Published online Dec 15, 2016. doi: 10.4251/wjgo.v8.i12.805
Peer-review started: May 3, 2016
First decision: June 13, 2016
Revised: August 4, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: December 15, 2016
It is well established that colorectal cancer develops from a series of precursor epithelial polyps, including tubular adenomas, villous/tubulovillous adenomas (VA/TVA), sessile serrated adenomas (SSA) and traditional serrated adenomas (TSA). Of these, TSAs are least common and account for only 5% of all serrated polyps. TSAs are characterised by the presence of a “pinecone-like” architecture, granular eosinophilic cytoplasm, luminal serrations, ectopic crypt foci (ECF) and elongated, pencillate nuclei. However, the distinct slit-like luminal serrations, reminiscent of small bowel mucosa, appear to be the most unique and reproducible feature to distinguish TSAs from other polyps. There is a contention that TSAs are not inherently dysplastic and that the majority do not show cytological atypia. Two types of dysplasia are associated with TSA. Serrated dysplasia is less well recognised and less commonly encountered than adenomatous dysplasia. In addition, it is now becoming increasingly evident that TSAs can be admixed with HP, SSA and VA/TVA. At a genetic level, polyps may switch phenotype as they accumulate genetic changes, evolving from a serrated pathway to a more conventional one, which could be the basis for a spectrum theory starting out with a TSA with serration and ECF evolving into a TSA with conventional dysplasia and, eventually, to a well-developed conventional adenoma. Nevertheless, there is an exigency for future studies to provide further illumination and bridge the gaps in our present understanding.
Core tip: Traditional serrated adenoma (TSA) is the least common type of the serrated polyps and is characterized by a constellation of distinct cytomorphological features. TSAs are thought to be precursors to the biologically aggressive, BRAF mutated, microsatellite stable, colorectal cancer. It is becoming increasingly evident that TSAs can co-exist with other serrated polyps including hyperplastic polyps and sessile serrated adenomas. In addition, TSAs may also be seen with adenomatous polyps. In this review, we wish to highlight the issues around nomenclature, diagnostic criteria, coexistence with other polyp types, the occurrence of dysplasia and molecular pathways involved in the neoplastic progression of TSAs.
- Citation: Kalimuthu SN, Chelliah A, Chetty R. From traditional serrated adenoma to tubulovillous adenoma and beyond. World J Gastrointest Oncol 2016; 8(12): 805-809
- URL: https://www.wjgnet.com/1948-5204/full/v8/i12/805.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i12.805
It is well established that colorectal cancer (CRC) develops from a series of precursor epithelial polyps[1-5], which include conventional adenomas, incorporating tubular adenomas and villous/tubulovillous adenomas (VA/TVA) and serrated polyps, incorporating hyperplastic polyps (HP), sessile serrated adenomas (SSA) and traditional serrated adenomas (TSA)[1,2,4,6,7]. CRC is known to develop through three putative molecular pathways with the conventional adenoma-carcinoma sequence (chromosomal instability pathway), the mismatch repair and serrated pathways, accounting for the molecular pathogenesis of most CRCs[2,4]. VA/TVAs are thought to be the advanced precursors in the “adenoma-carcinoma” pathway[2]. Obversely, CRCs arising from serrated polyps (which can be visible at the edge or intimately associated with the invasive tumour), are thought to be portentous of the “serrated pathway”[1,4,8-10]. All three serrated polyps stay true to their epithet by characteristically demonstrating luminal serrations. Of these, TSAs are the least common and account for only 5% of all serrated polyps[1].
The historical provenance and journey taken to recognise TSAs in their present guise has been an interesting one. TSAs were first recognised by Longacre and Fenoglio-Preiser[11,12] and were grouped together under the broad term of “serrated adenomas”, as these polyps were thought to be conventional adenomas with a serrated luminal profile. Torlakovic et al[13] further refined this definition by appending the term “traditional” to the appellation, specifically to distinguish TSAs from SSAs and later highlighted several distinct morphological features specific to TSAs[14].
TSAs are equally distributed between the genders and usually present in the sixth to seventh decade of life[11]. They range from 9-14 mm in maximum dimension and endoscopically have a pinecone-like appearance or may exhibit a fernlike/stellate pit pattern on chromoendoscopy[11].
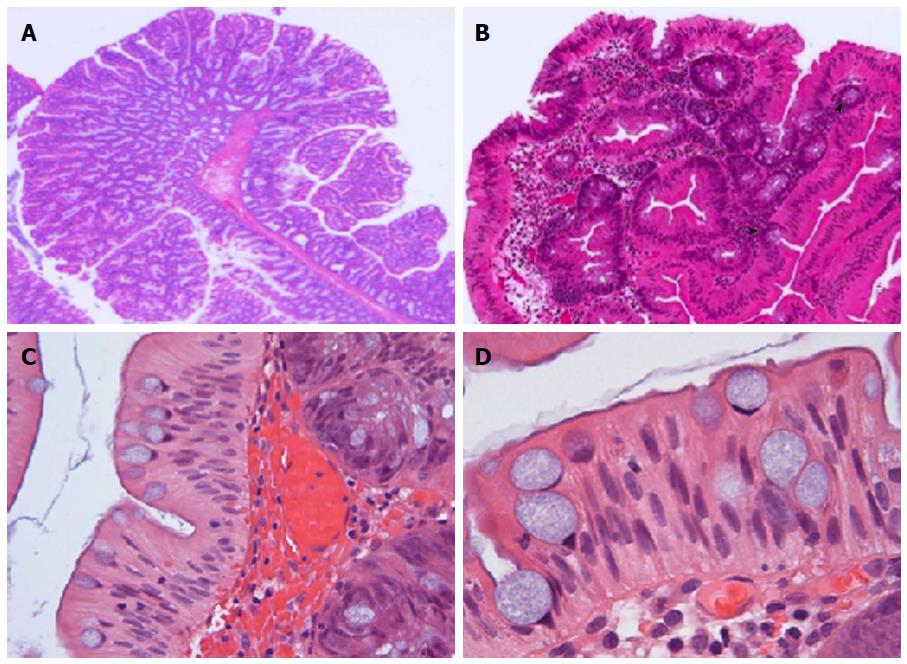
TSAs can be both sessile and pedunculated, the former being more common in the more proximal lesions[11]. Histologically, TSAs are exemplified by a constellation of characteristic cytomorphological features, which include a tubulovillous, “pinecone-like” architecture, striking granular eosinophilic cytoplasm, presence of ectopic crypt foci (ECF), distinct luminal serrations, elongated, pencillate nuclei with evenly dispersed chromatin and small inconspicuous nucleoli and haphazardly distributed goblet cells (Figure 1)[2,4,6,9,10,14]. Of these lineaments, the distinct slit-like luminal serrations with mushroom/jigsaw puzzle-like broad luminal fronds, reminiscent of small bowel mucosa, appear to be the most unique and reproducible feature to distinguish TSAs from other polyps[6,8,11]. Interestingly, Bettington et al[5] have recently described a “serrated” TVA, which occurs more frequently in a proximal location and in essence, morphologically resembles a conventional TVA but at least > 50% of the polyp displays prominent serrations. The authors argue that this represents a distinct entity, with more frequent KRAS mutations and CpG island methylation; however, we surmise that the “undulating” or “maze-like” serrations described may merely represent a morphological spectrum seen within TVAs and could possibly be secondary to mechanical compression due to luminal spatial constriction. This latter conjecture could possibly elucidate why this particular type of serration may, at least focally, be observed in larger polyps harbouring a villous configuration, albeit almost never seen in TSAs. Nevertheless, it is important to distinguish serrated TVA from TSA, particularly in the scenario when TSAs co-exist with conventional TVAs[6].
ECF have been defined as abnormal development of crypts, secondary to inactivating mutations in the bone morphogenetic protein 4 signalling pathway, causing loss of orientation towards the muscularis mucosae resulting in these short disorientated abortive crypts that fail to reach the muscularis mucosae (Figure 1)[2,6,14]. ECF were previously touted to be a prerequisite for the diagnosis of TSA[14]. However, these “maelstrom-like” or whorled clusters may not always be seen in TSAs[2,6,8,15], particularly in smaller lesions (< 10 mm) and have also been documented in VA/TVAs (34%)[2], albeit to a lesser frequency than observed in TSAs.
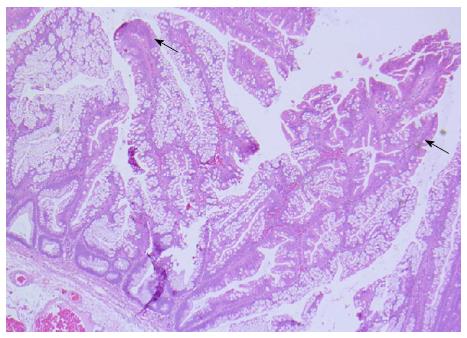
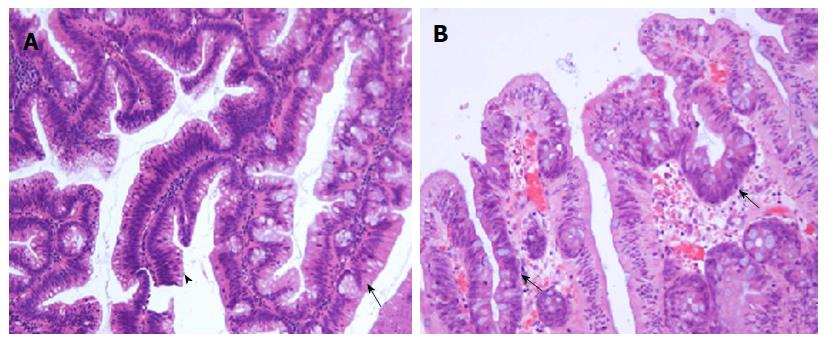
When most of the aforementioned hallmark features are represented, a diagnosis TSA can be made with minimal difficulty. However, it is becoming increasingly evident that TSAs can be admixed with HP, SSA and VA/TVA[1,2,6,16,17]. The documented rate of co-existent HP/SSA and VA/TVA with TSA range from 31%-52%, 14%-17% and 17%-43%[1,2,15,16], respectively. There is presently sufficient evidence to suggest that a significant proportion of TSAs contain precursor lesions in the form SSAs (Figure 2)[3,4,17], the former being more common of the two. These lesions are often seen intimately associated with the TSA. In contrast, the adenomatous polyps are sharply delineated from the TSAs and appear separate and morphologically distinct (Figure 3A). It is important not to over-document a co-existent adenomatous component when adenomatous dysplastic glands occur within a TSA (Figure 3). It is helpful to recognise that dysplastic glands in TSAs more or less retain the characteristic serrations and will be more replete with ECFs than expected in VA/TVAs.
The subject of dysplasia in TSA has long been a contentious one. Historically, TSAs were considered to be inherently dysplastic, owing to the close cytological resemblance to tubular adenomas or TVA[12]. However, this axiom has since been challenged and there is an alternate view proposed[8,10,11,15]. TSAs are unquestionably neoplastic; however, the absence of overt cytological atypia, infrequent or absent mitoses, low Ki-67 proliferation index, consistent B-catenin and p53 negativity, and retention of p16 staining, suggest TSAs are not intrinsically dysplastic[6,10,11,15]. Instead, the cells of TSAs may represent metaplastic or senescent cells[10,15]. It is noteworthy that there are two forms of dysplasia that can occur in TSAs and indeed in the other two serrated polyps[4,9,10,17]. The first is the well-accepted conventional adenomatous dysplasia, which can be readily recognised with minimal difficulty. The second, less well recognised and controversial, is serrated dysplasia. Serrated dysplasia manifests secondary to activation of the serrated pathway, which is initiated by BRAF mutations[4,7,10,11,18]. Similar to adenomatous dysplasia, this form of can be graded as low grade and high grade based on both cytological and architectural features. Low grade serrated dysplasia can be subtle and is characterised by ovoid enlarged nuclei, vesicular dispersed chromatin with low mitotic activity. In addition, scattered dystrophic goblet cells may also be observed[6,1,18]. In contrast, high grade dysplasia is readily recognisable as a result of severe cytological and architectural atypia. Currently, the biological significance of low grade serrated dysplasia is poorly understood. As such, in practice, it is recommended that only high grade serrated dysplasia be reported.
While the majority of the adenomatous polyps encountered may only display a single phenotype, in our experience, closer scrutiny often reveals isolated TSA-like glands or SSA/HP like areas. However, from a practical point of view, the sensible approach would be to name the polyp after the most dominant histological type, followed by any secondary or tertiary component identified with an accompanying comment as to the presence or absence of dysplasia.
At a molecular level, the morphological alteration in phenotype may possibly be secondary to the transition from the serrated pathway to a more conventional one. For example, when conventional dysplasia ensues in a TSA, it is possible that the Wnt signalling pathway typically associated with chromosomal instability alters its morphology[4]. Hence, the classic TSA with serrations and ECF, evolves into a TSA with conventional dysplasia and eventually, focally or entirely, resembling a conventional well-differentiated adenoma. This presupposes that molecular aberrations result in consistent morphological appearances. Jass et al[3] was the first to propose the theory where separate to the adenoma-carcinoma and serrated pathway, there may be “fusion” pathways that combine mechanisms associated with both adenomatous and serrated polyps[3,4]. However, further studies are required to consolidate this assertion.
Overall, it stands to reason that there is a considerable morphological overlap between different polyp types, albeit the molecular basis to which remains to be elucidated. Although conjectural, recent evidence raises the important question as to whether these polyps truly represent separate entities or are they merely manifestations at different stages of a morphological and/or molecular continuum. Nevertheless, there is an exigency for future studies to provide further illumination and bridge the gaps in our present understanding.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bettington M, Coriat R, Fiori E, Takeuchi Y S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Chetty R, Hafezi-Bakhtiari S, Serra S, Colling R, Wang LM. Traditional serrated adenomas (TSAs) admixed with other serrated (so-called precursor) polyps and conventional adenomas: a frequent occurrence. J Clin Pathol. 2015;68:270-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Hafezi-Bakhtiari S, Wang LM, Colling R, Serra S, Chetty R. Histological overlap between colorectal villous/tubulovillous and traditional serrated adenomas. Histopathology. 2015;66:308-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Jass JR, Baker K, Zlobec I, Higuchi T, Barker M, Buchanan D, Young J. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a “fusion” pathway to colorectal cancer. Histopathology. 2006;49:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, Klein K, Leggett B, Whitehall V. Serrated tubulovillous adenoma of the large intestine. Histopathology. 2016;68:578-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, Whitehall V, Leggett B. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol. 2014;38:158-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Bettington ML, Walker NI, Rosty C, Brown IS, Clouston AD, McKeone DM, Pearson SA, Klein K, Leggett BA, Whitehall VL. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Bateman AC. Pathology of serrated colorectal lesions. J Clin Pathol. 2014;67:865-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 10. | Bettington ML, Chetty R. Traditional serrated adenoma: an update. Hum Pathol. 2015;46:933-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 413] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65-81. [PubMed] [Cited in This Article: ] |
| 13. | Torlakovic EE, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, Snover DC. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol. 2008;32:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Kim MJ, Lee EJ, Suh JP, Chun SM, Jang SJ, Kim DS, Lee DH, Lee SH, Youk EG. Traditional serrated adenoma of the colorectum: clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol. 2013;140:898-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, Watanabe H. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5-9. [PubMed] [Cited in This Article: ] |
| 16. | Tsai JH, Liau JY, Lin YL, Lin LI, Cheng YC, Cheng ML, Jeng YM. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Lazarus R, Junttila OE, Karttunen TJ, Mäkinen MJ. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol. 2005;123:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol. 2006;125:132-145. [PubMed] [Cited in This Article: ] |