Published online Feb 15, 2019. doi: 10.4251/wjgo.v11.i2.139
Peer-review started: April 21, 2018
First decision: May 29, 2018
Revised: August 24, 2018
Accepted: October 23, 2018
Article in press: October 23, 2018
Published online: February 15, 2019
Long non-coding RNAs (lncRNAs) are a kind of single-stranded RNA of more than 200 nucleotides in length and have no protein-coding function. Amounting studies have indicated that lncRNAs could play a vital role in the initiation and development of cancers, including gastric cancer (GC). Considering the crucial functions of lncRNAs, the identification and exploration of novel lncRNAs in GC is necessary.
To identify independent prognostic markers for the whole gastroenteropancreatic neuroendocrine tumor (GEP-NET) group.
Ninety-three patients diagnosed with GEP-NETs within a specified period were included in this study. Patient data were retrospectively analyzed. The relationships between all independent variables and 5-year survival status calculated during the follow-up period (months) were assessed. In addition, the relationships between the independent variables were investigated.
When 5-year survival rate was compared, a statistically significant relationship between the age at diagnosis, male gender, tumor size, tumor stage, liver and/or distant metastasis, and tumor grade determined by the Ki-67 level and mitotic count, and the level of C-reactive protein (CRP), was observed. The mean survival (overall survival) of the study group was 102.5 ± 6.3 (SD) mo. The percentages of 1, 3 and 5-year survival were 90%, 72%, and 61%, respectively. In 63 of 93 patients, Ki-67 and the mitotic count determined the same grade. The Ki-67 levels in 29 patients and the mitotic count in only 1 patient were in the higher grade. The risk of death increased by 4% for every 1 year increase at the diagnosis age and was 2.0-fold higher for male patients, 3.0-fold higher for G3 according to the mitotic count, 3.7-fold higher for G3 according to the Ki-67 level, 12.7-fold higher for cases with tumor stage 3 or 4 by a 1 cm increase in the ratio of 9% in tumor size, and 6.1-fold higher for patients with liver metastasis for every 1 mg/dL increase in the ratio of 1.5% in CRP level. There was a significant difference between pancreatic and stomach NETs in favor of stomach tumors in terms of survival.
Tumor site, stage, grade and Ki-67 level affected patient survival, and it was observed that CRP affected disease progression (particularly if it was > 20 mg/dL). However, a relationship between surgical resection of the lesion and survival was not shown. Larger scale prospective studies are required to determine whether CRP level may be a poor prognostic factor for the entire GEP-NET group.
Core tip: Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) constitute a heterogeneous group of tumors with variable clinical presentations, different growth rates, and unpredictable prognoses. In our study, we aimed to identify the independent prognostic markers for the whole GEP-NET group. It was observed that the biochemical parameter, C-reactive protein (CRP), affected disease progression (particularly if it was > 20 mg/dL). However, larger scale prospective studies are required to determine whether CRP level may be a poor prognostic factor for the entire GEP-NET group.
- Citation: Komaç Ö, Bengi G, Sağol Ö, Akarsu M. C-reactive protein may be a prognostic factor for the whole gastroenteropancreatic neuroendocrine tumor group. World J Gastrointest Oncol 2019; 11(2): 139-152
- URL: https://www.wjgnet.com/1948-5204/full/v11/i2/139.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i2.139
Neuroendocrine tumors are a group of heterogeneous tumors that can develop in almost all locations in the body with the malignant transformation of various neuroendocrine cells rarely seen; however, their frequency is gradually increasing. These tumors are most commonly seen in the gastrointestinal system (GIS). This group of tumors can be defined by their different degrees of differentiation and slow growth rates, and can lead to clinical syndromes some due to excess secretion of hormones, which are functionally active and show a lower malignancy potential compared to most epithelial tumors[1]. The prognosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) is difficult to predict due to differences in tumor type, diversity of molecular mechanisms responsible for pathology, uncertainties in the effective oncogenic pathways and scarcity of large-scale and prospective randomized studies. In general, the mean time to diagnosis of these tumors is 5-7 years as they have vague symptoms and may not be correctly managed. The 5-year survival rate varies between 15% and 95% based on factors such as location of the primary tumor, changes in the tumor biology, extensiveness of the tumor at diagnosis, treatment options and competence of the center in which the patient receives treatment[2,3]. With the increase in treatment perspectives, it is necessary to determine the best treatment approach in patients with GEP-NETs. Therefore, the determination of prognostic factors affecting patient survival, achieving standardization, early identification of patients with tumors which have an aggressive course and treatment planning are essential.
It is thought that C-reactive protein (CRP) level, an indicator of inflammation, markedly increases in hematopoietic and some solid malignancies, and plays an important role in disease pathogenesis and progression[4,5]. The prognostic importance of CRP level has been shown in malignancies such as pancreatic and esophageal adenocarcinoma, soft tissue sarcoma and chronic lymphocytic leukemia[6-8].
In this pioneering study, we aimed to identify the independent prognostic markers for the whole GEP-NET group.
Ninety-three patients aged 18 years and over diagnosed with a GEP-NET who underwent surgical resection and/or non-surgical treatment in Dokuz Eylul University Hospital between January 2002 and June 2012 were included in this study. The patients’ data at the time of diagnosis were retrospectively reviewed.
Independent variables such as gender, age, primary tumor location, tumor diameter, non-surgical treatment, surgical resection, liver metastasis, extrahepatic distant metastasis, tumor stage (according to the AJCC 2010 criteria, TNM)[9], tumor grade determined according to the mitotic count and Ki-67 level (WHO 2010 criteria, as grade 1-2-3)[10], hemoglobin (HGB) level (g/dL), anemia (WHO criteria M < 13, F < 12 g/dL), albumin (ALB) level (g/dL), hypoalbuminemia (WHO criteria, < 3.5 g/dL), lactate dehydrogenase (LDH) level (U/L), CRP level (mg/L), elevated CRP (< 5 mg/L, between 5-20 mg/L, > 20 mg/L), erythrocyte sedimentation rate (ESR) (mm/h), and ESR elevation (Westergren method) were recorded.
Tissue sections were immunostained with the Ki-67 antibody (Ventana; anti-Ki67 30-9). The Ki-67 labeling index was calculated as a percentage of the Ki-67 immunoreactive cells from a total of 2000 neoplastic cells counted in hotspot areas. The counts were manually performed from camera images. The mitotic index was calculated as the number of mitotic cells in at least 50 high power fields in hotspot areas. Other diseases affecting the patients’ data (such as CRP) were screened and data determined to be affected were not included in the study.
Dependent variables were designated as the 1, 3 and 5-year survival rate and time (months) from diagnosis date to outcome date. In order to create a 5-year follow-up period, patients diagnosed after June 15, 2012, were not included in the study. The date of death for patients who died during the study was determined as the outcome date, and was established as June 15, 2017, for the other patients.
As some tissue samples were smaller than 1 cm and small quantities of material were obtained, patients diagnosed by endoscopic excisional biopsy, endoscopic incisional biopsy, fine-needle aspiration biopsy, and full layer biopsy were classified as the non-surgical group.
The SPSS 22 program was used for statistical analysis. The descriptive statistics, figures, and percentages for the categorical variables, mean and standard deviation values for the numeric variables were determined. Crosstabs were created for the categorical data, and the Chi-Square test was used in the multiple and pairwise group comparisons. The Kaplan-Meier method was used for the survival analyses. Spearman’s rho correlation analysis was carried out for independent variables. The factors affecting survival were determined and multivariate models were created. The Cox proportional hazards test was performed to determine the prognostic values of these factors. The ROC curve analysis was carried out using the numerical variables affecting survival. Sensitivity and specificity levels were determined and cut-off values were calculated[11,12]. In the single-variable analysis using the log-rank test; the age, gender, tumor diameter, multiple metastases, liver metastasis, CRP level, Ki-67 grade, mitosis grade, tumor grade, and clinical stage were significantly related with survival. As the independent variables showing a significant relationship between each other will change the model direction, the independent variables for the multivariate model were tested. Age and gender were distributed normally and the other independent variables did not show a significant relationship with multiple metastases, liver metastasis, Ki-67 grade, mitosis grade, tumor grade, CRP level and none of the clinical stage variables. However, Ki-67 grade, mitosis grade, tumor grade and clinical stage variables were significantly related. Both liver metastasis and multiple metastases were also related to each other. A model to explain death containing both the independent variables and showing a significant relationship with each other would be faulty. Therefore, the Cox proportional hazards model was constructed by adding each parameter (Ki-67 grade, mitotic index grade, tumor grade, clinical stage, liver metastasis, CRP level, tumor diameter) individually to the age and gender variables. When the alpha level of significance was below 0.05, it was accepted as significant. The ROC curve analysis was carried out using survival status and CRP level. The sensitivity and specificity levels were determined and cut-off values were calculated for CRP. This study was approved by the non-invasive clinical research ethics committee of Dokuz Eylül University Faculty of Medicine (22.06.2017/2017/17-48). Patient information was confidential and the study was conducted according to the Helsinki declaration.
The mean age of the whole group at the time of diagnosis was 53.45 ± 13.46 years. The youngest patient included in the study was 18 and the oldest was 83 years. A significant negative relationship was determined between patient age at the time of diagnosis and 5-year survival (P = 0.019). 55% of patients were female. A significant relationship was determined between female gender and 5-year survival (P = 0.014). The mean primary tumor diameter was 3.1 ± 3.45 cm. A significant positive relationship between tumor diameter and 5-year survival was observed (P = 0.013). The relationships between the patients’ demographic data and numeric independent variables and 5-year survival are provided in Table 1.
| Patient group, n = 93 | 5-yr survival | P value | ||
| Yes (alive), n = 57 | No (dead), n = 36 | |||
| Age (yr) | Mean: 53.45 | Mean: 50.9 | Mean: 57.4 | 0.02 |
| SD: 13.46 | SD: 12.2 | SD: 13.6 | ||
| Gender (F/M) | F 54.8% | F 64.9% | F 38.9% | 0.01 |
| M 45.2% | M 35.1% | M 61.1% | ||
| Tumor diameter (cm) | Mean: 3.1 | Mean: 2.39 | Mean: 4.20 | 0.01 |
| SD: 3.45 | SD: 3.56 | SD: 3.03 | ||
| Hgb level (g/dL), n = 89 | Mean: 12.21 | Mean: 12.31 | Mean: 12.05 | 0.54 |
| SD: 1.99 | SD: 1.86 | SD: 2.22 | ||
| Albumin level (g/dL), n = 87 | Mean: 4.08 | Mean: 4.16 | Mean: 3.94 | 0.07 |
| SD: 0.53 | SD: 0.56 | SD: 0.44 | ||
| LDH level (U/L), n = 83 | Mean: 291.1 | Mean: 255.6 | Mean: 347.5 | 0.11 |
| SD: 213.1 | SD: 95.11 | SD: 316.5 | ||
| CRP level (mg/L), n = 72 | Mean: 22.5 | Mean: 14.63 | Mean: 37.31 | 0.02 |
| SD: 33.8 | SD: 25.48 | SD: 42.25 | ||
| ESR (mm/h), n = 63 | Mean: 37.7 | Mean: 33.905 | Mean: 45.429 | 0.09 |
| SD: 25.9 | SD: 24.3329 | SD: 27.8039 | ||
The mean HGB level was 12.21 g/dL ± 1.99 and mean plasma ALB level was 4.08 g/dL ± 0.53. The mean LDH level was 291.1 U/L ± 213.1 (SD), the lowest LDH level was 109 U/L and the highest LDH level was 1659 U/L. The mean ESR was 37.7 mm/h ± 25.9. No statistically significant differences were found between the 5-year survival and HGB, ALB, LDH and ESR levels in these patients (P = 0.54, P = 0.07, P = 0.11, P = 0.09). The mean CRP level was 22.5 mg/dL ± 33.8, and a statistically significant relationship between CRP level and 5-year survival was observed (P = 0.02).
The mean survival (MS) time of patients was 102.5 ± 6.3 mo. The 1, 3 and 5-year survival percentages were determined to be 90%, 72%, and 61%, respectively. There were no statistically significant relationships between non-surgical treatment, surgical resection or both procedures (17 patients) and 5-year survival (P = 0.25, P = 0.62, P = 0.38). The same tumor grade was determined in 13 of these 17 patients, and the resected material predicted a higher grade in 4 patients. A strong negative relationship between 5-year survival and liver metastasis and between 5-year survival and extrahepatic distant metastasis (P < 0.001, P < 0.001) was determined. According to tumor stage, the MS was 132.8 ± 4.3 mo in stage 1 and 2 patients and was 69.7 ± 8.5 mo in stage 3 and 4 patients. When the mitotic count was taken into account when determining the tumor grade, the MS was 111 ± 6.2 mo in G1 patients and was 35.1 ± 11.3 mo in G3 patients. When the Ki-67 level was taken into account, the MS was 124.6 ± 6.1 (SD) mo in G1 patients and was 54.4 ± 12.7 (SD) in G3 patients (P < 0.001). The Ki-67 level was numerically stated (30%, 37%, 45%, 80%, and 90%) in 5 of 20 G3 patients, and the Ki-67 level in 15 patients was categorically stated (> 20%). Consistency was achieved in 63 (68%) patients between the tumor grades determined according to the mitotic count and Ki-67 level, and the mitotic count predicted a higher grade than the Ki-67 level in 29 of the remaining 30 patients.
The MS was 92.9 ± 8.7 mo (P = 0.34) in anemic patients, 91 ± 15.7 mo (P = 0.60) in those with hypoalbuminemia, 93.5 ± 9.5 mo (P = 0.28) in those with elevated ESR, 118.1 ± 8.2 mo in those with a CRP level < 5 mg/L, 118.6 ± 12.2 mo in those with a CRP level between 5 and 20 mg/L, and 72.3 ± 10.5 mo in those with a CRP level > 20 mg/L (P = 0.009). The relationships between the patients’ categorical independent variables and survival times are shown in Table 2.
| Mean survival (mo) | Standard deviation (± mo) | 1-yr survival (%) | 3-yr survival (%) | 5-yr survival (%) | P value | ||
| All patients | n = 93 | 102.5 | 6.3 | 90 | 72 | 61 | |
| Non-surgical (non-invasive) group | Yes (n = 56) | 101.9 | 7.3 | 91 | 75 | 66 | 0.25 |
| No (n = 37) | 93.2 | 10.3 | 89 | 67 | 54 | ||
| Surgical resection | Yes (n = 54) | 99.6 | 8.4 | 89 | 70 | 59 | 0.62 |
| No (n = 39) | 97 | 8.5 | 92 | 74 | 64 | ||
| Liver metastasis | Present (n = 38) | 61.3 | 9.5 | 81 | 47 | 29 | < 0.001 |
| Absent (n = 55) | 122.6 | 5.5 | 96 | 89 | 83 | ||
| Extrahepatic metastasis | Present (n = 23) | 32 | 5.8 | 78 | 30 | 08 | < 0.001 |
| Absent (n = 70) | 124 | 6 | 94 | 85 | 78 | ||
| Stage | 1 (n = 35) | 132.8 | 4.3 | 100 | 94 | 91 | < 0.001 |
| 2 (n = 7) | 100 | 100 | 100 | ||||
| 3 (n = 13) | 69.7 | 8.5 | 84 | 70 | 54 | ||
| 4 (n = 38) | 81 | 47 | 29 | ||||
| Grade mitotic count | 1 (n = 62) | 111 | 6.2 | 97 | 84 | 72 | < 0.001 |
| 2 (n = 21) | 87.1 | 14.4 | 81 | 57 | 52 | ||
| 3 (n = 10) | 35.1 | 11.3 | 70 | 30 | 10 | ||
| Grade Ki-67 level | 1 (n = 43) | 124.6 | 6.1 | 97 | 90 | 86 | < 0.001 |
| 2 (n = 30) | 83.9 | 9.9 | 90 | 66 | 50 | ||
| 3 (n = 20) | 54.4 | 12.7 | 75 | 40 | 25 | ||
| Anemia | Present (n = 42) | 92.9 | 8.7 | 90 | 71 | 57 | 0.34 |
| Absent (n = 47) | 110 | 8.6 | 90 | 76 | 68 | ||
| Hypoalbuminemia | Present (n = 10) | 91 | 15.7 | 100 | 70 | 60 | 0.60 |
| Absent (n = 77) | 105.4 | 6.8 | 89 | 75 | 63 | ||
| ESR elevation | Present (n = 30) | 93.5 | 9.5 | 93 | 76 | 60 | 0.28 |
| Absent (n = 33) | 117.7 | 9.2 | 93 | 81 | 72 | ||
| CRP elevation (mg/L) | < 5 (n = 28) | 118.1 | 8.2 | 100 | 85 | 78 | 0.009 |
| 5-20 (n = 20) | 118.6 | 12.2 | 90 | 80 | 75 | ||
| > 20 (n = 24) | 72.3 | 10.5 | 83 | 66 | 41 |
The correlations between the independent variables are displayed in Table 3. A statistically significant correlation of varying levels was observed between the tumor grades determined according to tumor diameter, tumor stage, mitotic count and Ki-67 level. As the mitotic count predicted a higher grade in only 1 of 93 patients, there was a statistically significant excellent correlation (99%) between the tumor grades calculated based on the Ki-67 level (P < 0.001). There was also a statistically significant correlation between CRP levels and tumor diameter and tumor stage. No statistically significant correlations were found between tumor grades based on the Ki-67 level or mitotic count and CRP level. The five-year survival rate is shown in Table 4 according to primary tumor locations. The most frequent primary organs involved were the stomach, pancreas, and colon, respectively. The survival percentages were determined to be 79%, 48%, and 54%, respectively. The survival difference between pancreatic and stomach NETs is shown in Table 5. A statistically significant difference was noted between the two tumor groups in terms of survival (P = 0.016).
| Correlation coefficients (r) | Tumor diameter (cm) | Tumor stage | Tumor grade (mitotic index) | Tumor grade (Ki-67 level) | Tumor grade (determined) | CRP (mg/L) |
| Tumor diameter (cm) | - | |||||
| Tumor stage | 0.76b | - | ||||
| Tumor grade (mitotic count) | 0.37b | 0.39b | - | |||
| Tumor grade (Ki-67 level) | 0.59b | 0.55b | 0.75b | - | ||
| Tumor grade (determined) | 0.57b | 0.53b | 0.78b | 0.99b | - | |
| CRP (mg/L) | 0.25a | 0.26a | 0.22 | 0.17 | 0.17 | - |
| Tumor location, n = 93 | N | % | Survivors at the end of 5 yr (%) |
| Appendix | 3 | 3.2 | 100 |
| Duodenum | 2 | 2.2 | 100 |
| Ileum | 7 | 7.5 | 43 |
| Liver | 2 | 2.2 | 50 |
| Colon | 13 | 14 | 54 |
| Stomach | 29 | 31.2 | 79 |
| Esophagus | 1 | 1.1 | 0 |
| Pancreas | 25 | 26.9 | 48 |
| Periampullary | 7 | 7.5 | 43 |
| Rectum | 4 | 4.3 | 75 |
| Tumor location, n = 54 | 5-yr survival | P value | ||
| Yes, n = 35 | No, n = 19 | |||
| Stomach | 53.7% | 65.7% | 31.5% | 0.016 |
| Pancreas | 46.3% | 34.3% | 68.5% | |
The risk of death in unit changes for determined variables are displayed in Table 6. The Cox proportional hazards model was created using patient age, gender, mitotic count or tumor grade calculated according to the Ki-67 level, tumor diameter, tumor stage and presence of liver metastasis. Each 1-year period showed an increased risk of death of 4% and was 2.0-fold higher in males than in females. The risk of death was 3.0-fold higher in patients with G3 tumors compared to G1 or G2 tumors when based on the mitotic count, 3.7-fold higher in patients with G3 tumors compared to G1 or G2 tumors when based on the Ki-67 level, 3.7-fold higher compared to G1 or G2 tumors when G3 was indicated in the report, and 12.7-fold higher than those with a tumor stage of 3-4 or 1-2. For each 1 cm increase in tumor diameter the risk of death increased by 9%, and increased 6.1-fold in the presence of liver metastasis.
| Significance level (P) | Relative increase in risk of death according to unit change (fold) | 95%CI | ||
| Lower limit | Upper limit | |||
| Age (yr) | 0.041 | 1.04 | 1.007 | 1.066 |
| Gender (M/F) | 0.015 | 2.0 | 1.028 | 3.976 |
| Tumor diameter (cm) | 0.02 | 1.09 | 1.014 | 1.169 |
| Tumor grade (Ki-67) (3/1-2) | < 0.001 | 3.6 | 1.873 | 7.206 |
| Tumor grade (mitosis) (3/1-2) | 0.006 | 3.0 | 2.823 | 13.362 |
| Determined tumor grade (3/1-2) | < 0.001 | 3.6 | 1.873 | 7.206 |
| Tumor stage (3-4/1-2) | < 0.001 | 12.7 | 3.869 | 41.708 |
| CRP level (mg/L ) | 0.005 | 1.01 | 1.022 | 1.253 |
| Liver metastasis (P/A) | < 0.001 | 6.1 | 2.823 | 13.362 |
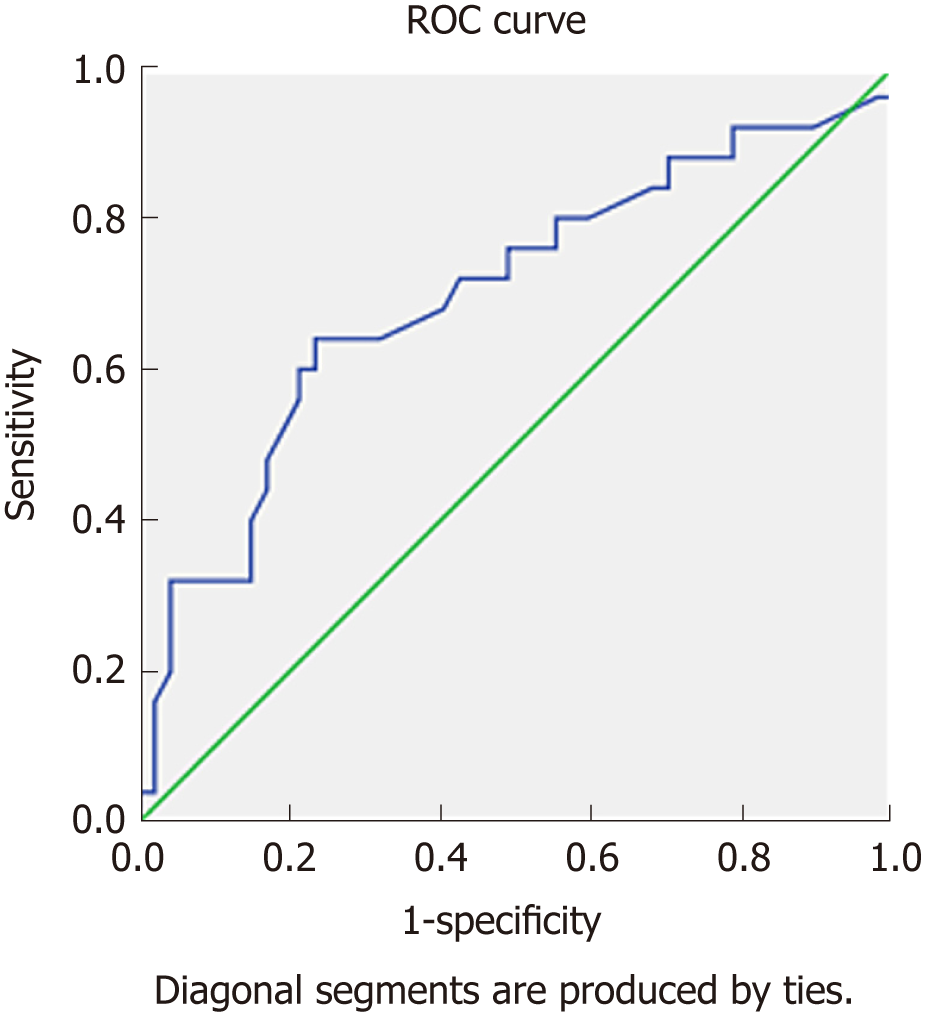
The sensitivity and specificity were determined to be 80% and 45%, respectively, when determining mortality over the 5-year follow-up period when the CRP “cut off” value was 3.85 mg/L; the sensitivity and specificity were 76% and 47%, respectively, when the CRP “cut off” value was 5 mg/L (the universal “cut off” value for CRP), and the sensitivity and specificity were 56% and 79% when the CRP “cut off” value was 20 mg/L. It was also shown that a CRP level over 20 mg/L increased the risk of death by 3.2-fold. As shown in Figure 1, 0.7 units remained under the curve.
Despite the general assumption that GEP-NETs are quite rare, benign and slow-growing tumors, it was shown that GEP-NETs were more prevalent than thought and were serious in some patients. Due to the nonspecific nature of the symptoms and findings, these tumors are generally misinterpreted and diagnosis is delayed. Consequently, metastases are seen in approximately 65% of commonly seen GEP-NET groups[13]. Although anatomic imaging is useful for accurate diagnosis, the lack of sensitive and specific plasma or genetic markers that can be used to screen early lesions or micrometastases poses a major obstacle in the diagnosis and treatment of these tumors. Therefore, increasing the number and reliability of available prognostic and diagnostic factors is important in the correct management of patients[14].
In patients with GEP-NETs, 5-year survival varies between 15% and 95% based on location of the primary tumor, changes in the tumor biology, extensiveness of the tumor at the time of diagnosis, treatment options and competence of the center in which the patient is treated[2,3]. The 5-year survival in the heterogeneous tumor group in our study was similar to that in the literature at 61%.
In a meta-analysis conducted by Jensen et al[15], increased age at the time of diagnosis, male gender, the presence of liver metastasis and increased tumor diameter were shown to indicate a poor prognosis. Similar results were also observed in our study. These parameters may represent poor prognosis in all tumor groups; however, it is not yet known why male gender increases the risk of death in the GEP-NET group, and males making up 56% of the patients in the stage 4 group in our study may be the reason for this finding. As stated in the meta-analysis by Yao et al[2], increased tumor grade, a decrease in tumor differentiation and the presence of distant metastasis also indicated poor prognosis, which was also found in our study. However, these prognostic factors are not unique to NETs.
In a study on GEP-NETs, it was shown that the risk of metastasis development increased as tumor grade increased, and tumor differentiation decreased[16]. Similarly, in our study, a positive moderately significant correlation was observed between tumor stage and grade (P < 0.001 rho =0.53). It was seen that this correlation rose to 97% particularly between stage 3-4 and G3 tumors. Thus, the parameters defining tumor grade are good indicators of the proliferative process and prognosis.
In a study in which the effect of tumor grade on prognosis was evaluated in metastatic GEP-NETs[16], 5-year survival was found to be 87% in patients with G1 tumors, 38% in those with G2 tumors, and 0% in those with G3 tumors. In our study, 5-year survival in the metastatic group was 66% in patients with G1 tumors, 25% in those with G2 tumors and 8% in those with G3 tumors. Thus, the stage and grade have an impact on survival independent of each other.
It is known that the organ in which metastases are most frequently seen in the GEP-NET group is the liver. In a study on GEP-NETs, the rate of liver metastasis in stage 4 patients was 89%[16], and in our study, the rate was 100%. This can be explained by invasive GEP-NET cells reaching the liver first via the portal venous system.
In another study, the Ki-67 level determined in advanced stage pancreatic NETs (PNET) was the most important prognostic factor, and it was stated that the presence of liver metastasis independent of the Ki-67 level was also an important determinant of survival[17]. In our study, when the whole GEP-NET group was evaluated, a 3.6-fold increase in the risk of death was observed in patients with G3 tumors according to the Ki-67 level compared to those with G1-G2 tumors, and the risk of death increased to 6.1-fold in the presence of liver metastasis. This indicates that the Ki-67 level and the presence of liver metastasis are important prognostic factors in the whole GEP-NET group. In a study of well-differentiated GEP-NETs[15], contrary to our findings, the results showed that the Ki-67 level reflected the proliferative activity, but did not affect survival.
Some studies have indicated that the mitotic count in the GEP-NET group had prognostic significance close to that of the Ki-67 level[18,19], and other studies have shown that the Ki-67 level is a stronger prognostic factor[20]. In a study of GEP-NET patients, it was shown that the Ki-67 level indicated high grade tumors in 87% of cases and this was verified by the survival data when concordance between these two parameters could not be achieved. In our study, the Ki-67 level also predicted higher grade tumors in 29 patients. The mitotic count can be affected by conditions such as tissue fixation and sample thickness, indistinguishability of apoptotic cells during counting and the possibility of counting only a small part of the cells in the proliferative phase. In the same study[21], concordance could not be achieved between the Ki-67 level and mitotic count with respect to tumor grade in nonsurgical samples in 65% of cases, and this was due to the small sample size and the possibility of erroneous sample collection from tumor tissue with a low metabolic ratio. In another study, the cytologic and histologic materials from 27 GEP-NET patients were evaluated, and the same tumor grades were determined[22]. In our study, nonsurgical samples in 16 of 30 cases were discordant, and no significant differences were observed between these samples.
In the study by Sorbye et al[23], when G3 NETs were divided into two groups using a cut-off value of 55% for the Ki-67 level, a 4-month significant statistical difference was found in terms of survival and response to treatment. In another similar study, it was shown that survival without progression decreased as the Ki-67 level increased independently of disease stage[20]. In our study, while the Ki-67 level determined G3 tumors in 5 of 20 patients and was numerically indicated (30%, 37%, 45%, 80%, 90%), the Ki-67 value in the remaining 15 patients was categorically (> 20%) indicated. The 5-year survival of these five patients was 0%; however, as the numeric Ki-67 levels in the other patients were not available, a statistical analysis of Ki-67 level increase and survival could not be performed.
In another study, it was demonstrated that the tumor grade of NETs was a progression marker of higher priority than tumor stage[20]; however, in our study, although the confidence interval was wide, it was shown that in patients with tumor stage 3 or 4, the risk of death was 12.7-fold higher and the risk of death in patients with grade 3 tumors increased 3.6-fold. Therefore, tumor stage has more prognostic importance than tumor grade.
In a study of well-differentiated GEP-NETs, while lymph node positivity was determined in 44% of ileal NETs smaller than 1 cm, lymph node metastasis was not found in any of the appendix NETs smaller than 1 cm; these findings indicated that tumor dimensions provided information on clinical course; however, tumor location was also important[24]. In our study, lymph node metastasis was found in only 6% of 34 cases with a tumor diameter 1 cm or less. This may have been caused by the heterogeneity of our study group and early-stage stomach NET determined coincidentally by endoscopy in 22 patients[25].
The only curative treatment for GEP-NETs is surgical resection[14]; however, in our study, no significant relationships were determined between nonsurgical treatment, surgical resection and survival (P = 0.25, P = 0.62). The reason for this may have been due to the heterogeneity of the groups who underwent nonsurgical treatment and those who underwent surgical resection. For example, while nonsurgical treatment was used only for diagnostic purposes in the advanced stage inoperable patient group, it was a curative method in patients with early-stage stomach tumors. Surgery for palliative or diagnostic purposes in patients who underwent resection might have altered the expected survival relationship.
A study[11] showed that primary NETs were most commonly found in the GIS (about 60% of all cases), followed by the bronchopulmonary system (27%); and these tumors were most frequently seen in the small intestine (34%), followed by the rectum (23%), colon (19%), stomach (7.7%), pancreas (7.5%) and appendix (6.6%). In our study, NETs were most frequently found in the stomach (31.2%), pancreas (26.7%), colon (14%) and ileum (7.5%). The reasons for this difference may have been due to an inability to determine some of the GEP-NETs that we had planned to include in the study, frequent detection of incidental stomach NETs, our institution being a reference center for pancreas fine needle aspiration sampling by EUS, and difficulties in sampling ileal NETs using endoscopic imaging.
In the study by Grin et al[26] on GEP-NETs, it was asserted that endoscopic excisional biopsy was generally sufficient for stomach tumors smaller than 1 cm, and which rarely show malignant progression. These authors indicated that the risk of malignant progression in the same group of tumors gradually increased with tumor diameter. In our study, while the 5-year survival of patients with a tumor diameter less than 1 cm was 90%, 5-year survival was 40% in patients with stomach NETs greater than 1 cm (P = 0.01).
When the survival relationship between pancreatic and stomach NETs was evaluated, a statistically significant difference in favor of stomach NETs was determined between the two tumor groups with regard to survival (P = 0.016). This result was also found in another study[27], and may have been due to the determination of nonfunctional PNETs at a more advanced stage, their progression due to being generally less differentiated and an increased number of early-stage stomach NETs incidentally determined due to an increase in the frequency of endoscopic imaging.
In a recent study by Freis et al[28] involving 100 GEP-NEC patients, a relationship between elevated lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) levels and survival was observed. No relationships were found between blood hemoglobin (HGB) and albumin (ALB) levels and survival. Erythrocyte sedimentation rate (ESR) was not evaluated in the study. In our study, no relationships were identified between LDH, ALB, HGB and ESR levels and survival (P = 0.11, P = 0.07, P = 0.28, P = 0.09, respectively).
CRP is an acute phase protein produced in the liver. The acute phase response is stimulated by IL-6 released from macrophages and T cells. Any acute or chronic inflammatory conditions may cause an increase in CRP due to activation of IL-6. These conditions include infections, autoimmune diseases, and malignancies. As a result, CRP is a sensitive, but nonspecific marker[29]. For this reason, the CRP level in 21 patients with elevated CRP, which was found to be due to non-tumor related causes was not taken into account in this study. In our study, a significant negative relationship between increased CRP level and survival was observed for the whole GEP-NET group (P = 0.009). The mean survival was 118.1 mo in the patient group with a CRP level < 5 mg/L, was 118.6 mo in the group with a CRP level between 5 and 20 mg/L and was 72.3 mo in the group with a CRP level > 20 mg/L. It was noted that each increase in the CRP value of 1 mg/L increased the risk of death by 1.5%. It was observed that the risk of death increased 3.2-fold when the CRP level was greater than 20 mg/L, and when this value was taken as the cut-off, its sensitivity was 56% and specificity was 79% for the occurrence of death. According to these results, the CRP level, especially above 20 mg/L, is a prognostic factor for the entire GEP-NET group. This was shown for the entire GEP-NET group for the first time. Wiese et al[30] in a study of PNET patients, showed a relationship between CRP level at the time of diagnosis and survival, and indicated that a high CRP value may be an independent prognostic factor in PNET patients. Moreover, when a correlation between the Ki-67 level and survival was not determined, CRP level was found to be an independent prognostic factor for the whole GEP-NET group.
This study was retrospective and thus did not take into account treatment methods other than tumor resection, did not evaluate tumor functionality status, did not determine some patient data such as the indication in the GEP-NET group according to the current ICD-10 diagnostic coding (for example; if a malignant neoplasm of the stomach diagnostic code was assigned to a patient with stomach NET, the patient may be evaluated as having stomach adenocarcinoma), not using the planned statistics according to tumor location, which was low in some sub-groups and Ki-67 level was not specified in the pathological data, especially in patients evaluated before 2010. These factors are limitations of the present study.
Throughout this study, a significant relationship was observed between age at diagnosis, gender, tumor size, tumor stage, tumor grade specified individually according to Ki-67 and mitotic count, the presence of liver metastasis and extrahepatic distant metastasis with survival as indicated in the literature. Nevertheless, it was shown that tumor location can also affect survival, especially in patients with stomach and pancreatic NETs. Our study differs from other studies in the literature in that tumor stage was also found to be an important parameter, at least as important as tumor grade. In addition, different to other studies, the prognostic effects of Ki-67 level and mitotic count were similar in our study. The Ki-67 level was an important grading parameter and a more efficient prognostic factor as it determined the tumor grade in almost all of our patient group, increased the risk of death compared to the mitotic count and had a higher error margin in terms of the technical aspects while calculating the mitotic count.
In conclusion, there are small-scale studies in which patients were included according to the involved sites, the presence of metastasis, stage or grade in the literature; however, the number of studies approaching the GEP-NET group holistically is very few. As it is known that all GEP-NETs can metastasize independent of tumor grade and stage, follow-up parameters that can be used for the whole GEP-NET group and factors that affect clinical course and determine the confidence level of the available parameters are important. It was shown in our study that the CRP level negatively affected the disease course, especially in patients with CRP levels > 20 mg/dL. We propose that the CRP level is an independent prognostic factor in the whole GEP-NET group. Further prospective and controlled studies are required to determine whether patients progress according to the CRP level at the time of diagnosis to ensure appropriate treatment planning, and CRP level can also be monitored during follow-up.
Neuroendocrine tumors develop as a result of the malignant transformation of neuroendocrine cells, they can be found in all areas of the body and their frequency is gradually increasing. They are most commonly seen in the gastrointestinal system (GIS), and may cause various clinical syndromes with different malignancy potentials. In general, these tumors are less malignant than epithelial tumors. The 5-year survival varies between 15% and 95% based on factors such as location of the primary tumor, changes in the tumor biology, extensiveness of the tumor at the time of diagnosis and treatment options. The determination of a high C-reactive protein (CRP) level, a marker of inflammation, in hematopoietic and some solid malignancies suggests a poor prognosis, and may have prognostic significance. It has been shown in several studies that the CRP level has prognostic importance in malignancies such as pancreatic adenocarcinoma, soft tissue sarcoma, and chronic lymphocytic leukemia.
The prognosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) is difficult to predict due to differences in tumor type, the diversity of molecular mechanisms responsible for pathology, a scarcity of large-scale and prospective randomized studies, and the possible development of various clinical syndromes. In general, the mean time to diagnosis of these tumors is 5 to 7 years as they have vague symptoms and may not be correctly managed. Therefore, the determination of prognostic factors affecting survival and achieving standardization of these tumors is important in terms of early identification due to their aggressive course, and treatment planning.
To specify the survival status and time of the patients included in this study, the relationship between tumor location, tumor extensiveness, size, pathological characteristics, sampling method and laboratory data were evaluated. The prognostic importance of certain variables were also assessed.
Ninety-three patients aged 18 years and over, diagnosed with GEP-NETs who underwent surgical resection and/or non-surgical treatment in Dokuz Eylul University Hospital between January 2002 and June 2012 were included in this study. Patient data at the time of diagnosis was retrospectively reviewed. Independent variables such as demographic, radiological, surgical, pathological and specific laboratory data were assessed and recorded. Dependent variables were designated as the 1, 3 and 5-year survival and time (months) from diagnosis date to outcome date. In order to create a 5-year follow-up period, patients diagnosed after June 15, 2012, were not included in the study. The date of death in patients who died during the study was determined as the outcome date, and was June 15, 2017 for the other patients. As some tumor diameters were smaller than 1 cm and small quantities of material were obtained, the patients diagnosed by the endoscopic excisional biopsy, endoscopic incisional biopsy, fine-needle aspiration biopsy, and full layer biopsy were classified as the non-surgical group.
A significant negative relationship was observed between patient age at the time of diagnosis and 5-year survival (P = 0.019). 55% of patients were female. A significant relationship between female gender and 5-year survival was determined (P = 0.014). The mean primary tumor diameter was 3.1 ± 3.45 (SD) cm. A significant positive relationship between tumor diameter and 5-year survival was observed (P = 0.013). The mean CRP level was 22.5 mg/dL ± 33.8. There was a statistically significant relationship between CRP level and 5-year survival (P = 0.02). The mean survival (MS) time was (overall survival) 102.5 ± 6.3 mo. The 1, 3, and 5-year survival rates were 90%, 72%, and 61%, respectively. A strong negative relationship was observed between survival and both liver metastasis and and extrahepatic distant metastasis (P < 0.001, P < 0.001). When the mitotic count was taken as the basis for determining tumor grade, the MS was 111 ± 6.2 mo in the G1 group and was 35.1 ± 11.3 mo in the G3 group. When the Ki-67 level was taken as the basis for determining tumor grade, the MS was 124.6 ± 6.1 mo in the G1 group and was 54.4 ± 12.7 mo in the G3 group (P < 0.001). The MS was 118.1 ± 8.2 mo in those with a CRP level < 5 mg/L, was 118.6 ± 12.2 mo in those with a CRP between 5 and 20 mg/L, and was 72.3 ± 10.5 mo in those with a CRP level > 20 mg/L (P = 0.009). When pancreatic and stomach NETs were compared, the survival time in patients with pancreatic NETs was significantly lower (P = 0.016). When assessing mortality during the 5-year follow-up period, sensitivity and specificity were 80% and 45%, respectively, when the CRP “cut off” value was 3.85 mg/L; the sensitivity and specificity were 76% and 47%, respectively, when the CRP “cut off” value was 5 mg/L (the universal “cut off” value for CRP), and the sensitivity and specificity were 56% and 79% when the CRP “cut off” value was 20 mg/L. It was also observed that CRP levels greater than 20 mg/L with a 1 mg/dL increase, increased the risk of death 3.2-fold (95% CI: 14.5-7.1).
CRP is an acute phase protein produced in the liver. The acute phase response is stimulated by IL-6 released from macrophages and T cells. Any acute or chronic inflammatory conditions may cause an increase in CRP due to the activation of IL-6. These conditions include infections, autoimmune diseases, and malignancies. As a result, CRP is a sensitive, but nonspecific marker[29]. For this reason, the CRP level in 21 patients whose elevation in CRP was determined to be due to non-tumor related causes was not taken into account in this study. In our study, a significant negative relationship between increased CRP level and survival was observed for the whole GEP-NET group (P = 0.009). Mean survival was 118.1 mo in patients with a CRP level < 5 mg/L, was 118.6 mo in those with a CRP level between 5 and 20 mg/L and was 72.3 mo in those with a CRP level > 20 mg/L. It was shown that each increase in the CRP value of 1 mg/L increased the risk of death by 1.5%. It was observed that the risk of death increased 3.2-fold when CRP level was greater than 20 mg/L, and when this value was taken as the cut off, the sensitivity was 56% and specificity was 79% for the occurrence of death. According to these results, the CRP level, especially when it is above 20 mg/L, is a prognostic factor for a poor outcome in the GEP-NET group. This was shown for the entire GEP-NET group for the first time. Wiese et al[30] performed a study on a PNET group and found a relationship between the CRP level at the time of diagnosis and survival, which indicated that a high CRP value may be an independent prognostic factor in the PNET group. Moreover, as a correlation between Ki-67 level and survival was not observed, the CRP level was shown to be an independent prognostic factor for the whole GEP-NET group.
Small-scale studies have been carried out in which patients were included according to the involved sites, the presence of metastasis, and stage or grade; however, only a few studies have analyzed the GEP-NET group holistically. It is known that all GEP-NETs can metastasize independent of tumor grade and stage; thus, follow-up parameters that can be used for the whole group, factors that affect clinical course and determination of confidence levels of available parameters are important. It was demonstrated in our study that CRP level negatively affected the disease course, especially in patients with a CRP level > 20 mg/dL as it was shown that CRP level was a prognostic factor for the GEP-NET group. We propose that CRP level is an independent prognostic factor for the whole GEP-NET group. However, further prospective and controlled studies are required to verify disease progression according to the CRP level at the time of diagnosis, and treatment can be planned accordingly. CRP level can also be monitored during the follow-up period.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Sharma V, Zhang Z, Tomizawa M S- Editor: Ji FF L- Editor: Webster JR E- Editor: Bian YN
| 1. | Modlin IM, Champaneria MC, Bornschein J, Kidd M. Evolution of the diffuse neuroendocrine system--clear cells and cloudy origins. Neuroendocrinology. 2006;84:69-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3022] [Cited by in F6Publishing: 3036] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 3. | Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez A, Llanos-Muñoz M, Marazuela M, Alvarez-Escola C, Castellano D, Vilar E, Jiménez-Fonseca P, Teulé A, Sastre-Valera J, Benavent-Viñuelas M, Monleon A, Salazar R. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 2010;21:1794-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 4. | Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 706] [Cited by in F6Publishing: 720] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 5. | Zhang Z, Pan L, Deng H, Ni H, Xu X. Prediction of delirium in critically ill patients with elevated C-reactive protein. J Crit Care. 2014;29:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Lackner C, Ress AL, Seggewies FS, Gerger A, Hoefler G, Pichler M. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. 2014;110:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006;12:3746-3750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 78] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V, Lazarus HM, Litzow M, Loren A, Majhail NS, Maziarz RT, McCarthy P, Popat U, Saber W, Spellman S, Ringden O, Wickrema A, Pasquini MC, Cooke KR; from the Center for International Blood and Marrow Transplantation Research. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101:1426-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5537] [Cited by in F6Publishing: 6120] [Article Influence: 437.1] [Reference Citation Analysis (0)] |
| 10. | Bosman F, Carneiro F, Hruban R, Theise N, Bosman FT, Hruban RH, Theise ND, Bosnan FT, Carbeuri F, Organization WH, Bosmanm FT, Carmeiro F. WHO classification of tumours of the digestive system. IARC. 2010;1089. [Cited in This Article: ] |
| 11. | Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Zhang Z. Semi-parametric regression model for survival data: graphical visualization with R. Ann Transl Med. 2016;4:461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1131] [Cited by in F6Publishing: 1117] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 14. | Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, Wright NA, Kidd M. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust. 2010;193:46-52. [PubMed] [Cited in This Article: ] |
| 15. | Jensen R, Doherty G. Section 6. Carcinoid tumors and the carcinoid syndrome (Chapter 34.6 Cancer of the Endocrine System). In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. CancerPrinciples and Practice of Oncology. New York, NY: Lipppincott, New York, Williams and Wilkins, 2005. . [Cited in This Article: ] |
| 16. | Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Carlinfante G, Baccarini P, Berretti D, Cassetti T, Cavina M, Conigliaro R, De Pellegrin A, Di Tommaso L, Fabbri C, Fornelli A, Frasoldati A, Gardini G, Losi L, Maccio L, Manta R, Pagano N, Sassatelli R, Serra S, Camellini L. Ki-67 cytological index can distinguish well-differentiated from poorly differentiated pancreatic neuroendocrine tumors: a comparative cytohistological study of 53 cases. Virchows Arch. 2014;465:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G, Wiedenmann B. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 19. | Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013;31:420-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Dhall D, Mertens R, Bresee C, Parakh R, Wang HL, Li M, Dhall G, Colquhoun SD, Ines D, Chung F, Yu R, Nissen NN, Wolin E. Ki-67 proliferative index predicts progression-free survival of patients with well-differentiated ileal neuroendocrine tumors. Hum Pathol. 2012;43:489-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | van Velthuysen ML, Groen EJ, van der Noort V, van de Pol A, Tesselaar ME, Korse CM. Grading of neuroendocrine neoplasms: mitoses and Ki-67 are both essential. Neuroendocrinology. 2014;100:221-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, Imaoka H, Niwa Y, Tajika M, Kondo S, Tanaka T, Shimizu Y, Kinoshita T, Kohsaki T, Nishimori I, Iwasaki S, Saibara T, Hosoda W, Yatabe Y. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 641] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 24. | Zimmermann ME, Bosman FT. Proliferative activity of well differentiated neuroendocrine tumours of the gut. Histol Histopathol. 2003;18:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 25. | Frilling A, Akerström G, Falconi M, Pavel M, Ramos J, Kidd M, Modlin IM. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19:R163-R185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Grin A, Streutker CJ. Neuroendocrine tumors of the luminal gastrointestinal tract. Arch Pathol Lab Med. 2015;139:750-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1766] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 28. | Freis P, Graillot E, Rousset P, Hervieu V, Chardon L, Lombard-Bohas C, Walter T. Prognostic factors in neuroendocrine carcinoma: biological markers are more useful than histomorphological markers. Sci Rep. 2017;7:40609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 1141] [Article Influence: 54.3] [Reference Citation Analysis (2)] |
| 30. | Wiese D, Kampe K, Waldmann J, Heverhagen AE, Bartsch DK, Fendrich V. C-Reactive Protein as a New Prognostic Factor for Survival in Patients With Pancreatic Neuroendocrine Neoplasia. J Clin Endocrinol Metab. 2016;101:937-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |