Published online Feb 27, 2019. doi: 10.4240/wjgs.v11.i2.62
Peer-review started: November 14, 2018
First decision: November 29, 2018
Revised: January 6, 2019
Accepted: January 23, 2019
Article in press: January 23, 2019
Published online: February 27, 2019
The incidence of biliary injury after laparoscopic cholecystectomy (LC) has shown a declining trend though it may still be twice that as with open cholecystectomy. Major biliary or vasculobiliary injury is associated with significant morbidity. As prevention is the best strategy, the concept of a culture of safe cholecystectomy has been recently introduced to educate surgeons and apprise them of basic tenets of safe performance of LC. Various aspects of safe cholecystectomy include: (1) thorough knowledge of relevant anatomy, various anatomical landmarks, and anatomical variations; (2) an understanding of the mechanisms involved in biliary/vascular injury, the most important being the misidentification injury; (3) identification of various preoperative and intraoperative predictors of difficult cholecystectomy; (4) proper gallbladder retraction; (5) safe use of various energy devices; (6) understanding the critical view of safety, including its doublet view and documentation; (7) awareness of various error traps (e.g., fundus first technique); (8) use of various bailout strategies (e.g., subtotal cholecystectomy) in difficult gallbladder cases; (9) use of intraoperative imaging techniques (e.g., intraoperative cholangiogram) to ascertain correct anatomy; and (10) understanding the concept of time-out. Surgeons should be facile with these aspects of this culture of safety in cholecystectomy in an attempt to reduce the incidence of biliary/vascular injury during LC.
Core tip: Laparoscopic cholecystectomy (LC) is associated with higher risk of biliary injury. This complication is associated with prolonged morbidity, decreased overall survival and potential for litigation. Prevention remains the best strategy. With the understanding of underlying mechanisms related to this complication, a number of preventive strategies have been described. Besides proper training and use of optimal equipment, understanding relevant anatomy, identification of factors predicting difficult procedure, execution of correct surgical technique, use of the critical view of safety, judicious use of energy sources, understanding stopping rules, time-out and bailout techniques, and proper documentation are basic tenets of safe LC.
- Citation: Gupta V, Jain G. Safe laparoscopic cholecystectomy: Adoption of universal culture of safety in cholecystectomy. World J Gastrointest Surg 2019; 11(2): 62-84
- URL: https://www.wjgnet.com/1948-9366/full/v11/i2/62.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i2.62
A safe cholecystectomy is one that is “safe for both the patient (no bile duct/hollow viscus/vascular injury) and for the operating surgeon (no or minimal scope for litigation)”[1]. Laparoscopic cholecystectomy (LC) is one of the most commonly performed general surgical procedures worldwide. It is associated with an overall complication rate of approximately ten percent with a higher risk of biliary injury (0.1%-1.5%)[2-4] when compared to the open approach (0.1%-0.2%)[2,5]. This complication, if sustained, largely offsets the benefit of the minimally invasive approach. Recent data suggest a declining trend in the bile duct injury (BDI) rate (0.32%-0.52%) without any significant change in the morbidity or mortality after LC[5]. However, it is important to recognize the fact that most of these injuries are preventable, especially if a structured safe technical protocol is followed.
Given the immediate morbidity, higher mortality, decreased quality of life, and decreased long term survival associated with BDI or vasculobiliary injury (VBI), as well as its medicolegal implications[6-10], the importance of safe performance of LC cannot be underemphasized. As the insight into the mechanisms involved in BDI/VBI during LC has evolved[11-13], a large number of strategies have been proposed to safeguard against BDI/VBI[14-18]. The present review focuses on most effective strategies a surgeon should be familiar with during LC. Reduced port or single port approaches for LC are not covered in this review as currently they are not the standard of care[19].
Factors predisposing to BDI/VBI are related to anatomy, disease related pathology, structural misidentification and improper techniques[12,20]. The most common mechanism of such injuries involves the misidentification of the common bile duct (CBD) or common hepatic duct (CHD) as the cystic duct or the misidentification of the hepatic artery as the cystic artery[16].
Basic tenets of performing a safe LC include: (1) Thorough knowledge of surgically relevant anatomy; (2) Identification of factors predictive of difficult cholecystectomy; (3) Understanding and execution of correct technique that includes: Correct exposure/display of hepatocystic (HC) triangle in preparation of dissection; Judicious use of energy sources; Achieving the critical view of safety (CVS); Remembering error traps; (4) Strategies to handle a difficult situation: Stopping rules; Second opinion/surgical assistance; Use of intraoperative imaging to clarify the anatomy; Bail-out procedures; and (5) Documentation.
It is crucial to have a thorough knowledge of the relevant anatomy as the procedure is performed in an area adjacent to many vital structures (portal vein, hepatic artery and extrahepatic biliary tract). Furthermore, the surgeon needs to be mindful of common anatomical variations, and the anatomical distortion due to pathological processes (e.g., acute/chronic cholecystitis). Safe execution of LC requires clear understanding of following anatomical terms/landmarks.
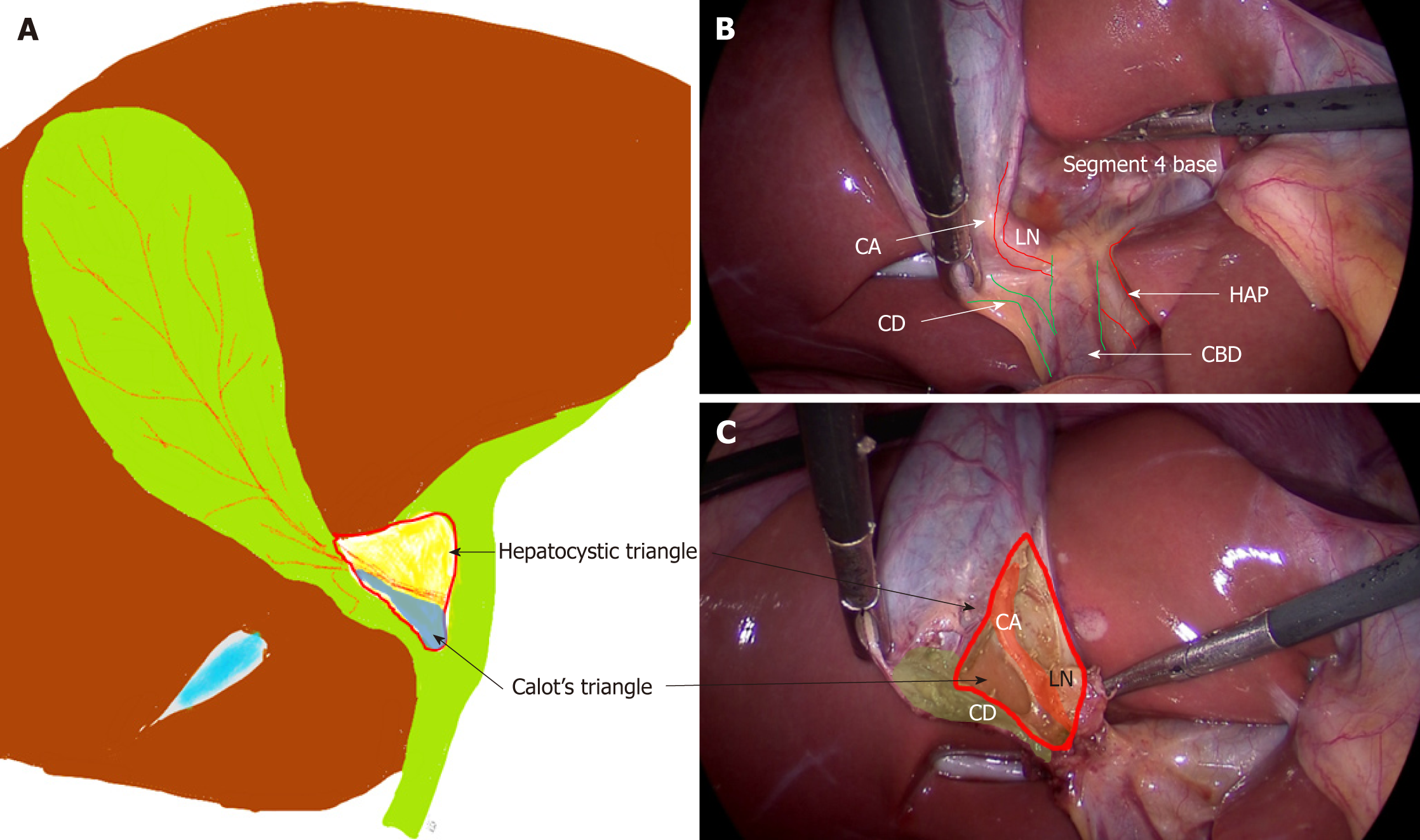
This is an area on the under surface of the liver on the right side of the hepatic hilum. It has CHD on the medial side, cystic duct caudally, and liver under surface cranially (Figure 1)[21,22]. This triangle contains the cystic artery, a variable portion of right hepatic artery, the cystic lymph node, lymphatics, and a variable amount of fibro-fatty connective tissue[21].
Clinical significance: This is the target area for dissection during LC. This triangle is different from Calot’s triangle where the cystic artery forms cephalad boundary instead of the liver surface[21,22] (Figure 1). In literature, these terms have been used interchangeably but in present review only the term “HC triangle” has been used to maintain uniformity as the actual dissection takes place in this triangle, not in Calot’s triangle[21]; The cystic lymph node often lies superficial to the cystic artery and acts a landmark to locate this artery[23]. The artery should be divided on the right side of this lymph node close to the gallbladder to avoid injury to the right hepatic artery. This landmark is quite useful in difficult cases. This area may be affected by the inflammatory process during acute or chronic cholecystitis, and it may appear thick, fibrotic, or scarred, which may create difficulty in anatomical identification and/or dissection.
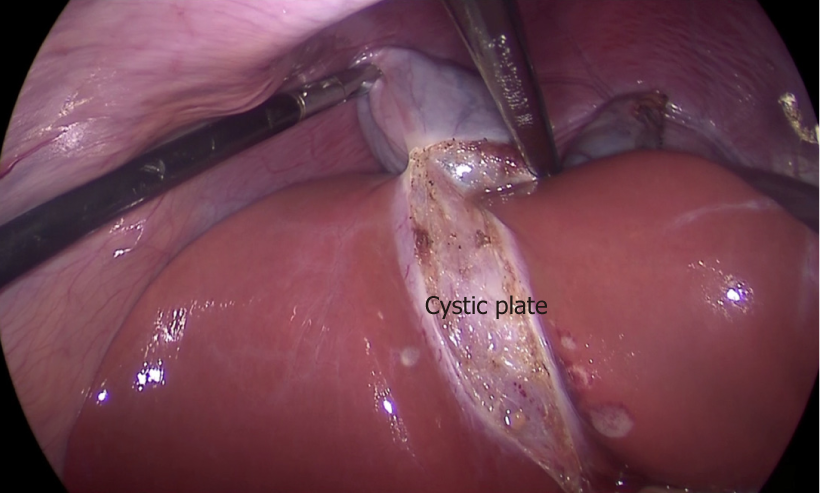
This is a flat ovoid fibrous sheet located in the gallbladder bed[24,25]. It is a part of sheath/plate system of the liver[24]. It is continuous with liver capsule of segment 4 (medially) and segment 5 (laterally). Postero-medially towards the hepatic hilum, it narrows to become a stout cord like structure that is continuous with the sheath of the right portal pedicle[24].
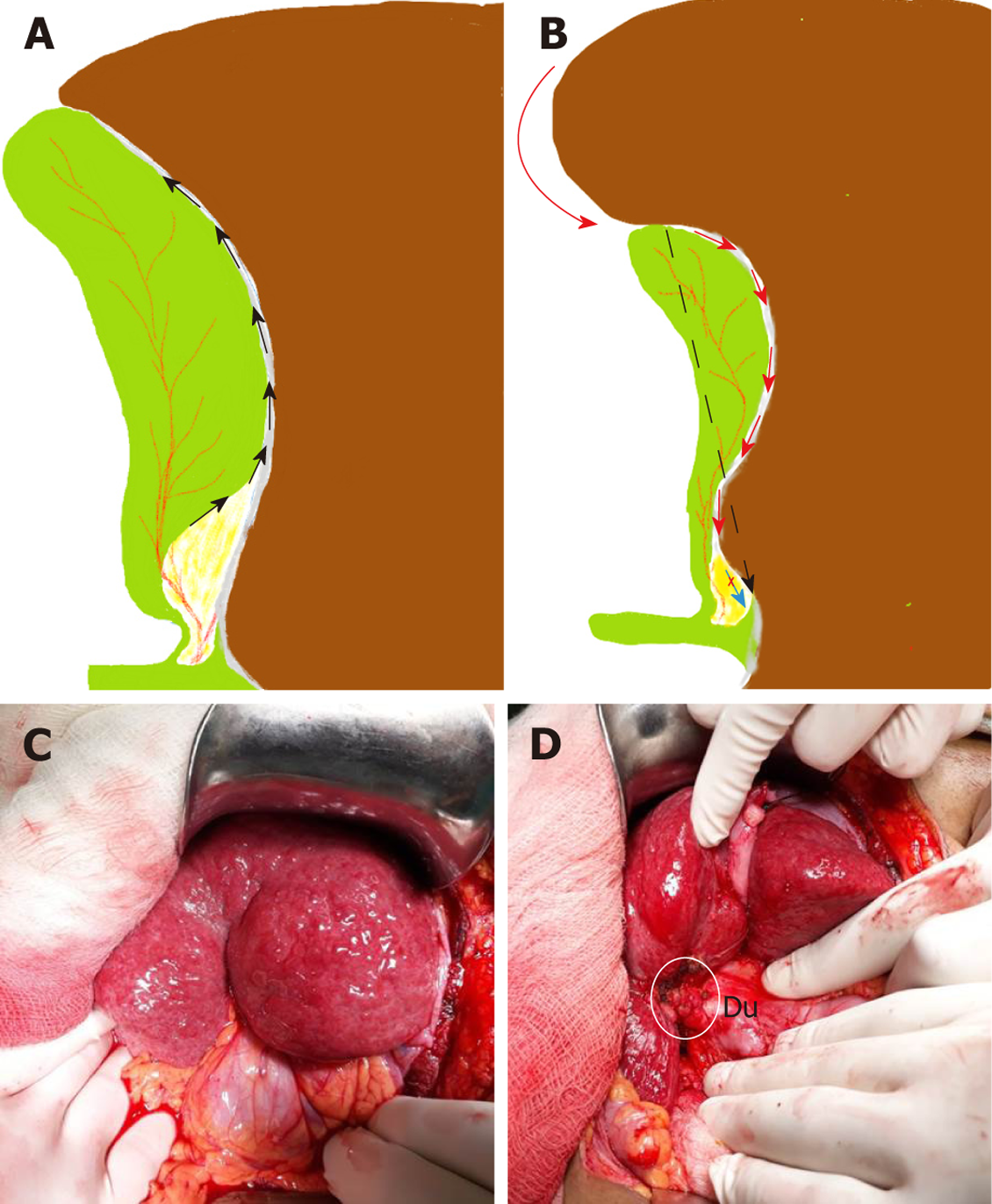
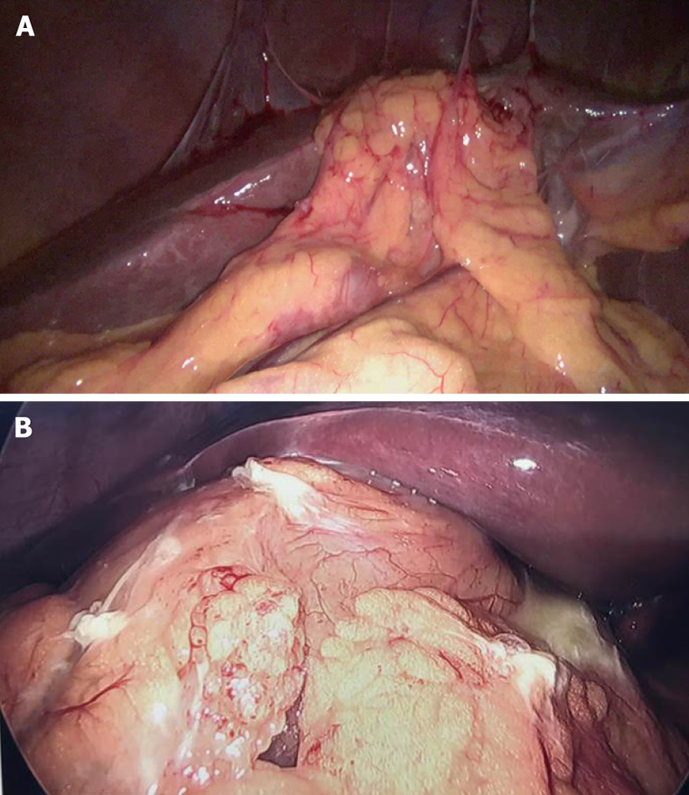
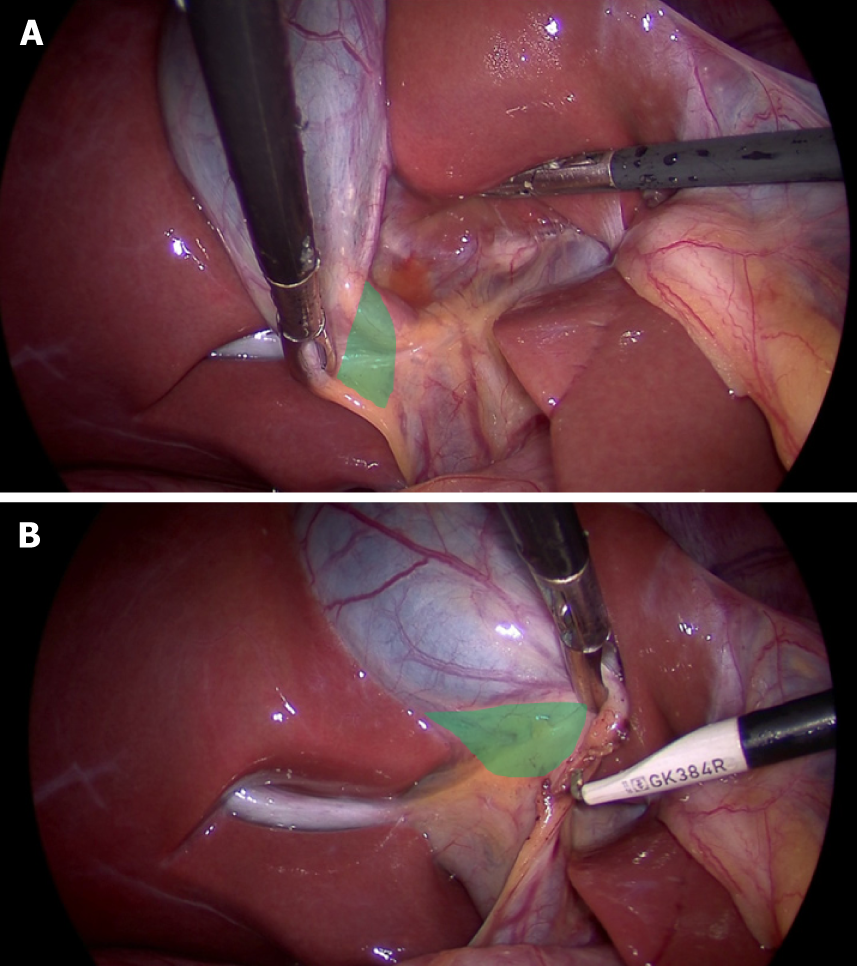
Clinical significance: With gallbladder in-situ, the cystic plate is not visible as it is covered by the gallbladder. It is exposed as whitish/greyish structure once the gallbladder is dissected off of the liver (Figure 2). It is important to expose the lower part of the cystic plate while achieving the CVS as discussed subsequently in this article. The CVS is considered incomplete without this exposure as an anomalous duct (e.g., right posterior sectional duct) may exit the liver or an anomalous right hepatic artery may enter the liver in this area. These structures may be at risk of injury if not identified properly. While the fundus and most of the body of the gallbladder remains closely adherent to the cystic plate, a layer of loose areolar tissue between the gallbladder wall and the cystic plate thickens towards the hilum[24] (Figure 3A). It is important to remain close to the gallbladder wall while dissecting it off the liver leaving behind the areolar tissue that is attached to the cystic plate while dissecting the neck of the gallbladder and the cystic duct. It is important not to breach the cystic plate while dissecting the gallbladder off the liver. Breaching the cystic plate during this step may cause two problems. First, there might be troublesome bleeding from liver parenchyma, especially if the terminal tributaries of the middle hepatic vein (which lie in this location) are injured. Second, sub-vesical bile ducts (coursing superficially close to gallbladder fossa) may be injured, causing a postoperative bile leak[26]. Such a breach is more likely to occur in chronic cholecystitis where the gallbladder may be densely adherent to the underlying liver without distinct dissection planes. In chronic cholecystitis with a small and contracted gallbladder, the longitudinal length of the cystic plate from the fundus to its attachment with right portal pedicle sheath becomes short (Figure 3B). Without appreciating this pathologic shortening, the surgeon may enter into the right portal pedicle sheath soon after the dissecting the fundus/body of the gallbladder if the fundus first technique is attempted. This may cause injure to the right portal pedicle structures causing serious VBI[27].
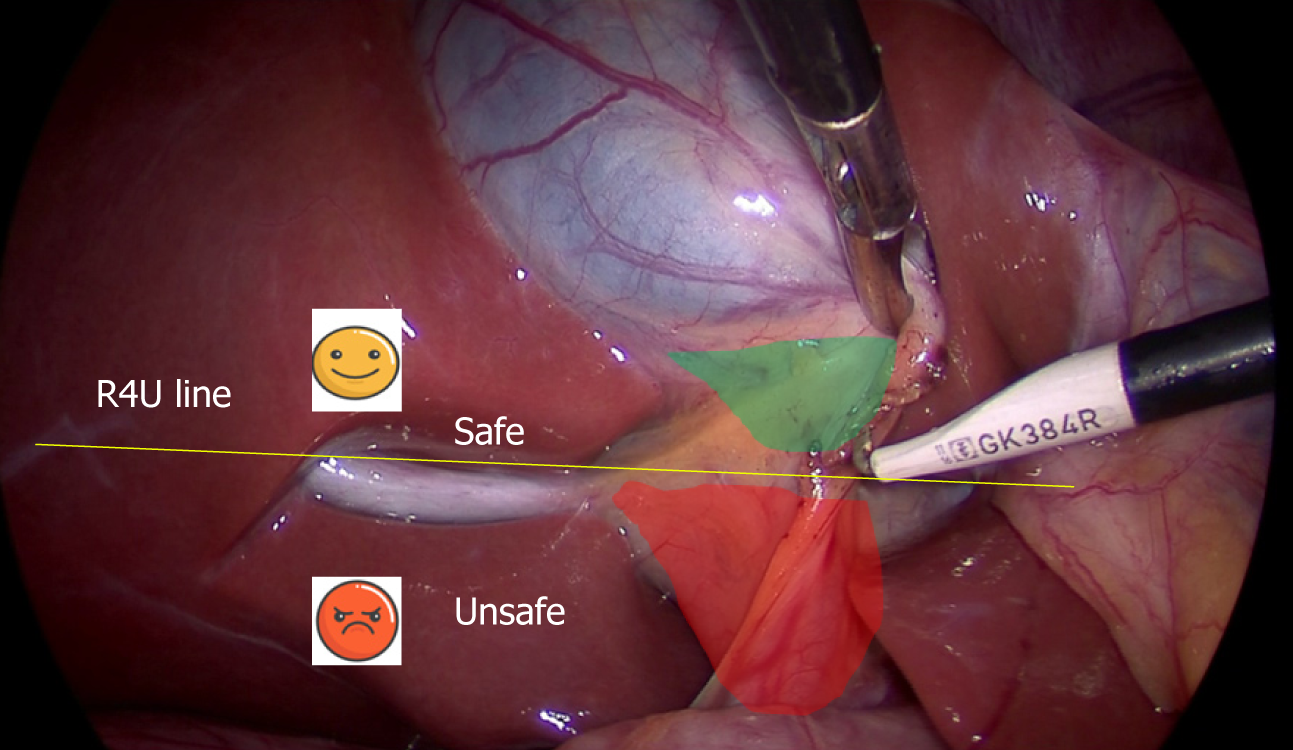
This sulcus is 2-5 cm long and is present on the under surface of the right lobe of the liver, running to the right of the hepatic hilum[28-31] (Figure 4). It is easily visible in most (80%) of the cases where it remains open (partly or fully) and it usually contains right portal pedicle or its branches[28-30]. During LC, it is best seen when the gallbladder neck is retracted towards the umbilical fissure (Figure 5).
Clinical significance: The visible open sulcus acts as a fixed anatomical landmark during LC. It reliably indicates the plane of the CBD thus helps to reorient surgeon in cases of difficultly. All the dissection during the LC must be done ventral and cephalad to the line joining the roof of this sulcus and base of segment 4 (R4U line) (Figures 4 and 5) as discussed later. During posterior dissection in the HC triangle, the dissection may be safely started by dividing the peritoneum immediately ventral and cephalad to the sulcus[28] (Figure 5).
This is a fissure between the left lateral section (segments 2, 3) and left medial section (segment 4) where the falciform ligament and ligamentum teres lie.
Clinical significance: This also acts as a fixed anatomical landmark, and helps the operating surgeon to reorient in difficult situations.
This is the left medial section of the liver[32]. During LC it is identified easily by its location between the umbilical fissure and the gallbladder (Figure 4).
Clinical significance: The base of segment 4 acts as a fixed landmark. All the dissection in the HC triangle should be done cephalad to the R4U line (Figures 4 and 5). The initial assessment of size of this segment is important. This can be easily done by looking at the distance between the umbilical fissure and the gallbladder. Certain congenital conditions like segment 4 hypoplasia and ectopic gallbladder may need to be assessed correctly before the dissection begins to avoid inadvertent injury to bile duct[33,34].
As the HC triangle is the primary surgical field during LC, it is important to know the clinically significant anatomic variations occurring in this area. These variations may pose challenges to the unfamiliar surgeon with a potential for BDI/VBI.
Vascular anomalies: The cystic artery and the right hepatic artery (RHA) are two important vessels of concern during LC.
The cystic artery is usually single (approximately 79%), originates from RHA, and most commonly (81.5%) traverses the HC triangle to supply the gallbladder through its two branches - superficial and deep[23]. However, this artery may have variations in its origin, number, or course. The most important of these variations includes: (1) the cystic artery passing anterior to the common hepatic/bile duct (17.9%); (2) a short (< 1 cm) cystic artery (9.5%); (3) multiple cystic arteries (8.9%); and (4) the cystic artery located inferior to the cystic duct (4.9%)[23].
Clinical importance: If the presence of a short cystic artery is not appreciated during surgery, the right hepatic artery may be clipped and divided in a manner similar to that of the classic BDI. Keeping the dissection of the artery close to the gallbladder on the right side of the cystic lymph node (a fixed landmark) may prevent injury to the right hepatic artery. When the cystic artery arises from the gastroduodenal or left hepatic artery, it doesn’t pass through the HC triangle so can’t be localised during dissection in this triangle[23]. When it arises from the gastroduodenal artery, the cystic artery passes inferior to the cystic duct (low lying cystic artery)[21].
Anatomic variations of RHA are common. It usually passes behind the CHD (87%) before entering the HC triangle[22]. An aberrant right hepatic artery (replaced or accessary) is also not uncommon. A replaced right hepatic artery, after coursing from behind the portal vein and CBD, comes to lie on the right side of the bile duct, and then travels close to the cystic duct and gallbladder and joins the right pedicle high up in the HC triangle[21,23].
Clinical importance: During dissection in the HC triangle, a replaced right hepatic artery may appear as a large cystic artery, and might be injured if not identified correctly[23]. The right hepatic artery may take a tortuous course (Caterpillar turn/Moynihan’s hump) within the HC triangle, and it may lie very close to the gallbladder and the cystic duct before giving off a short cystic artery[21]. Again, this aberrant course makes the right hepatic artery prone to injury during cholecystectomy.
Important ductal anomalies relevant to LC involve variations in the cystic duct and right hepatic ductal system[35].
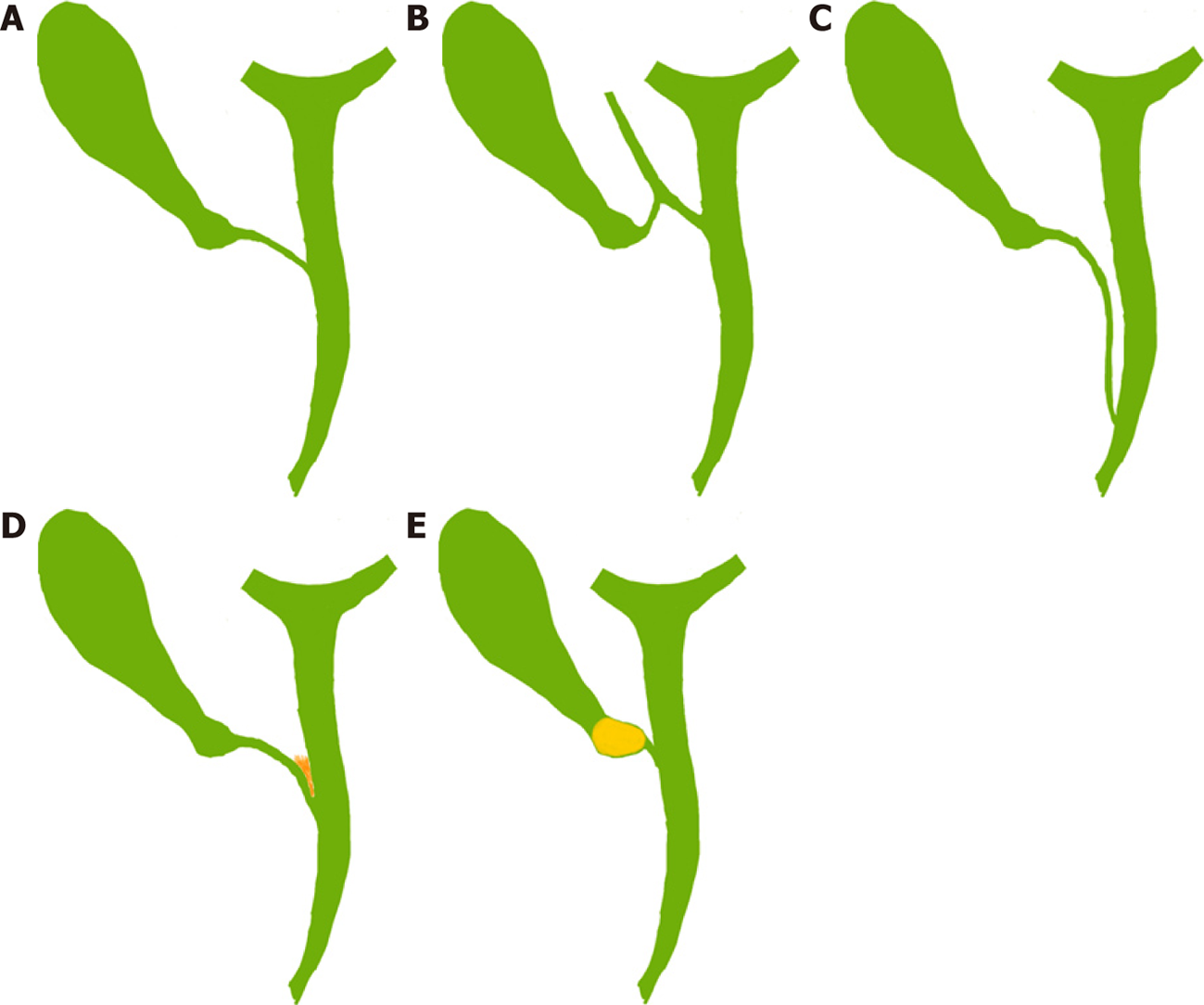
The cystic duct is usually 2-4 cm long and 2-3 mm wide[21,36]. It may be congenitally absent (very rare) or very long (5 cm or more)[21,36]. Usually it joins the CHD at an angle (angular insertion, 75%) but its course may be parallel (20%) or spiral (5%)[21,24,35]. It usually enters the CHD but there are variations: it may enter the right hepatic duct (0.6%-2.3%), anomalous right sectional duct, or CHD quite low near the ampulla (Figure 6)[21,22]. An anomalous right sectional duct, especially a right posterior sectional duct, may join the biliary tree at a level lower than usual. Rarely, there might be duplication of the CBD[37].
Clinical importance: The length and course of the cystic duct and its joining pattern with CHD are variable (Figure 6). However, its entire course and its junction with the CBD do not need to be delineated during LC as it is not required and it will put the bile duct at risk of injury specially in case of parallel insertion where the part of the cystic duct may be adherent to the CHD due to inflammation (Figure 6D). A congenitally absent cystic duct is a very rare condition. If the cystic duct is not apparent during cholecystectomy then either it is short or it may be effaced by the stone or the Mirizzi syndrome is present (Figure 6E). The surgeon should be careful while dissecting in the HC triangle in such situation and may need to resort to one of the bail-out techniques as discussed later. Similarly, if two cystic ducts are visible, surgeon should be very careful in labelling this as double cystic ducts and dividing these structures[24]. The anatomy should be clarified [e.g., with intraoperative cholangiography (IOC)] as these two ducts may be CBD and the CHD with a very short or effaced cystic duct[24], indicating that the dissection has gone behind the bile duct rather than through the HC triangle. The cystic duct diameter may be as much as 5 mm due to the passage of stone, and in this situation it may be confused with CBD. Inability to completely occlude the cystic duct with a medium-large clip should raise the suspicion of it to be CBD[38] and this should be clarified (with proper display of CVS, and/or intraoperative imaging) before clipping and division. Anomalous low insertion of a right sectional duct (usually posterior), especially when the cystic duct joins it (Figure 6B), will put this sectional duct at risk of injury[22,35] if the surgeon is unaware of this variation and does not achieve CVS as discussed subsequently.
A difficult LC (difficult gallbladder) creates a prolonged operative time and a higher likelihood of conversion to an open procedure. A number of parameters have been evaluated to predict such difficulty[39-41]. Male gender and higher age have been found to be consistent predictors of difficult procedure in both acute cholecystitis and in elective cases[39,40] (Table 1).
| History |
| Male gender |
| Higher age (> 65 yr) |
| Increased interval between onset and presentation (> 72-96 h) in acute cholecystitis |
| Previous multiple attacks of biliary colic |
| History of acute cholecystitis |
| Upper abdominal surgery |
| Prior attempt at cholecystectomy (including cholecystostomy) |
| Physical examination |
| Fever |
| Higher ASA score |
| Morbid obesity |
| Laboratory tests |
| Raised leucocyte count (> 18000/mm3) |
| Raised C-reactive protein |
| Imaging (USG/CT/MRI-MRCP) |
| Thick walled gallbladder (> 4-5 mm) |
| Small contracted gallbladder |
| Distended gallbladder with impacted stone in neck |
| Gangrenous gallbladder/gallbladder perforation |
| Mirizzi syndrome/Cholecystoenteric fistula |
| Cirrhosis/extrahepatic portal vein obstruction (portal cavernoma) with portal hypertension |
| Intraoperative |
| Small shrunken gallbladder not visualized on initial exploration |
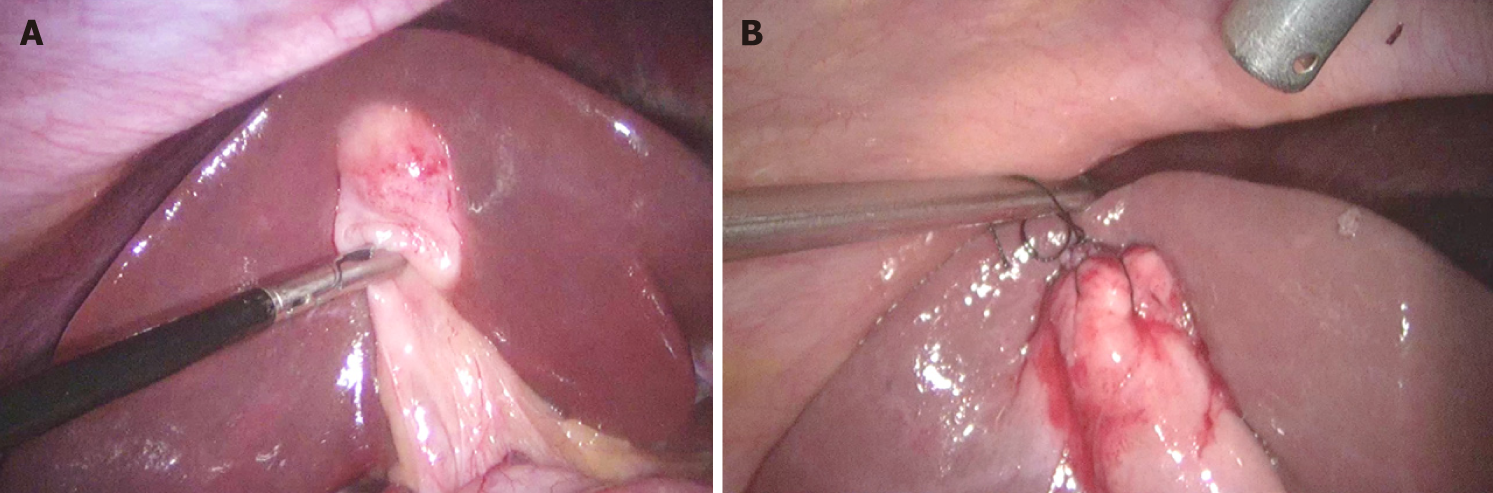
| Liver edge retracted with fissure/depression/puckering near fundus (Liver pucker sign, Figure 3C) |
| Fatty/firm cirrhotic liver (difficulty in retraction) |
In cases with acute cholecystitis, the severity of the inflammation in the HC triangle and adjoining area (as indicated by raised total leucocyte counts, and C-reactive protein levels), remains the most common reason for conversion[39].
In cases with chronic cholecystitis, presence of thick wall gallbladder, presence of Mirizzi syndrome, small contracted gallbladder, and impacted large stone at neck are important predictors of difficult procedure[39,40].
An acute or chronic inflammatory process leads to local changes within and around the gallbladder. It may lead to scarring in the HC triangle, the primary surgical field of interest, to such an extent that it may be difficult to ascertain the actual anatomy and safely identify the cystic duct and the cystic artery.
During surgery, certain intraoperative findings can predict the subsequent operative difficulty. These intraoperative findings which are considered high on difficulty score include diffuse dense peri-gallbladder adhesions (Figure 7), partial or diffuse fibrosis/scarring in the HC triangle, diffuse scarring in the gallbladder bed with a small shrunken lumen-less gallbladder, easy bleeding at dissection around the gallbladder or in HC triangle, gallbladder perforation (contained perforation or cholecysto-enteric fistula), Mirizzi syndrome, and necrotic changes in the HC triangle/around the gallbladder[14]. A comprehensive scoring system, e.g., CLOC score, may also be useful in clinical practice to predict conversion to open cholecystectomy[41].
As most of these predictive factors are non-modifiable, the surgeon must review all these factors preoperatively to suitably plan the operation and prepare for the higher likelihood for conversion or need for subtotal cholecystectomy (STC) or other bailout techniques. In addition, the presence of these risk factors should alert surgeons with limited experience (e.g., those who are early in their career) to be prepared to request help from a more experienced surgeon, as this is one of the strategies to decrease the risk of biliary injuries[1,42].
The basic essential steps of LC cholecystectomy include gallbladder retraction (to open up and expose HC triangle in preparation for next step), dissection in the HC triangle to achieve the CVS, clipping and division of the cystic duct and the cystic artery, and dissection of the gallbladder from its bed (Figure 8).
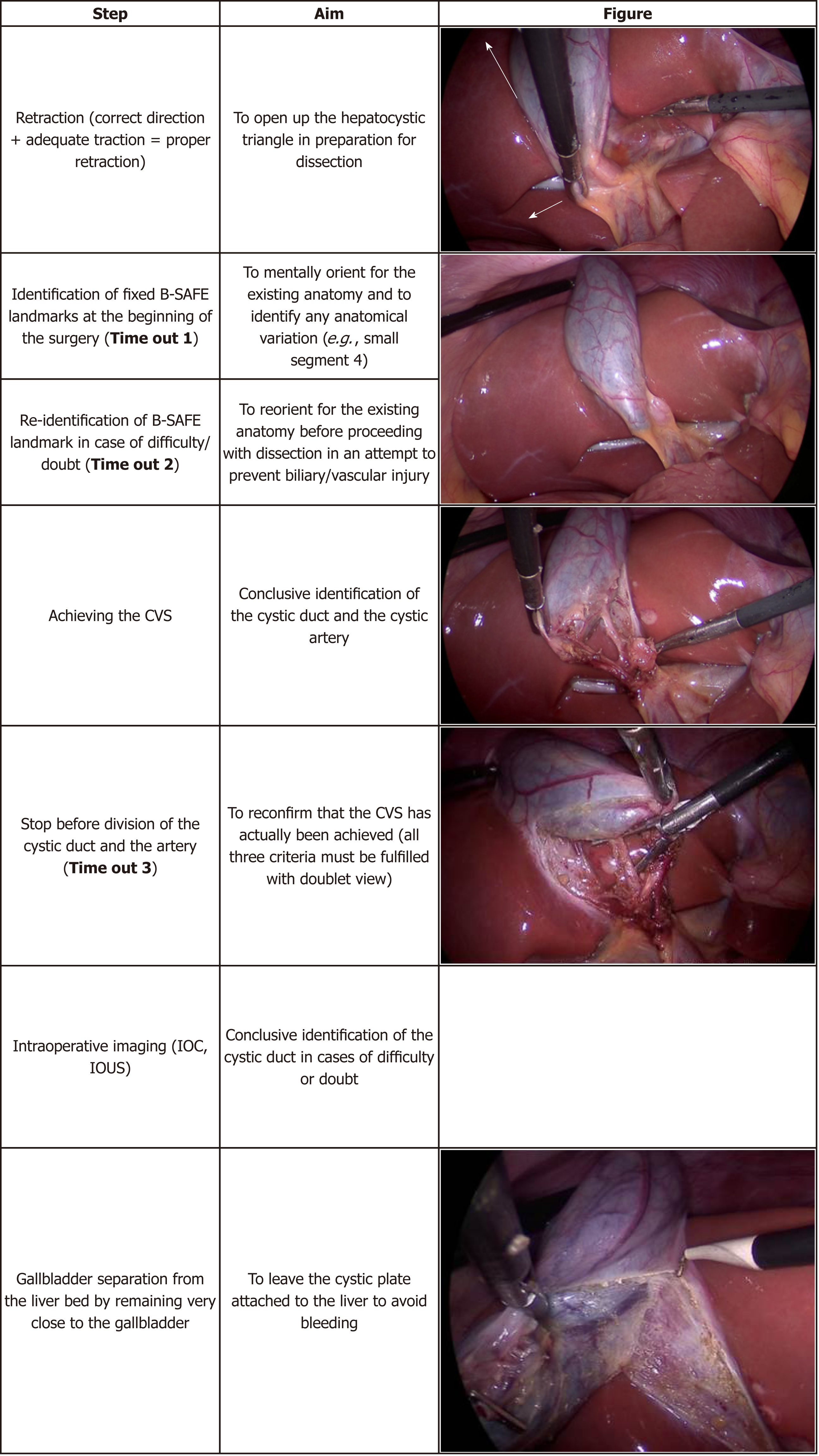
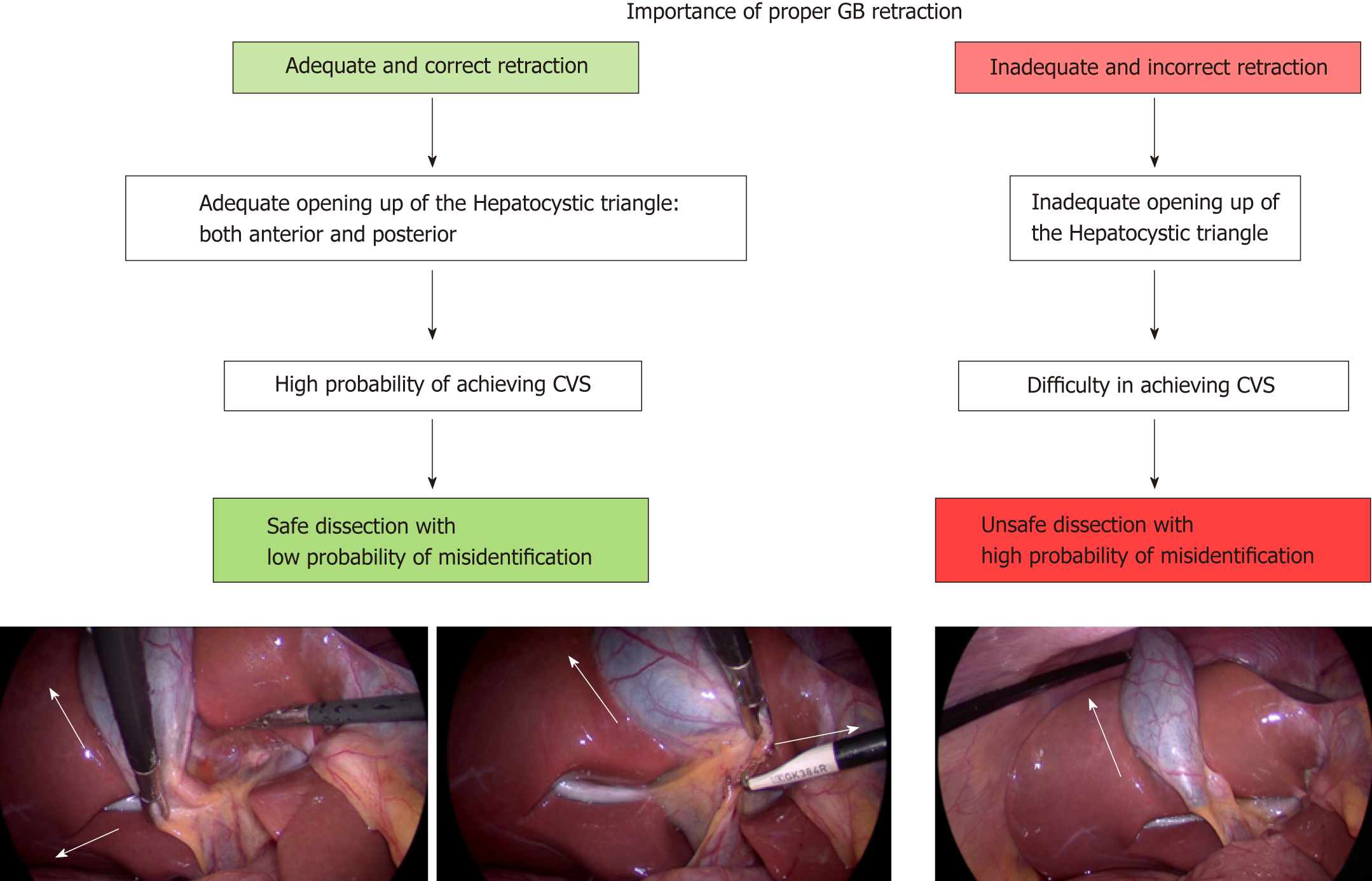
The HC triangle is the primary surgical field of interest in the LC. It is essential to expose this area before the actual dissection begins. This requires proper retraction of the gallbladder. Proper retraction means adequate retraction of the gallbladder in the correct directions.
The fundus should be retracted towards the right shoulder of the patient and the infundibulum should be retracted infero-laterally towards the right side of the patient (Figure 9)[43]. This will expose the anterior peritoneal layer over the HC triangle. The cystic lymph node can be seen, which serves as a fixed anatomical landmark for the cystic artery. In a thin patient without inflammation and without much fibro-fatty tissue in the HC triangle, the cystic duct and the artery may be easily visible at this stage.
When the infundibulum (neck) is retracted towards the umbilical fissure, the posterior aspect of the HC triangle can be seen (Figure 10). If the infundibulum is not retracted adequately in the correct direction, the CBD will be pulled towards the right upper quadrant of the patient, resulting in parallel alignment of the cystic duct and the CBD (Figure 10). In this situation the CBD may be mistaken for the cystic duct and may be injured if care is not taken to correctly identify the anatomy. Infundibular traction opens up the HC triangle and increases the angle between the cystic duct and CBD. An adequate retraction will distort the HC triangle and it may then appear as diamond shape that gradually become more and more appreciable as the dissection proceeds (especially when the gallbladder is separated from the liver to expose lower part of the cystic plate) and the critical view is gradually achieved (Figure 1B).
When there is difficulty in grasping and retracting the fundus (due to a tensely distended gallbladder as in acute cholecystitis or mucocele, thick walled gallbladder, or impacted stone in the fundus), the gallbladder may be decompressed by aspiration, or a stay suture may be taken to facilitate retraction (Figure 11). A firm liver, as in cirrhosis, may also create difficulty in fundal retraction.
Similarly an impacted large stone in the Hartmann’s pouch may interfere with retraction, and may needs to be dislodged before the gallbladder can be grasped and retracted properly. It is important to use angled laparoscope (30/45 degree) during surgery as these scopes allow surgical field to be visualized from different angles with better assessment, especially the assessment of CBD that lies parallel to visual axis of 0 degree scope[43].
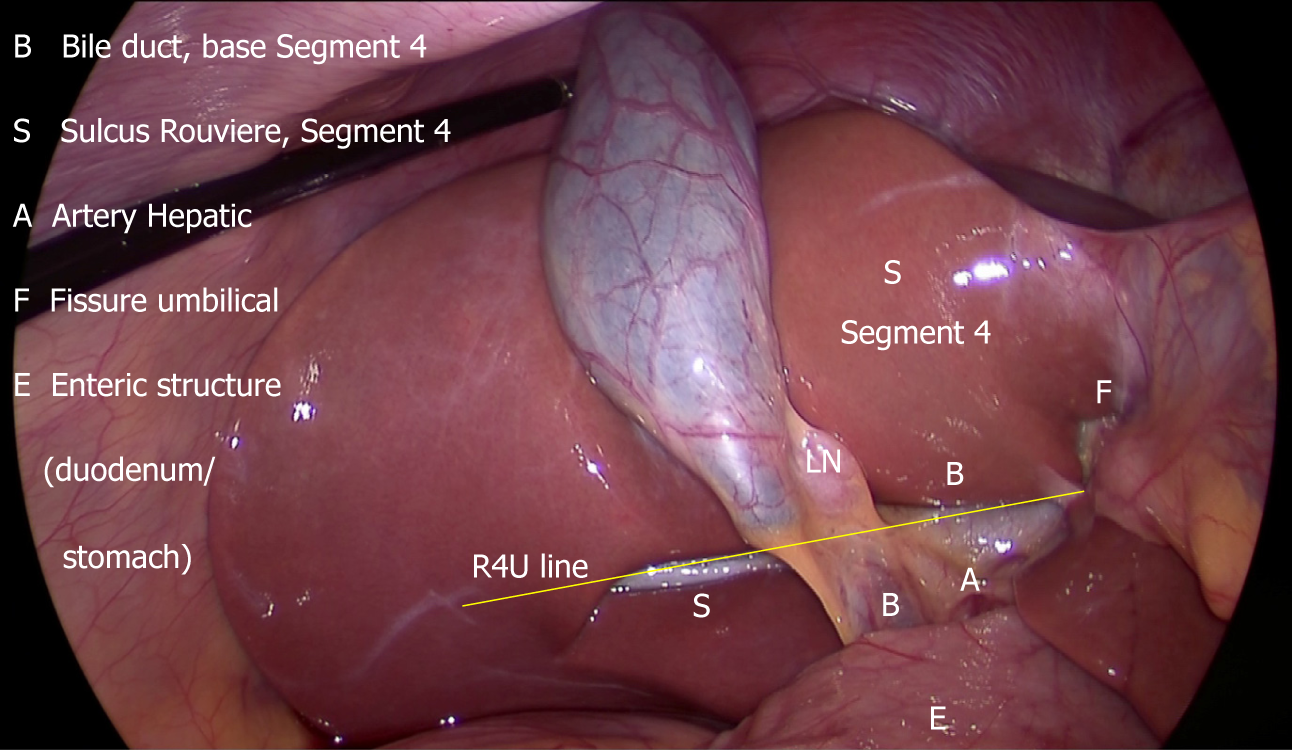
It is essential for the surgeon to know the safe zone of dissection to delineate the cystic duct and the cystic artery. Looking at the fixed anatomical landmark (B-SAFE) will help the surgeon to identify and remain in the safe zone of dissection. These fixed anatomical landmarks include bile duct and base of segment 4 (B), Rouviere’s sulcus and segment 4 (S), hepatic artery (A), umbilical fissure (F), and enteric viscera (E), e.g., duodenum, pylorus[44].
The safe zone of dissection lies cephalad to a line extending from the roof the Rouviere’s sulcus to the umbilical fissure across the base of the segment 4[28]. This safety line, as we refer it to as R4U line (Rouviere’s sulcus→Segment 4→Umbilical fissure), separates the safe zone (cephalad to this line) from danger zone (caudal to this line) (Figures 4 and 5).
Dissection on the posterior aspect of the HC triangle (which is exposed by the medial retraction of the infundibulum/neck towards the umbilical fissure) can be safely started immediately ventral and cephalad to the sulcus and then continued in a triangular area bounded by the plane of the sulcus, the neck of the gallbladder and the liver surface (Figures 5 and 10)[28].
Similar to any other surgical procedure, the safe performance of LC consists of many well-defined steps. Surgeon must realize the importance of these steps and that omission of any of them may result in a less than desirable outcome (Figure 8).
In difficult gallbladder operation, the surgeon may become disoriented and may enter the zone of danger. To avoid this, the concept of the time out has been introduced[44].
The surgeon should take a pause, reorient him/herself, get stock of the situation, and then proceed. To reorient, surgeon should look for five important B-SAFE anatomic landmarks (Figure 4). These landmarks are present and can be easily identified in almost every patient.
It’s a natural tendency for operating surgeon to ask the camera operator to move the laparoscope closer to surgical field in an attempt to get a better view during a difficult LC. This may facilitate better dissection and structure identification. However, it may also result in non-visualization of normal clues/landmarks necessary for correct orientation[38,44].
To look at these cues/landmarks, the camera operator should withdraw the laparoscope to give a panoramic view of the surgical field, and then the surgeon should reorient him/herself with the help of B-SAFE landmarks.
The surgeon should routinely perform a time-out during following steps of the procedure: (1) immediately after entry in to the abdomen; (2) before dissection of HC triangle; (3) when faced with anatomic ambiguity, anomalous anatomy, or in case of any doubt or difficulty; and (4) before the cystic duct and artery are clipped and divided after achieving the CVS[44] (Table 2). For a safer operation, it is important for the entire surgical team to be cognizant of this concept and adhere to these basic principles on a regular basis (Table 2 and Figure 12).
| Use B-SAFE to be safe |
| To be safe: Use time-out |
| Aim: Reorientation/reassessment |
| What to do: Stop→Wait→Reassess→Act |
| What to see: B-SAFE |
| When to see |
| 1 Before beginning dissection in hepatocystic triangle |
| 2 Whenever there is any doubt about anatomy |
| 3 After achieving CVS and before dividing cystic duct and artery (define, decide and then divide) |
Energy devices used to dissect the HC triangle and to separate the gallbladder from its bed are monopolar cautery, bipolar cautery and ultrasonic energy devices. While the monopolar cautery is used most commonly, current evidence is not sufficient to recommend one over the other in terms of safety[17] though the operative time may be shorter with ultrasonic device[45-47]. All these energy sources are considered appropriate for safe cholecystectomy. The operating surgeon must be aware of safe handling of these energy devices.
If a monopolar energy device (most with hook cautery) is used, it is important to: (1) keep it at low setting (≤ 30W) to avoid arcing of current to the bile duct; (2) divide a small amount of tissue at a time after a gentle pull to avoid injury to deeper structures by the heel of the hook cautery; (3) use intermittent short bursts of current at 2-3 s intervals avoid thermal spread to the bile duct; and (4) avoid blind use of cautery in the case of brisk bleeding[48-50].
While lateral thermal spread is less with an ultrasonic energy source, it may be cumbersome to use the long and relatively straight jaws to dissect in the HC triangle. While this may be used to divide the cystic artery, division of the cystic duct using the ultrasonic device is not the standard practice despite some recent reports indicating its feasibility[45-47,51]. Bipolar cautery is useful to control bleeding in the HC triangle and in the liver bed.
A common cause of biliary injury during LC is misidentification of structures in the HC triangle. The CBD or an aberrant right sectional duct may be misidentified as the cystic duct and then, if not correctly appreciated, may be clipped and divided[50]. Similarly the right hepatic artery may be misidentified as the cystic artery if the latter is short or if the right hepatic artery has an aberrant course.
To avoid such misidentification injury, it is of utmost importance that these two structures (the cystic artery and the cystic duct) must be identified conclusively before they are clipped and divided. The concept of the “CVS” was introduced in an attempt to decrease the misidentification injury[12].
The aim of the CVS is conclusive identification of the cystic duct and the cystic artery (two targets) to avoid misidentification injury[48,52,53].
It is not the dissection technique. It is the final view that is achieved after a thorough dissection of the HC triangle to delineate the cystic duct and the cystic artery before they are clipped and divided[12,48,49]. The CVS has three components (Figure 13), and all must be met before the surgeon declares that the CVS has been achieved[12].
1 Clearance of the HC triangle: The HC triangle should be cleared of all the fibro-fatty and soft areolar tissue. Once adequately cleared of all fibro-fatty tissue, the under surface of the liver is easily seen across this triangle.
2 Exposure of the lower cystic plate: The gallbladder should be separated from its liver bed to expose at least the lower third of the cystic plate.
3 Two and only two tubular structures should be seen entering the gallbladder: the Cystic duct and the cystic artery.
It is important to remember the following points: Merely creating two windows alone in the HC triangle is not the CVS as it does not fulfil all three criteria[48]. While all fibro-fatty tissue in the HC triangle needs to be cleared, surgeon should not aim to expose the cystic duct-CBD junction, as such attempt will put the CBD at risk of injury[54,55]. The surgeon should expose at least the lower third of the cystic plate (liver bed of the gallbladder) to ensure that only two target structures (the cystic artery and the cystic duct) are entering the gallbladder[17]. This allows safe identification of any third abnormal structure (non-target structure, aberrant duct or artery) in the HC triangle that needs to be preserved. It is better to separate the gallbladder from its bed as much as possible while achieving the CVS provided a sufficient part of the gallbladder at fundus/body remains attached to the liver to avoid its twisting (extended HC/hepatobiliary triangle). In this extended hepatobiliary triangle, the lower boundary is now formed by the part of the gallbladder along with the cystic duct and upper boundary is also formed by the cystic plate along with the liver surface (Figure 1). Achieving this extended hepatobiliary triangle enhances the safety margin against misidentification of any aberrant structure, and also saves time during subsequent dissection after the cystic duct and the artery have been divided. The CVS should be seen clearly both from front and the back to have complete circumferential visualization of cystic duct and artery (doublet view). The anterior view is easily visible by retracting the infundibulum infero-laterally towards right (with segment 5 surface visible across window) while the posterior view (inverted/reverse HC triangle) requires the infundibulum to be retracted towards the umbilical fissure (with segment 4/quadrate lobe surface visible across window) (Figure 13). Once the surgeon thinks that the CVS has been achieved, he/she should stop at that stage (time out) and reconfirm (with his team/ second surgeon) that the CVS has actually been achieved. At this stage, the CVS (with both the views) may be documented by photographs and/or video recordings[56].
Among all techniques, the CVS approach has been recognized as most effective method of preventing BDI as reported in many recent studies[55,57-59]. Thus, the surgeon must strive to incorporate the CVS in the routine practice of LC. It is important to understand the concept of the CVS and use it correctly. Despite its description more than twenty years ago, surgeons have not been using it frequently[60], or have been interpreting or understanding it incorrectly[49,61]. A recent Dutch study, on reviewing the operative notes and videos of LC, revealed that the CVS was actually achieved in only 10.8% cases despite the fact that it was mentioned to have been achieved in 80% of cases[61]. If a surgeon faces any difficulty in achieving the CVS, he/she should take this difficulty as a warning that proceeding with further dissection may be hazardous with an increased risk of biliary and/or vascular injury[48]. In this situation alternate strategies to complete the cholecystectomy should be considered as discussed in bailout techniques section. Thus, the CVS itself acts as barrier to biliary/vascular injury. As a method of target identification, it needs to be achieved before the cystic duct and the artery are divided. Failure to achieve the CVS after a reasonable attempt indicates a difficult situation with a higher risk of injury with an ongoing attempt at dissection. This approach prevents injury due to misidentification, but not due to direct injury if dissection is continued in a difficult situation in an attempt to identify structures[48].
There are several techniques of target identification during LC. Apart from CVS, surgeons have been replying on several other techniques for identifying anatomy during LC, including infundibular techniques, fundus first technique, and the intraoperative cholangiogram[62]. However, these techniques, especially the infundibular and the fundus first, may be misleading at times and then may act as error traps for the unsuspecting surgeon[63].
In the infundibular technique, cystic duct identification is based on the appearance of the infundibulum-cystic duct junction as a funnel[48,52,63]. When this junction is circumferentially exposed, the surgeon confirms cystic duct identification and then proceeds with its division. Complete dissection in the HC triangle is not performed at this stage. In certain situations this technique can be misleading. When the cystic duct is fused with CHD due to acute or chronic inflammation, when the cystic duct is very short or effaced by a large stone impacted in the infundibulum, or when there is difficultly in exposing the HC triangle due to inadequate retraction (e.g., due to fibrosis), the CBD may be misidentified as the cystic duct[63]. Circumferential dissection then goes around the CHD/CBD rather than around the cystic duct across the HC triangle. This leads to classical BDI where the bile duct is divided twice before the gallbladder could be completely separated from the liver. Thus this technique of cystic duct identification does not protect against biliary injury in difficult situations. Surgeons using this technique should be aware of this error trap.
In the fundus first technique (also known as the dome-down technique) the gallbladder is dissected off its liver bed/cystic plate, and then the cystic duct and the artery are identified and divided[64-66]. Used commonly in the open cholecystectomy, this technique poses technical challenge in handling the gallbladder as it tends to twist once separately completely from the liver. Additionally, there is difficulty in retracting liver.
Not commonly used in LC, this method lays another error trap for the surgeon in certain situations when surgeon does not appreciate the local anatomy well, especially that of the cystic plate.
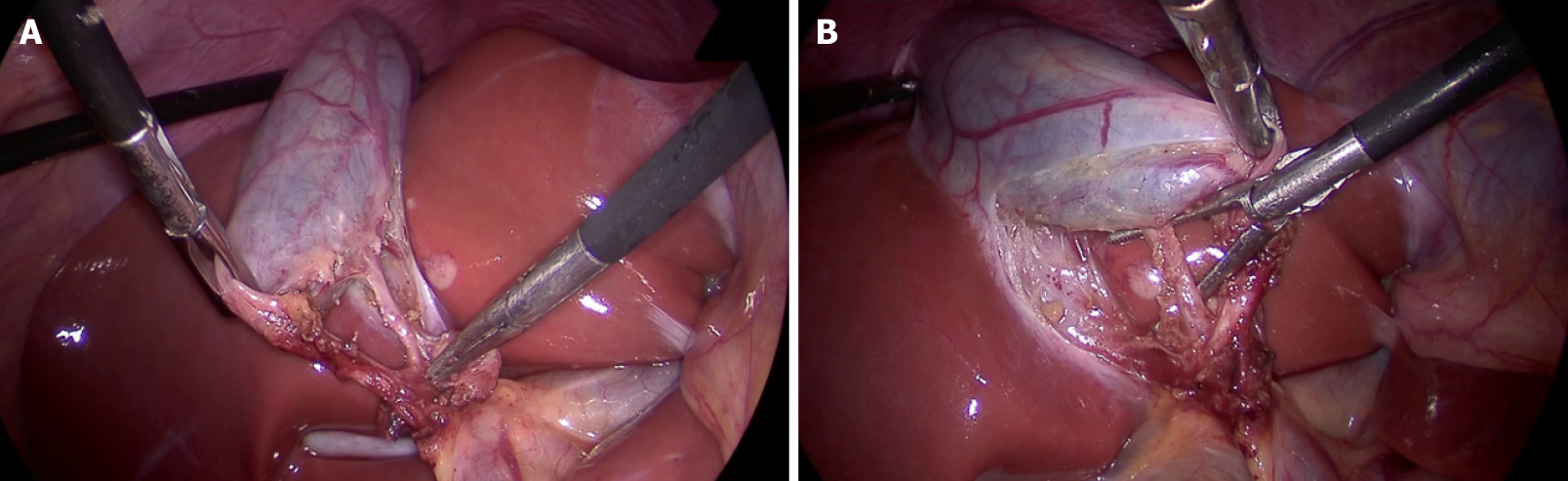
Using this technique in the usual uncomplicated cholecystectomy, the surgeon keeps the dissection close to the gallbladder, moving from the fundus towards the HC triangle. Dissection within the triangle then allows identification of both the cystic duct and the cystic artery. In case of severe inflammation, especially in chronic cholecystitis with a small shrunken gallbladder with a fused and scarred HC triangle, the surgeon may get in to the wrong plane while dissecting down towards the cystic duct. There may be pathological shortening of the cystic plate (Figure 3) so that the distance between the fundus (where the cystic plate starts) and the right portal pedicle (where the cystic plate ends) may decrease so much so that the surgeon may encounter the right portal pedicle or hilar area soon after the dissection begins[27] (Figure 3B). Secondly, fused planes with distorted anatomy may lead the surgeon to dissect close to liver rather than close to the gallbladder in the HC triangle area, which puts hilar structure at risk of injury (Figure 3B). Severe vasculobiliary injuries have been reported in such situations with this technique[27,63].
This approach has been described as an alternative technique to complete LC in the presence of severe inflammation in the HC triangle[17]. However the surgeon must be wary of this technique, should have clear understanding of the cystic plate anatomy and pathological alterations affecting it, should remain very close to the gallbladder throughout the dissection, and when such dissection does not seem possible, should resort to bailout techniques like STC[17].
It is important for the operating surgeon to be able to recognize during the procedure when the dissection is becoming unsafe with a high potential for biliary/vascular injury.
Prudence lies in realizing this danger much before the procedure continues in to the zone of great risk[52] so that the procedure can be stopped at a point of safe return. Thus the operating surgeon should be able to identify or pre-empt the difficult situation that might increase the risk of biliary/vascular injury with the help of certain red flags (Table 3). The difficulty may be due to severe adhesions (Figure 7), severe acute inflammation, a large impacted stone in the neck of the gallbladder, Mirizzi syndrome, or chronic inflammation with fibrosis or scarring. Such conditions may lead to failure of timely progression of the dissection, anatomic disorientation, and difficulty in visualization of operative field. These operative clues are important indicators of a “zone of great danger” lying ahead. The surgeon should recognize these clues, stop the dissection, and then to decide the strategy for a safe operation before the dissection starts again (use “time-out”).
| Stopping rules |
| More than 2 tubular structures entering gallbladder |
| Unusually large presumed cystic artery (this may be hepatic artery) |
| Large artery pulsations present behind the presumed cystic duct (this duct may be common hepatic/bile duct) |
| Medium-large clip fails to occlude ductal lumen (this duct may be hepatic/bile duct) |
| Large ductal structure that can be traced behind the duodenum (this duct is common bile duct) |
| Excessive fibrofatty/lymphatic tissue noted around the presumed cystic duct (this may be common hepatic/bile duct) |
| Bile leak seen with intact gallbladder |
| Bleeding requiring blood transfusion |
It is advisable that operating surgeon should take a pause and seek a second opinion from another surgeon in event of any unexpected finding, a difficult gallbladder, unusual anatomy, or a difficult dissection[1,42]. Misidentification is the major cause of biliary/vascular injury, and most commonly (65%) the CBD/CHD is misidentified as the cystic duct or the hepatic artery is misidentified as the cystic artery (10%)[16]. However, such misidentification may be prevented from causing biliary/vascular injury with the advice from a second surgeon in as many as 18% of cases[16], which emphasizes the importance of a second opinion. The operating surgeon should not hesitate to seek a second opinion whenever needed, and this should be considered as a sign of good clinical practice rather than a sign of surgical ineptitude.
Various methods have been described for intraoperative assessment of biliary anatomy that may result in decreased incidence of BDI. Although many studies suggest the protective effect of these approaches, future studies are necessary to recommend routine use of these techniques.
Intraoperative cholangiography (IOC): It is the most commonly performed and most studied method utilized for the intraoperative assessment of the biliary anatomy, identification and assessment of extent of biliary injury, and possible prevention of biliary ductal injury. Several large retrospective data sets report association of IOC with lower rates of BDI[67].
It is a safe (minimally invasive) technique with 90%–95% success rate, and has the additional advantage of detection of asymptomatic CBD stones. However, IOC may be cumbersome at times. Ductal cannulation can be difficult in patients with short and thin cystic ducts. This additional procedure adds to the operative time and cost, and there is learning curve involved for correct interpretation. There is also the need for radiographic equipment.
Current status: Many studies have demonstrated that IOC may result in lower chances of BDI and it may also lead to early recognition of such injury. However, its various disadvantages may preclude it from being a part of routine clinical practice. It remains a matter of debate as to whether IOC should be performed routinely or selectively; routine IOC cannot be recommended based on the available literature[43].
It is imperative for surgeons to know the indications for and technique of performing IOC, and surgeons should keep a low threshold for its use.
Laparoscopic ultrasound: Many outcome studies support laparoscopic ultrasound (LUS) for prevention of BDI[68]. Being non-invasive, it is very safe. Some advantages of LUS compared to IOC are its non-invasiveness, shorter procedure time, higher success rates and lack of radiation exposure. However, it is less accurate for the assessment of the intrapancreatic and intrahepatic parts of the biliary system. Additionally, it is associated with a long learning curve.
Current status: LUS is a reasonable alternative to IOC for the diagnosis of CBD stones[55]. However, it is not clear whether its use prevents BDI.
Near infrared fluorescent cholangiography: Near infrared fluorescent cholangiography is the most recent addition to the armamentarium for intraoperative assessment of biliary tract. Its efficacy and safety have been confirmed in various studies[69]. When compared to IOC this technique takes less time, and it is cheaper and safer. However, as it is a new technique, its use in various biliary pathologies has not yet been evaluated.
Current status: Currently there is not enough evidence to recommend its routine use to detect CBD stones or BDI recognition[17]. In summary, among the currently available imaging methods, there is no one method that is superior to others. IOC or LUS can be performed routinely or selectively, based on operating surgeon’s discretion.
A simple, uneventful cholecystectomy is akin to a safe journey by airplane in clear weather where there is absolutely no deviation from the standard protocol related to take-off, navigation and landing[70]. However, when the weather is bad, such a journey needs to be modified depending on the degree of weather disturbance. Perhaps the flight is cancelled altogether until the weather improves (if such information is available beforehand). If inclement weather develops en route, the pilot has several options to choose a different course. The pilot may choose to return to the source of origin, to fly around the bad weather to reach the desired destination, or divert the flight to an alternative destination[70]. The primary aim in all of these alternatives is the safety of all passengers. It is most important to protect the lives of the passengers rather than to reach the desired destination (i.e., safety first).
Similarly, in a situation of a difficult gallbladder, it is not important to push ahead with the goal of complete cholecystectomy while risking patient safety due to potential of biliary/vascular injury in such situations. Rather it is important to perform an alternate procedure (bailout techniques) that allows the surgeon to complete the procedure in a safe manner[14,16,17,70,71].
There are five bailout strategies for a difficult gallbladder[14,17,71]: (1) Abort the procedure altogether; (2) Convert to an open procedure; (3) Tube cholecystostomy; (4) Subtotal cholecystectomy (STC, open/laparoscopic); and (5) Fundus first cholecystectomy.
The surgeon will need to use clinical judgement while performing the cholecystectomy in choosing a specific bailout technique. The best choice will depend on the clinical situation and the experience/expertise of the surgeon.
The safest bailout strategy is to abort the procedure altogether. Dense pericholecystic adhesions due to severe acute or chronic inflammation with non-visualization of the gallbladder may force the surgeon to resort to this option. Continuing antibiotics (with percutaneous cholecystostomy if required) postoperatively and reattempting the cholecystectomy after a long waiting period (2-3 mo or more) may be helpful in such scenario[72,73].
Conversion to an open procedure is another safe option, but with a word of caution. It is important to realize that simply converting to an open procedure does not safeguard against bile duct/vascular injury. A difficult procedure may remain difficult even after conversion to open with no effect on postoperative complications[72]. Recent evidence suggests a nearly 100 fold increase in BDI rate in converted cases[73].
Tube cholecystostomy is a simple bridge procedure to provide symptomatic relief until the time a definitive procedure can be performed. It can be performed laparoscopically or after conversion to open procedure. It is important to remember that the interval LC may again be difficult, with a higher rate of conversion and morbidity[74].
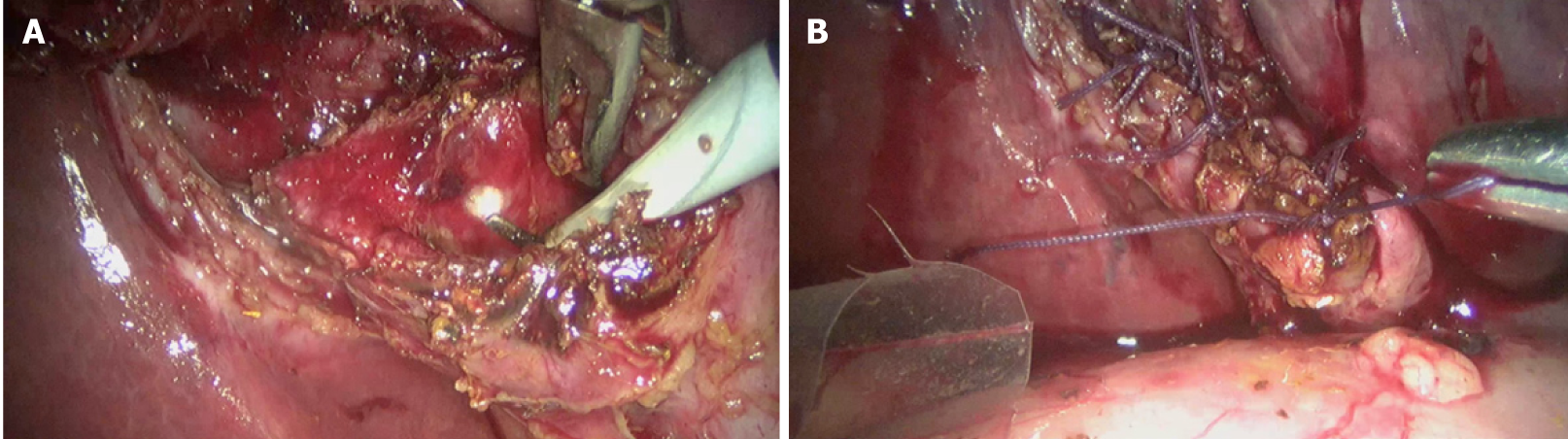
When complete gallbladder removal is not possible due to a frozen, scarred, or fibrotic HC triangle or due to severe inflammation, STC remains a valid and safe alternative to total cholecystectomy[17]. Leaving behind a part of the gallbladder is always safer than a difficult dissection in the HC triangle with a potential for BDI in an attempt to remove the entire gallbladder.
STC can be performed laparoscopically (Figure 14) or after conversion to open[74]. It is important to remove all the stones from the gallbladder, to ablate the mucosa of the gallbladder stump (with diathermy or argon plasma coagulator), and to leave this stump as small as possible. There are two types of STC depending upon on whether the stump is closed or is left open[75,76]. This stump may vary in length and part of the gallbladder wall may be left attached to the liver in both types. In subtotal reconstituting cholecystectomy, the stump is closed, either with sutures or with staples. In the subtotal fenestrating cholecystectomy, the stump is left open, with or without closing the cystic duct opening. In both techniques, the gallbladder mucosa is ablated. Both of these techniques are safe and are acceptable alternatives to total cholecystectomy. Intraoperative conditions (degree of inflammation, tissue friability, extent of scarring, etc.) may dictate which type to choose; usually this decision relies on the judgement of the surgeon[77].
While both types of STC reduce the risk of biliary/vascular complications, these complications are not entirely preventable[75,77]. In addition, STC is associated with specific postoperative problems. The incidence of biliary events (recurrent cholelithiasis in gallbladder stump, cholangitis, choledocholithiasis, and biliary pancreatitis) is higher with subtotal reconstituting cholecystectomy. Fenestrating STC is associated with a higher risk of postoperative bile leak[76,77]. Due to persistent bile leak, endoscopic management may be required in approximately 10% of cases[77], and completion cholecystectomy may be required for recurrent cholelithiasis in 5% of cases[78].
Both the current IRCAD recommendations and TG-18 guidelines consider STC as an important and safe alternative procedure in cases where the dissection is difficult to avoid serious biliary or vascular injury[14,17]. The surgeon should be familiar with this bailout technique, its technical aspect, and the complications and consequences. The surgeons must accurately document this procedure in the operative note.
Fundus first technique (dome down, fundus down, retrograde) has been described as a bailout technique[14,17] though its safety is not conclusively proven[14] and it may work as an error trap as described earlier[63]. The surgeon should resort to this technique only when he/she has clear understanding of normal cystic and hilar plate anatomy and their pathological alterations due to acute severe or chronic inflammation involving HC triangle and or gallbladder. The dissection must remain very close to the gallbladder wall[15,17]. Besides decreasing the rate of conversion to open cholecystectomy in difficult cases, it can also facilitate performing STC if complete cholecystectomy is not possible or is considered unsafe even after resorting to fundus first technique.
As the clinical evidence is insufficient at present to suggest superiority of one specific bailout procedure over another[16], the surgeon should use his/her own judgement to select a specific bailout procedure depending on the intraoperative findings and his/her experience. In practice, most surgeons prefer to convert to an open procedure or perform a STC. Cholecystostomy remains the least preferred bailout technique[16].
Biliary injury remains the most common reason for a malpractice claim in gastrointestinal surgery[4]. Thus, the concept of “safe cholecystectomy” also entails safety for the operating surgeon. Besides being vigilant during surgery, vigilance during writing operative notes is also important. Literature is scant about how closely operative notes reflect what was actually performed during the operation. A recent study suggests that descriptions of key elements of adequate HC triangle dissection were described in the operative records of uncomplicated cholecystectomy in only 24.8% cases and in none of cases where a biliary injury occurred[54]. Such practice of inadequate documentation is another “error-trap” as 20%-30% of laparoscopic biliary injury result in some sort of malpractice litigation[4]. The surgeon should describe the measures taken (e.g., achieving CVS) to safeguard the CBD, preferably with photo/video documentation if possible, and describe if STC was performed, including its type and the size of gallbladder stump. Such documentation is also important for future reference, especially if STC has been performed and the patient might develop recurrent stones in the stump many years after the index surgery.
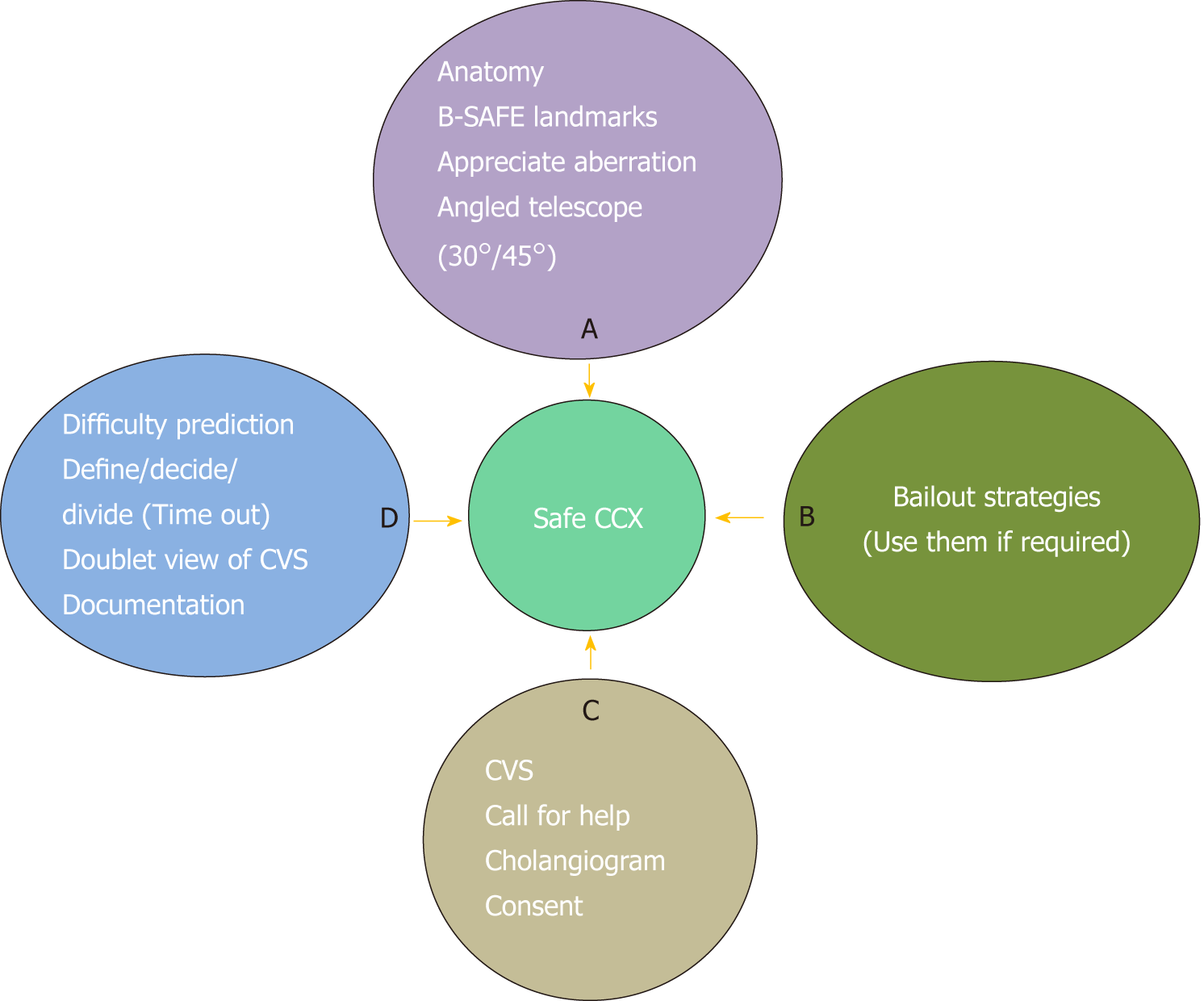
Biliary injuries after LC can result in significant morbidity. The surgical team involved in LC should be aware of the concept of “culture of safety in cholecystectomy” (COSIC)[70]. This universal culture of safety should be routinely adopted by the whole surgical team during every case. The key components of the COSIC, summarised as “ABCD of a safe LC”[1] include: (1) a clear understanding of relevant anatomy; (2) appropriate and timely use of bailout techniques; (3) obtaining CVS prior to division of cystic duct and artery in every case; (4) recognizing the importance of time-out; (5) use of intraoperative imaging; (6) obtaining a second opinion in difficult cases; and (7) importance of proper documentation (Figure 15). In addition, surgeon should be able to anticipate the operative difficulty based on various preoperative predictors, should adhere to basic principles of surgery including safe use of energy devices, instrumentation, and hemostasis, and must confine the dissection in the safe zone of dissection with the help of R4U line.
The surgeon should remember that a 95% cholecystectomy (i.e., STC) is always safer than a 100+% cholecystectomy where variable portion of the bile duct is also excised along with gallbladder, and bile leak from gallbladder is always safer (i.e., dissection very close to gallbladder wall) then from the bile duct. Routine adoption of COSIC may help reduce the incidence of post cholecystectomy biliary/vascular injury to pre-laparoscopic era, if not eliminate them entirely.
We sincerely acknowledge Dr. Richard Sidwell, MD, FACS, Program Director, General Surgery Residency, Iowa Methodist Medical Centre and Adjunct Clinical Professor, Department of Surgery, University of Iowa, United States for critically revising the article for language.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Komatsu S, Martini F, Poskus T S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Gupta V. ABCD of Safe Laparoscopic Cholecystectomy: Imbibing Universal Culture of Safety in Cholecystectomy. Indian J Surg. 2018;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (2)] |
| 2. | Berci G, Hunter J, Morgenstern L, Arregui M, Brunt M, Carroll B, Edye M, Fermelia D, Ferzli G, Greene F, Petelin J, Phillips E, Ponsky J, Sax H, Schwaitzberg S, Soper N, Swanstrom L, Traverso W. Laparoscopic cholecystectomy: first, do no harm; second, take care of bile duct stones. Surg Endosc. 2013;27:1051-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 3. | Fédération de chirurgie viscérale et digestive. Risk management to decrease bile duct injury associated with cholecystectomy: measures to improve patient safety. J Visc Surg. 2014;151:241-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Barrett M, Asbun HJ, Chien HL, Brunt LM, Telem DA. Bile duct injury and morbidity following cholecystectomy: a need for improvement. Surg Endosc. 2018;32:1683-1688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Pucher PH, Brunt LM, Davies N, Linsk A, Munshi A, Rodriguez HA, Fingerhut A, Fanelli RD, Asbun H, Aggarwal R; SAGES Safe Cholecystectomy Task Force. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc. 2018;32:2175-2183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 130] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 6. | Booij KAC, de Reuver PR, van Dieren S, van Delden OM, Rauws EA, Busch OR, van Gulik TM, Gouma DJ. Long-term Impact of Bile Duct Injury on Morbidity, Mortality, Quality of Life, and Work Related Limitations. Ann Surg. 2018;268:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Rystedt JM, Montgomery AK. Quality-of-life after bile duct injury: intraoperative detection is crucial. A national case-control study. HPB (Oxford). 2016;18:1010-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Hariharan D, Psaltis E, Scholefield JH, Lobo DN. Quality of Life and Medico-Legal Implications Following Iatrogenic Bile Duct Injuries. World J Surg. 2017;41:90-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Dominguez-Rosado I, Mercado MA, Kauffman C, Ramirez-del Val F, Elnecavé-Olaiz A, Zamora-Valdés D. Quality of life in bile duct injury: 1-, 5-, and 10-year outcomes after surgical repair. J Gastrointest Surg. 2014;18:2089-2094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Ejaz A, Spolverato G, Kim Y, Dodson R, Sicklick JK, Pitt HA, Lillemoe KD, Cameron JL, Pawlik TM. Long-term health-related quality of life after iatrogenic bile duct injury repair. J Am Coll Surg. 2014;219:923-932.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Hunter JG. Avoidance of bile duct injury during laparoscopic cholecystectomy. Am J Surg. 1991;162:71-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 182] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hugh TB. New strategies to prevent laparoscopic bile duct injury--surgeons can learn from pilots. Surgery. 2002;132:826-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Wakabayashi G, Iwashita Y, Hibi T, Takada T, Strasberg SM, Asbun HJ, Endo I, Umezawa A, Asai K, Suzuki K, Mori Y, Okamoto K, Pitt HA, Han HS, Hwang TL, Yoon YS, Yoon DS, Choi IS, Huang WS, Giménez ME, Garden OJ, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Liu KH, Su CH, Misawa T, Nakamura M, Horiguchi A, Tagaya N, Fujioka S, Higuchi R, Shikata S, Noguchi Y, Ukai T, Yokoe M, Cherqui D, Honda G, Sugioka A, de Santibañes E, Supe AN, Tokumura H, Kimura T, Yoshida M, Mayumi T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:73-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 15. | Honda G, Hasegawa H, Umezawa A. Universal safe procedure of laparoscopic cholecystectomy standardized by exposing the inner layer of the subserosal layer (with video). J Hepatobiliary Pancreat Sci. 2016;23:E14-E19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Iwashita Y, Hibi T, Ohyama T, Umezawa A, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Han HS, Hwang TL, Suzuki K, Yoon YS, Choi IS, Yoon DS, Huang WS, Yoshida M, Wakabayashi G, Miura F, Okamoto K, Endo I, de Santibañes E, Giménez ME, Windsor JA, Garden OJ, Gouma DJ, Cherqui D, Belli G, Dervenis C, Deziel DJ, Jonas E, Jagannath P, Supe AN, Singh H, Liau KH, Chen XP, Chan ACW, Lau WY, Fan ST, Chen MF, Kim MH, Honda G, Sugioka A, Asai K, Wada K, Mori Y, Higuchi R, Misawa T, Watanabe M, Matsumura N, Rikiyama T, Sata N, Kano N, Tokumura H, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Delphi consensus on bile duct injuries during laparoscopic cholecystectomy: an evolutionary cul-de-sac or the birth pangs of a new technical framework? J Hepatobiliary Pancreat Sci. 2017;24:591-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Conrad C, Wakabayashi G, Asbun HJ, Dallemagne B, Demartines N, Diana M, Fuks D, Giménez ME, Goumard C, Kaneko H, Memeo R, Resende A, Scatton O, Schneck AS, Soubrane O, Tanabe M, van den Bos J, Weiss H, Yamamoto M, Marescaux J, Pessaux P. IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2017;24:603-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Connor SJ, Perry W, Nathanson L, Hugh TB, Hugh TJ. Using a standardized method for laparoscopic cholecystectomy to create a concept operation-specific checklist. HPB (Oxford). 2014;16:422-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Gurusamy KS, Vaughan J, Rossi M, Davidson BR. Fewer-than-four ports versus four ports for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;CD007109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Callery MP. Avoiding biliary injury during laparoscopic cholecystectomy: technical considerations. Surg Endosc. 2006;20:1654-1658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 21. | Abdalla S, Pierre S, Ellis H. Calot's triangle. Clin Anat. 2013;26:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Skandalakis JE, Skandalakis PN, Skandalakis LJ. Surgical anatomy and technique. 2nd ed. Springer-Verlag. 2000;573-612. [DOI] [Cited in This Article: ] |
| 23. | Andall RG, Matusz P, du Plessis M, Ward R, Tubbs RS, Loukas M. The clinical anatomy of cystic artery variations: a review of over 9800 cases. Surg Radiol Anat. 2016;38:529-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 24. | Strasberg SM. Liver and biliary anatomy. In: Zyromski NJ. Handbook of Hepato-Pancreato-Biliary Surgery. Wolters Kluwer 2015; 254-270. [Cited in This Article: ] |
| 25. | Kawarada Y, Das BC, Taoka H. Anatomy of the hepatic hilar area: the plate system. J Hepatobiliary Pancreat Surg. 2000;7:580-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Schnelldorfer T, Sarr MG, Adams DB. What is the duct of Luschka?--A systematic review. J Gastrointest Surg. 2012;16:656-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Strasberg SM, Gouma DJ. 'Extreme' vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford). 2012;14:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Hugh TB, Kelly MD, Mekisic A. Rouvière's sulcus: a useful landmark in laparoscopic cholecystectomy. Br J Surg. 1997;84:1253-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Dahmane R, Morjane A, Starc A. Anatomy and surgical relevance of Rouviere's sulcus. ScientificWorldJournal. 2013;2013:254287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Lockhart S, Singh-Ranger G. Rouviere's sulcus-Aspects of incorporating this valuable sign for laparoscopic cholecystectomy. Asian J Surg. 2018;41:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Peti N, Moser MA. Graphic reminder of Rouviere's sulcus: a useful landmark in cholecystectomy. ANZ J Surg. 2012;82:367-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, Makuuchi M, Strong RW. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2000;2:333-339. [DOI] [Cited in This Article: ] |
| 33. | Mercado MA, Franssen B, Arriola JC, Garcia-Badiola A, Arámburo R, Elnecavé A, Cortés-González R. Liver segment IV hypoplasia as a risk factor for bile duct injury. J Gastrointest Surg. 2011;15:1589-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Gupta V, Chandra A. Segment IV hypoplasia: defining criteria, their reliability, and association with biliary injury. J Gastrointest Surg. 2012;16:1080-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Blumgart LH, Schwartz LH, DeMatteo RP, Jarnagin WR, Allen PJ, Chapman WC, D'Angelica MI, DeMatteo RP. Surgical and radiological anatomy of the liver, biliary tract, and pancreas. Jarnagin WR, Allen PJ, Chapman WC, D'Angelica MI, DeMatteo RP. Blumgart’s Surgery of the liver, biliary tract, and pancreas. Philadelphia: Elsevier 2017; 32-59. [Cited in This Article: ] |
| 36. | Adkins RB, Chapman WC, Reddy VS. Embryology, anatomy, and surgical applications of the extrahepatic biliary system. Surg Clin North Am. 2000;80:363-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Gupta V, Chandra A. Duplication of the extrahepatic bile duct. Congenit Anom (Kyoto). 2012;52:176-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Stewart L, Dixon E, Vollmer CM Jr, May GR. Perceptual errors leading to bile duct injury during laparoscopic cholecystectomy. Dixon E, Vollmer CM Jr, May GR. Management of benign biliary stenosis and injury. Switzerland: Springer 2015; 165-186. [DOI] [Cited in This Article: ] |
| 39. | Panni RZ, Strasberg SM. Preoperative predictors of conversion as indicators of local inflammation in acute cholecystitis: strategies for future studies to develop quantitative predictors. J Hepatobiliary Pancreat Sci. 2018;25:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Philip Rothman J, Burcharth J, Pommergaard HC, Viereck S, Rosenberg J. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery - A Systematic Review and Meta-Analysis of Observational Studies. Dig Surg. 2016;33:414-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Sutcliffe RP, Hollyman M, Hodson J, Bonney G, Vohra RS, Griffiths EA; CholeS study group, West Midlands Research Collaborative. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: a validated risk score derived from a prospective U.K. database of 8820 patients. HPB (Oxford). 2016;18:922-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Society of American Gastrointestinal and Endoscopic Surgeons. The SAGES safe cholecystectomy program. Available from: URL: https://www.sages.org/safe-cholecystectomy-program/. [Cited in This Article: ] |
| 43. | Eikermann M, Siegel R, Broeders I, Dziri C, Fingerhut A, Gutt C, Jaschinski T, Nassar A, Paganini AM, Pieper D, Targarona E, Schrewe M, Shamiyeh A, Strik M, Neugebauer EA; European Association for Endoscopic Surgery. Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2012;26:3003-3039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 44. | Sutherland F, Ball CG, Dixon E, Vollmer CM Jr, May GR. The heuristic and psychology of bile duct injuries. Dixon E, Vollmer CM Jr, May GR. Management of benign biliary stenosis and injury. Switzerland: Springer 2015; 191-198. [DOI] [Cited in This Article: ] |
| 45. | Kandil T, El Nakeeb A, El Hefnawy E. Comparative study between clipless laparoscopic cholecystectomy by harmonic scalpel versus conventional method: a prospective randomized study. J Gastrointest Surg. 2010;14:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | El Nakeeb A, Askar W, El Lithy R, Farid M. Clipless laparoscopic cholecystectomy using the Harmonic scalpel for cirrhotic patients: a prospective randomized study. Surg Endosc. 2010;24:2536-2541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Jain SK, Tanwar R, Kaza RC, Agarwal PN. A prospective, randomized study of comparison of clipless cholecystectomy with conventional laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2011;21:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 2010;211:132-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 276] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 49. | Singh R, Brunt L. Critical view of safety-its feasibility and efficacy in preventing bile duct injuries. Ann Laparosc Endosc Surg. 2018;3. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Strasberg SM. Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9:543-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | van Dijk AH, van Roessel S, de Reuver PR, Boerma D, Boermeester MA, Donkervoort SC. Systematic review of cystic duct closure techniques in relation to prevention of bile duct leakage after laparoscopic cholecystectomy. World J Gastrointest Surg. 2018;10:57-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Strasberg SM. Biliary injury in laparoscopic surgery: part 2. Changing the culture of cholecystectomy. J Am Coll Surg. 2005;201:604-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Strasberg SM. A perspective on the critical view of safety in laparoscopic cholecystectomy. Ann Laparosc Endosc Surg. 2017;2. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Stewart L, Hunter JG, Wetter A, Chin B, Way LW. Operative reports: form and function. Arch Surg. 2010;145:865-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Buddingh KT, Nieuwenhuijs VB, van Buuren L, Hulscher JB, de Jong JS, van Dam GM. Intraoperative assessment of biliary anatomy for prevention of bile duct injury: a review of current and future patient safety interventions. Surg Endosc. 2011;25:2449-2461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Sanford DE, Strasberg SM. A simple effective method for generation of a permanent record of the Critical View of Safety during laparoscopic cholecystectomy by intraoperative "doublet" photography. J Am Coll Surg. 2014;218:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Yegiyants S, Collins JC. Operative strategy can reduce the incidence of major bile duct injury in laparoscopic cholecystectomy. Am Surg. 2008;74:985-987. [PubMed] [Cited in This Article: ] |
| 58. | Avgerinos C, Kelgiorgi D, Touloumis Z, Baltatzi L, Dervenis C. One thousand laparoscopic cholecystectomies in a single surgical unit using the "critical view of safety" technique. J Gastrointest Surg. 2009;13:498-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Tsalis K, Antoniou N, Koukouritaki Z, Patridas D, Christoforidis E, Lazaridis C. Open-access technique and "critical view of safety" as the safest way to perform laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Stefanidis D, Chintalapudi N, Anderson-Montoya B, Oommen B, Tobben D, Pimentel M. How often do surgeons obtain the critical view of safety during laparoscopic cholecystectomy? Surg Endosc. 2017;31:142-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Nijssen MA, Schreinemakers JM, Meyer Z, van der Schelling GP, Crolla RM, Rijken AM. Complications After Laparoscopic Cholecystectomy: A Video Evaluation Study of Whether the Critical View of Safety was Reached. World J Surg. 2015;39:1798-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Strasberg SM, Brunt LM. The Critical View of Safety: Why It Is Not the Only Method of Ductal Identification Within the Standard of Care in Laparoscopic Cholecystectomy. Ann Surg. 2017;265:464-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Strasberg SM. Error traps and vasculo-biliary injury in laparoscopic and open cholecystectomy. J Hepatobiliary Pancreat Surg. 2008;15:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Mahmud S, Masaud M, Canna K, Nassar AH. Fundus-first laparoscopic cholecystectomy. Surg Endosc. 2002;16:581-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Gupta A, Agarwal PN, Kant R, Malik V. Evaluation of fundus-first laparoscopic cholecystectomy. JSLS. 2004;8:255-258. [PubMed] [Cited in This Article: ] |
| 66. | Tuveri M, Calò PG, Medas F, Tuveri A, Nicolosi A. Limits and advantages of fundus-first laparoscopic cholecystectomy: lessons learned. J Laparoendosc Adv Surg Tech A. 2008;18:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Alvarez FA, de Santibañes M, Palavecino M, Sánchez Clariá R, Mazza O, Arbues G, de Santibañes E, Pekolj J. Impact of routine intraoperative cholangiography during laparoscopic cholecystectomy on bile duct injury. Br J Surg. 2014;101:677-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Machi J, Johnson JO, Deziel DJ, Soper NJ, Berber E, Siperstein A, Hata M, Patel A, Singh K, Arregui ME. The routine use of laparoscopic ultrasound decreases bile duct injury: a multicenter study. Surg Endosc. 2009;23:384-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Osayi SN, Wendling MR, Drosdeck JM, Chaudhry UI, Perry KA, Noria SF, Mikami DJ, Needleman BJ, Muscarella P 2nd, Abdel-Rasoul M, Renton DB, Melvin WS, Hazey JW, Narula VK. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc. 2015;29:368-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 70. | Strasberg SM. A teaching program for the "culture of safety in cholecystectomy" and avoidance of bile duct injury. J Am Coll Surg. 2013;217:751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Santos BF, Brunt LM, Pucci MJ. The Difficult Gallbladder: A Safe Approach to a Dangerous Problem. J Laparoendosc Adv Surg Tech A. 2017;27:571-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Borzellino G, Sauerland S, Minicozzi AM, Verlato G, Di Pietrantonj C, de Manzoni G, Cordiano C. Laparoscopic cholecystectomy for severe acute cholecystitis. A meta-analysis of results. Surg Endosc. 2008;22:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Mangieri CW, Hendren BP, Strode MA, Bandera BC, Faler BJ. Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg Endosc. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 74. | Suzuki K, Bower M, Cassaro S, Patel RI, Karpeh MS, Leitman IM. Tube cholecystostomy before cholecystectomy for the treatment of acute cholecystitis. JSLS. 2015;19:e2014.00200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Elshaer M, Gravante G, Thomas K, Sorge R, Al-Hamali S, Ebdewi H. Subtotal cholecystectomy for "difficult gallbladders": systematic review and meta-analysis. JAMA Surg. 2015;150:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 76. | Strasberg SM, Pucci MJ, Brunt LM, Deziel DJ. Subtotal Cholecystectomy-"Fenestrating" vs "Reconstituting" Subtypes and the Prevention of Bile Duct Injury: Definition of the Optimal Procedure in Difficult Operative Conditions. J Am Coll Surg. 2016;222:89-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 156] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 77. | van Dijk AH, Donkervoort SC, Lameris W, de Vries E, Eijsbouts QAJ, Vrouenraets BC, Busch OR, Boermeester MA, de Reuver PR. Short- and Long-Term Outcomes after a Reconstituting and Fenestrating Subtotal Cholecystectomy. J Am Coll Surg. 2017;225:371-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 78. | Henneman D, da Costa DW, Vrouenraets BC, van Wagensveld BA, Lagarde SM. Laparoscopic partial cholecystectomy for the difficult gallbladder: a systematic review. Surg Endosc. 2013;27:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |