise training and cardiovascular risk factors in type 1 diabetic adolescent girls. Pediatr Exerc Sci. 2007;19:408-419. [PubMed] [Cited in This Article: ] |
| 79. | Mosher PE, Nash MS, Perry AC, LaPerriere AR, Goldberg RB. Aerobic circuit exercise training: effect on adolescents with well-controlled insulin-dependent diabetes mellitus. Arch Phys Med Rehabil. 1998;79:652-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Roberts L, Jones TW, Fournier PA. Exercise training and glycemic control in adolescents with poorly controlled type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15:621-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Wallberg-Henriksson H, Gunnarsson R, Henriksson J, DeFronzo R, Felig P, Ostman J, Wahren J. Increased peripheral insulin sensitivity and muscle mitochondrial enzymes but unchanged blood glucose control in type I diabetics after physical training. Diabetes. 1982;31:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Wadén J, Forsblom C, Thorn LM, Saraheimo M, Rosengård-Bärlund M, Heikkilä O, Lakka TA, Tikkanen H, Groop PH. Physical activity and diabetes complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) Study. Diabetes Care. 2008;31:230-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Jakobsen J, Reske-Nielsen E. Diffuse muscle fiber atrophy in newly diagnosed diabetes. Clin Neuropathol. 1986;5:73-77. [PubMed] [Cited in This Article: ] |
| 84. | Fuchsjäger-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | Mason NJ, Jenkins AJ, Best JD, Rowley KG. Exercise frequency and arterial compliance in non-diabetic and type 1 diabetic individuals. Eur J Cardiovasc Prev Rehabil. 2006;13:598-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Kriska AM, LaPorte RE, Patrick SL, Kuller LH, Orchard TJ. The association of physical activity and diabetic complications in individuals with insulin-dependent diabetes mellitus: the Epidemiology of Diabetes Complications Study--VII. J Clin Epidemiol. 1991;44:1207-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 88. | Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1208] [Cited by in F6Publishing: 1160] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 89. | Nicolls MR, Haskins K, Flores SC. Oxidant stress, immune dysregulation, and vascular function in type I diabetes. Antioxid Redox Signal. 2007;9:879-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294-2303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 91. | Melstrom LG, Melstrom KA, Ding XZ, Adrian TE. Mechanisms of skeletal muscle degradation and its therapy in cancer cachexia. Histol Histopathol. 2007;22:805-814. [PubMed] [Cited in This Article: ] |
| 92. | Molanouri Shamsi M, Hassan ZH, Gharakhanlou R, Quinn LS, Azadmanesh K, Baghersad L, Isanejad A, Mahdavi M. Expression of interleukin-15 and inflammatory cytokines in skeletal muscles of STZ-induced diabetic rats: effect of resistance exercise training. Endocrine. 2014;46:60-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Ono T, Takada S, Kinugawa S, Tsutsui H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp Physiol. 2015;100:1052-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 94. | Lira FS, Koyama CH, Yamashita AS, Rosa JC, Zanchi NE, Batista ML, Seelaender MC. Chronic exercise decreases cytokine production in healthy rat skeletal muscle. Cell Biochem Funct. 2009;27:458-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Zanchi NE, Lira FS, de Siqueira Filho MA, Rosa JC, de Oliveira Carvalho CR, Seelaender M, Santos RV, Lancha AH. Chronic low frequency/low volume resistance training reduces pro-inflammatory cytokine protein levels and TLR4 mRNA in rat skeletal muscle. Eur J Appl Physiol. 2010;109:1095-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 337] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 97. | Carmeli E, Moas M, Lennon S, Powers SK. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp Physiol. 2005;90:613-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Yeghiazaryan M, Żybura-Broda K, Cabaj A, Włodarczyk J, Sławińska U, Rylski M, Wilczyński GM. Fine-structural distribution of MMP-2 and MMP-9 activities in the rat skeletal muscle upon training: a study by high-resolution in situ zymography. Histochem Cell Biol. 2012;138:75-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Kim JS, Lee YH, Kim JC, Ko YH, Yoon CS, Yi HK. Effect of exercise training of different intensities on anti-inflammatory reaction in streptozotocin-induced diabetic rats. Biol Sport. 2014;31:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 100. | Rosa JS, Oliver SR, Flores RL, Ngo J, Milne GL, Zaldivar FP, Galassetti PR. Altered inflammatory, oxidative, and metabolic responses to exercise in pediatric obesity and type 1 diabetes. Pediatr Diabetes. 2011;12:464-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 102. | Kim HC, Cho HY, Hah YS. Role of IL-15 in Sepsis-Induced Skeletal Muscle Atrophy and Proteolysis. Tuberc Respir Dis (Seoul). 2012;73:312-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Figueras M, Busquets S, Carbó N, Barreiro E, Almendro V, Argilés JM, López-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2004;569:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 296] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 105. | Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433-E437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 669] [Cited by in F6Publishing: 662] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 106. | McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2900] [Cited by in F6Publishing: 2822] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 107. | Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682-2688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1001] [Cited by in F6Publishing: 933] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 108. | Hulmi JJ, Silvennoinen M, Lehti M, Kivelä R, Kainulainen H. Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am J Physiol Endocrinol Metab. 2012;302:E307-E315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 109. | Chen Y, Cao L, Ye J, Zhu D. Upregulation of myostatin gene expression in streptozotocin-induced type 1 diabetes mice is attenuated by insulin. Biochem Biophys Res Commun. 2009;388:112-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Wieteska-Skrzeczynska W, Grzelkowska-Kowalczyk K, Jank M, Maciejewski H. Transcriptional dysregulation of skeletal muscle protein metabolism in streptozotocin-diabetic mice. J Physiol Pharmacol. 2009;60 Suppl 1:29-36. [PubMed] [Cited in This Article: ] |
| 111. | Wang F, Liao Y, Li X, Ren C, Cheng C, Ren Y. Increased circulating myostatin in patients with type 2 diabetes mellitus. J Huazhong Univ Sci Technolog Med Sci. 2012;32:534-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Brandt C, Nielsen AR, Fischer CP, Hansen J, Pedersen BK, Plomgaard P. Plasma and muscle myostatin in relation to type 2 diabetes. PLoS One. 2012;7:e37236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 113. | Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009;58:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 114. | Allen DL, Cleary AS, Lindsay SF, Loh AS, Reed JM. Myostatin expression is increased by food deprivation in a muscle-specific manner and contributes to muscle atrophy during prolonged food deprivation in mice. J Appl Physiol (1985). 2010;109:692-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 116. | Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. 2013;7:352-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 117. | Attie KM, Borgstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE, Kumar R, Willins DA, Seehra JS, Sherman ML. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47:416-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 118. | McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 119. | Zhao B, Wall RJ, Yang J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun. 2005;337:248-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 120. | Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, Sharma M, Kambadur R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 2012;55:183-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 121. | Guo T, Bond ND, Jou W, Gavrilova O, Portas J, McPherron AC. Myostatin inhibition prevents diabetes and hyperphagia in a mouse model of lipodystrophy. Diabetes. 2012;61:2414-2423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | Bonala S, Lokireddy S, McFarlane C, Patnam S, Sharma M, Kambadur R. Myostatin induces insulin resistance via Casitas B-lineage lymphoma b (Cblb)-mediated degradation of insulin receptor substrate 1 (IRS1) protein in response to high calorie diet intake. J Biol Chem. 2014;289:7654-7670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 123. | Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4:e4937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 124. | Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 125. | Brandt C, Hansen RH, Hansen JB, Olsen CH, Galle P, Mandrup-Poulsen T, Gehl J, Pedersen BK, Hojman P. Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice. Metabolism. 2015;64:283-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 126. | Zhang C, McFarlane C, Lokireddy S, Bonala S, Ge X, Masuda S, Gluckman PD, Sharma M, Kambadur R. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia. 2011;54:1491-1501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 127. | Takahashi H, Sato K, Yamaguchi T, Miyake M, Watanabe H, Nagasawa Y, Kitagawa E, Terada S, Urakawa M, Rose MT. Myostatin alters glucose transporter-4 (GLUT4) expression in bovine skeletal muscles and myoblasts isolated from double-muscled (DM) and normal-muscled (NM) Japanese shorthorn cattle. Domest Anim Endocrinol. 2014;48:62-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 128. | Cleasby ME, Jarmin S, Eilers W, Elashry M, Andersen DK, Dickson G, Foster K. Local overexpression of the myostatin propeptide increases glucose transporter expression and enhances skeletal muscle glucose disposal. Am J Physiol Endocrinol Metab. 2014;306:E814-E823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Sriram S, Subramanian S, Sathiakumar D, Venkatesh R, Salerno MS, McFarlane CD, Kambadur R, Sharma M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell. 2011;10:931-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 130. | Sriram S, Subramanian S, Juvvuna PK, Ge X, Lokireddy S, McFarlane CD, Wahli W, Kambadur R, Sharma M. Myostatin augments muscle-specific ring finger protein-1 expression through an NF-kB independent mechanism in SMAD3 null muscle. Mol Endocrinol. 2014;28:317-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 131. | Sriram S, Subramanian S, Juvvuna PK, McFarlane C, Salerno MS, Kambadur R, Sharma M. Myostatin induces DNA damage in skeletal muscle of streptozotocin-induced type 1 diabetic mice. J Biol Chem. 2014;289:5784-5798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 132. | Ploquin C, Chabi B, Fouret G, Vernus B, Feillet-Coudray C, Coudray C, Bonnieu A, Ramonatxo C. Lack of myostatin alters intermyofibrillar mitochondria activity, unbalances redox status, and impairs tolerance to chronic repetitive contractions in muscle. Am J Physiol Endocrinol Metab. 2012;302:E1000-E1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol. 2012;32:2871-2879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 134. | Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D, David J, Li X, Tomasian V, Reid CB. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014;55:375-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 135. | Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity (Silver Spring). 2013;21:1180-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int J Biochem Cell Biol. 2012;44:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 137. | Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27:1981-1989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 138. | Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 139. | Wang Q, Guo T, Portas J, McPherron AC. A soluble activin receptor type IIB does not improve blood glucose in streptozotocin-treated mice. Int J Biol Sci. 2015;11:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 140. | Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317-318. [PubMed] [Cited in This Article: ] |
| 141. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [PubMed] [Cited in This Article: ] |
| 142. | Blüher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes. 2007;14:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004;19:1607-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 144. | Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437-446. [PubMed] [Cited in This Article: ] |
| 145. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3149] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 146. | Hanaki K, Becker DJ, Arslanian SA. Leptin before and after insulin therapy in children with new-onset type 1 diabetes. J Clin Endocrinol Metab. 1999;84:1524-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 147. | Kratzsch J, Deimel A, Galler A, Kapellen T, Klinghammer A, Kiess W. Increased serum soluble leptin receptor levels in children and adolescents with type 1 diabetes mellitus. Eur J Endocrinol. 2004;151:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahrén B. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274:R1482-R1491. [PubMed] [Cited in This Article: ] |
| 149. | Sivitz WI, Walsh S, Morgan D, Donohoue P, Haynes W, Leibel RL. Plasma leptin in diabetic and insulin-treated diabetic and normal rats. Metabolism. 1998;47:584-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Ueno N, Inui A, Kalra PS, Kalra SP. Leptin transgene expression in the hypothalamus enforces euglycemia in diabetic, insulin-deficient nonobese Akita mice and leptin-deficient obese ob/ob mice. Peptides. 2006;27:2332-2342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Kojima S, Asakawa A, Amitani H, Sakoguchi T, Ueno N, Inui A, Kalra SP. Central leptin gene therapy, a substitute for insulin therapy to ameliorate hyperglycemia and hyperphagia, and promote survival in insulin-deficient diabetic mice. Peptides. 2009;30:962-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 152. | Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813-4819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 153. | Kruger AJ, Yang C, Lipson KL, Pino SC, Leif JH, Hogan CM, Whalen BJ, Guberski DL, Lee Y, Unger RH. Leptin treatment confers clinical benefit at multiple stages of virally induced type 1 diabetes in BB rats. Autoimmunity. 2011;44:137-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999;48:1275-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 155. | Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA. 2010;107:17391-17396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 156. | Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, Unger RH. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci USA. 2012;109:14972-14976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 157. | Lee Y, Berglund ED, Yu X, Wang MY, Evans MR, Scherer PE, Holland WL, Charron MJ, Roth MG, Unger RH. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci USA. 2014;111:13217-13222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 158. | Wang MY, Yan H, Shi Z, Evans MR, Yu X, Lee Y, Chen S, Williams A, Philippe J, Roth MG. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci USA. 2015;112:2503-2508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 159. | Cusin I, Zakrzewska KE, Boss O, Muzzin P, Giacobino JP, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Chronic central leptin infusion enhances insulin-stimulated glucose metabolism and favors the expression of uncoupling proteins. Diabetes. 1998;47:1014-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 160. | Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 161. | Toda C, Shiuchi T, Kageyama H, Okamoto S, Coutinho EA, Sato T, Okamatsu-Ogura Y, Yokota S, Takagi K, Tang L. Extracellular signal-regulated kinase in the ventromedial hypothalamus mediates leptin-induced glucose uptake in red-type skeletal muscle. Diabetes. 2013;62:2295-2307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 162. | Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070-14075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 163. | Ceddia RB, William WN, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord. 1999;23:75-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 164. | Hidaka S, Yoshimatsu H, Kondou S, Tsuruta Y, Oka K, Noguchi H, Okamoto K, Sakino H, Teshima Y, Okeda T. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 2002;16:509-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 165. | Muoio DM, Dohm GL, Fiedorek FT, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46:1360-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 272] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 166. | Steinberg GR, Parolin ML, Heigenhauser GJ, Dyck DJ. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab. 2002;283:E187-E192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 167. | Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1498] [Cited by in F6Publishing: 1421] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 168. | German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 169. | Kusakabe T, Tanioka H, Ebihara K, Hirata M, Miyamoto L, Miyanaga F, Hige H, Aotani D, Fujisawa T, Masuzaki H. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia. 2009;52:675-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 170. | Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:E1084-E1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 171. | Kraus D, Herman MA, Kahn BB. Leveraging leptin for type I diabetes? Proc Natl Acad Sci USA. 2010;107:4793-4794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 172. | Miyanaga F, Ogawa Y, Ebihara K, Hidaka S, Tanaka T, Hayashi S, Masuzaki H, Nakao K. Leptin as an adjunct of insulin therapy in insulin-deficient diabetes. Diabetologia. 2003;46:1329-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 173. | Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2259] [Cited by in F6Publishing: 2233] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 174. | Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1868] [Cited by in F6Publishing: 1797] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 175. | Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1118] [Cited by in F6Publishing: 1069] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 176. | Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 738] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 177. | Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 968] [Cited by in F6Publishing: 986] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 178. | Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79-83. [PubMed] [Cited in This Article: ] |
| 179. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1862] [Cited by in F6Publishing: 1912] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 180. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3510] [Cited by in F6Publishing: 3418] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 181. | Liu Y, Chewchuk S, Lavigne C, Brûlé S, Pilon G, Houde V, Xu A, Marette A, Sweeney G. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009;297:E657-E664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 182. | Imagawa A, Funahashi T, Nakamura T, Moriwaki M, Tanaka S, Nishizawa H, Sayama K, Uno S, Iwahashi H, Yamagata K. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care. 2002;25:1665-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 183. | Abi Khalil C, Mohammedi K, Aubert R, Travert F, Hadjadj S, Roussel R, Fumeron F, Marre M. Intensifying glycaemic control with insulin reduces adiponectin and its HMW isoform moderately in type 2, but not in type 1, diabetes. Diabetes Metab. 2011;37:259-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 184. | Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, Maahs DM. Adiponectin dysregulation and insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E642-E647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 185. | Stadler M, Storka A, Theuer EA, Krebs M, Vojtassakova E, Nowotny P, Pacini G, Kästenbauer T, Luger A, Prager R. Adipokines in type 1 diabetes after successful pancreas transplantation: normal visfatin and retinol-binding-protein-4, but increased total adiponectin fasting concentrations. Clin Endocrinol (Oxf). 2010;72:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 186. | Timar R, Timar B, Degeratu D, Serafinceanu C, Oancea C. Metabolic syndrome, adiponectin and proinflammatory status in patients with type 1 diabetes mellitus. J Int Med Res. 2014;42:1131-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 187. | Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623-33631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 188. | Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 447] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 189. | Ebeling P, Essén-Gustavsson B, Tuominen JA, Koivisto VA. Intramuscular triglyceride content is increased in IDDM. Diabetologia. 1998;41:111-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 190. | Ling AH, Donaghue KC, Howard NJ, Arrowsmith FE, Ward JA, Baur LA, Thompson CH. Intramyocellular lipid, adiposity, and muscle oxygen supply in prepubertal type 1 diabetes. Pediatr Diabetes. 2003;4:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 191. | Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol. 2008;295:C203-C212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 192. | Behre CJ. Adiponectin: saving the starved and the overfed. Med Hypotheses. 2007;69:1290-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |