Published online Apr 26, 2018. doi: 10.4252/wjsc.v10.i4.34
Peer-review started: March 6, 2018
First decision: March 13, 2018
Revised: March 21, 2018
Accepted: April 10, 2018
Article in press: April 10, 2018
Published online: April 26, 2018
To examine whether nuclear factor kappa B (NF-κB) activity regulates LIN28B expression and their roles in leukemia stem cell (LSC)-like properties.
We used pharmacological inhibitor and cell viability assays to examine the relation between NF-κB and LIN28B. Western blot and qRT-PCR was employed to determine their protein and mRNA levels. Luciferase reporter was constructed and applied to explore the transcriptional regulation of LIN28B. We manipulated LIN28B level in acute myeloid leukemia (AML) cells and investigated LSC-like properties with colony forming and serial replating assays.
This study revealed the relationship between NF-κB and LIN28B in AML cells through drug inhibition and overexpression experiments. Notably, inhibition of NF-κB by pharmacological inhibitors reduced LIN28B expression and decreased cell proliferation. We demonstrated that NF-κB binds to the -819 to -811 region of LIN28B promoter, and transcriptionally regulates LIN28B expression. LIN28B protein was significantly elevated in NFκB1 transfected cells compared to vector control. Importantly, ectopic expression of LIN28B partially rescued the self-renewal capacity impaired by pharmacological inhibition of NF-κB activity.
These results uncover a regulatory signaling, NF-κB/LIN28B, which plays a pivotal role in leukemia stem cell-like properties and it could serve as a promising intervening target for effective treatment of AML disease.
Core tip: In this study, we uncovered that LIN28B is regulated by nuclear factor kappa B (NF-κB) activity on transcriptional level and important for NF-κB-mediated leukemia-stem cell like properties of acute myeloid leukemia cells. NF-κB/LIN28B represents an attractive therapeutic target.
- Citation: Zhou J, Chooi JY, Ching YQ, Quah JY, Toh SHM, Ng Y, Tan TZ, Chng WJ. NF-κB promotes the stem-like properties of leukemia cells by activation of LIN28B. World J Stem Cells 2018; 10(4): 34-42
- URL: https://www.wjgnet.com/1948-0210/full/v10/i4/34.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i4.34
The nuclear factor kappa B (NF-κB) is an well-studied dimeric transcription factor, which regulates the expression of a plethora of downstream targets in action to different cellular conditions, including cell viability, differentiation, proliferation, adhesion, cell cycle and immune response[1,2]. Cross-species analysis demonstrates the optimal DNA binding motif of the human NF-κB orthologous is highly evolutionarily conserved, suggesting its functional importance[3,4]. The Rel homology domain of NF-κB contains the nuclear localization signal (NLS), which determines the its subcellular localization. In inactivation state, Inhibitor of kappa-B (I-κB) binds to NF-κB, and covers-up NLS, preserving the NF-κB complexes in the cytoplasm[5]. Upon activation, an upstream IB kinase (IKKβ) phosphorylates I-κB, then the phosphorylated I-κB is degraded by proteasome system[6]. These NF-κB dimers with open NLS are translocated to the nucleus to binds consensus NF-κB binding motif. In the past a few decades, a substantial number of literatures underscored the key role of the NF-κB pathway in the pathogenesis of solid tumors, hematological malignancies[7-10]. Moreover, Constitutive activation of NF-κB has been implicated in the chemo-resistance and radiation resistance of cancer cells[11].
Acute myeloid leukemia (AML) consists of a group of clonal disorders characterized by different subtypes of genetic and epigenetic abnormalities, which has diverse response to treatment and prognosis[12-14]. Constitutive activity of NF-κB has been demonstrated in approximate 40%[15] to 70% of AML cases[16]. Aberrant NF-κB activity upregulates a long list of anti-apoptotic target genes, such as MCL-1, XIAP, BCL-2, BCL-xL, FLIP, enabling AML cells to evade apoptosis and increase proliferation. Notably, aberrant activation of NF-κB has also been detected in CD34+CD38-CD123+ subpopulation, which is defined as leukemia stem cell (LSC), but not unstimulated normal CD34+ progenitors[15,17]. LSC, also known as leukemia initiating cells (LIC), is the first reported and most studied cancer stem cells (CSC). Although LSCs account for a small fraction of leukemia cells, they are responsible for the treatment failure and disease relapse. The bone marrow microenvironment provides sanctuary to LSC, which is the root source of treatment failure and relapse. Taken together, these evidences suggest that selectively targeting LSC might represent an important strategy towards curing AML. However, the underlying mechanisms of transformation of LSC remain elusive.
The RNA-binding protein, LIN28B, is a microRNAs (miRNA) regulator, which negatively regulates let-7 miRNA family. LIN28B protein belongs to LIN28 family, which is used to reprogram induced pluripotent stem cells (iPSCs). A plethora of clinical and laboratory studies firmly established LIN28B as oncogene in a variety of cancers and hematological malignancies. A number of studies have revealed that LIN28B induces the transformation of CSCs and LSCs and predicts poor prognosis and advanced cancer stages. However, the connection of NF-κB and LIN28B in the pathology of AML remains unacquainted.
In this study, we demonstrate that NF-κB positively regulates LIN28B. We identify a consensus NF-κB binding motif on the LIN28B promoter, which is functionally important. We also show LIN28B expression is the key for the NF-κB-mediated stem cell properties of AML cells.
TF-1a cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, United States) and grown in RPMI 1640 (Biowest, France), supplemented with 10% Fetal Bovine Serum (Biowest) and 1% of antibiotics (Biowest) in an incubator at 37 °C with 5% CO2. HEK-293T cells were maintained in Dublecoo’s modified Eagle’s medium (DMEM) (Biowest) with additional 10% FBS and 1% of antibiotics (Biowest).
We first designed the 5’ forward primers containing additional random sequence and 3’ reverse primer containing additional random sequence and a BagIII restriction site. Then, we used this pair of primers to amplify the human LIN28B promoter fragment containing potential NF-κB was amplified from genomic DNA and the purified PCR products were cloned into reporter pGL3.0 basic vector (Promega, Madison, WI, United States) to generate fusion genes. Sequencing analysis has confirmed these fusion genes. The consensus NF-κB binding motif GGCGATCCC (−819 to −811 relative to TSS) of the construct was mutated to GGCGATTTT using QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, United States). These mutations were confirmed by sequencing analysis.
HEK-293T Cells (2 × 105 cells) were chemically transfected (PEI) with a mix containing 0.5 µg pGL3.0, 2 µg pCMV6-XL4 NFκB1, 2 µg NFκB reporter gene, 10 ng Renilla vector in 1ml of DMEM growth medium for one day. 293T cells were rinsed with 1 x PBS and harvested in 250 µL of Passive Lysis Buffer (Promega, WI, United States). We performed dual luciferase assay by using a kit from Promega according to the manufacturer’s instruction. Briefly, firefly and renilla activity were recorded by a 20/20 luminomoter (Glomax). The relative intensity of firefly luciferase of each sample was normalized to its renilla luciferase. The reporter gene assays for the empty pGL3.0 vector as a control and LIN28N promoter vector were conducted in a similar fashion. These experiments were performed in triplication.
RNeasy Mini Kit (Qiagen Hilden, Germany) was purchased for RNA extraction. In brief, cDNA was synthesized by using the i-Script™ Reverse Transcription Supermix (Biorad, Hercules, CA, United States) from 1 µg of total RNA. The RT reaction was carried under the following conditions: 5 min at 25 °C, 30 min at 42 °C and 5 min at 85 °C in total 20 µL of reaction mixture. Quantitative real-time (qRT)-PCR was performed in a 7300 real-time PCR System (Thermo Fisher Scientific, Forster city, CA, United States). The reaction mixture consists of 0.8 µL of LIN28B or GAPDH primers in 10 µL of iTaq Universal SybrGreen mastermix (Biorad) to a total volume of 20 µL. The qRT-PCR cycle conditions are 2 min at 50 °C, 10min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1min at 60 °C. The primer sequences were described as the following: LIN28B Forward: 5’- GGATTTGGATTC ATCTCCATGATAA-3’, LIN28B Reverse: 5’-GAATTCCACTGGTTCTCCTTCTTTT-3’; GAPDH Forward: 5-GCACCACCAACTGCTTAGCA-3; and GAPDH Reverse: 5-GTCT TCTGGGTGGCAGTGATG-3. The relative quantification of LIN28B gene was determined by using the comparative CT (ΔΔCT) method as recommended by the manufacturer.
Bortezomib, MG-132, and IKK2 inhibitor IV were purchased form Sigma-Aldrich (St. Louis, MO, United States) were dissolved in either Dimethyl sulfoxide (DMSO) or PBS, depending on their solubility in the solvents. TF-1a cells were seeded at a density of 20000 viable cells per well in 96-well culture plates in triplication. As described previously, we used the CellTiter-Glo® Luminescent Cell Viability Assay, also known as CTG assay (Promega Corporation, Madison, WI) to study the effect of drugs on cell viability and proliferation[18]. The inhibitory concentration (IC50) for the cell line was estimated using CTG assay and respective concentrations of each drug were used to treat TF-1a cells. For Bortezomib, the concentrations used were 12.5 nmol/L and 25 nmol/L, while for MG-132 were 312 nmol/L and 625 nmol/L and IKK2 Inhibitor IV were 5.31 µmo l/L and 10.62 µmol/L. Cells were incubated at 24 h and 48 h prior protein extraction. Same batch of cells were added with either PBS or DMSO as controls. Each experiment was repeated 3 times.
Cells were lysed in Lysis buffer (1% Nonidet P-40, 50 mmol/L Tris, pH 8.0, 50 mM NaCl, 1 mM EDTA, 10% glycerol with protease and phosphatase inhibitors) and followed by protein extraction. The amount of proteins was quantified with Bradford assay (Biorad). The cell lysates were loaded into polyacrylamide denaturing gels (12%) and transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Kenilworth, NJ, United States). These membranes were then blocked in 5% milk with 0.1% Tween 20-PBS (PBS-T) solution for one hour. These primary antibodies were used: anti-NFκB-p65 and anti-β-actin antibody (HRP-conjugated) from Santa Cruz Biotechnology (Santa Cruz, CA, United States), anti-phosphoNFκB-P65 antibody from Cell Signaling Technologies, Danvers, MA, United States. Respective second antibodies were applied and washed before exposure for Chemiluminescence (Santa Cruz Biotechnology).
LIN28B gene was cloned into pEGFP vector (Clontech, Fremont, CA, United States) by standard method to create LIN28B-pEGFP vector. We transfected LIN28B-pEGPF and empty vector pEGFP into TF-1a cells by using a Neon™ transfection system (ThermoFisher Scientific, Waltham, MA, United States). The transfection was carried out as the following condition: 1 million cells with five microgram of each vector in 100 μL of Resuspension buffer R electroporation at 1200 V for 20 ms, three pulses. After transfection, cells were transferred into fresh culture medium and grown in the incubator.
Trypan Blue Exclusion method was applied to determine the cell viability of TF-1a and TF-1a-LIN28B cells. Briefly, ten microliter of trypan blue dye (concentration: 0.4% from Sigma-Aldrich) was mixed with equal volume of the cell suspension to obtain a 1 to 2 dilution for one minute. The mixture was put on a hemacytometer and viable cells were counted under a light microscope. We then applied 2 x 104 viable TF-1a and TF-1a-LIN28B cells into in human StemMACS HSC-CFU basic medium without cytokines (130-091-275, Miltenyi Biotec, Germany) in 6-well plates for seven days. Normally, colonies comprises of greater than 50 cells which were counted under an inverted microscope. Total 5 random 4 x 10 magnification fields were counted for colony numbers. The average of colonies number was determined by total numbers divided into five. In serial replating assay, the colony number was determined , followed by harvesting and diluting, and 20000 cells were replated in fresh methylcellulose medium and subjected to replating every 7 d. All these experiments were repeated twice.
T-test was applied to compare the mean ± SD between two groups. Two-way analysis of variance (ANOVA) was used to analyse the statistical significance when there were more than two groups. We considered P values less than 0.05 as statistically significant.
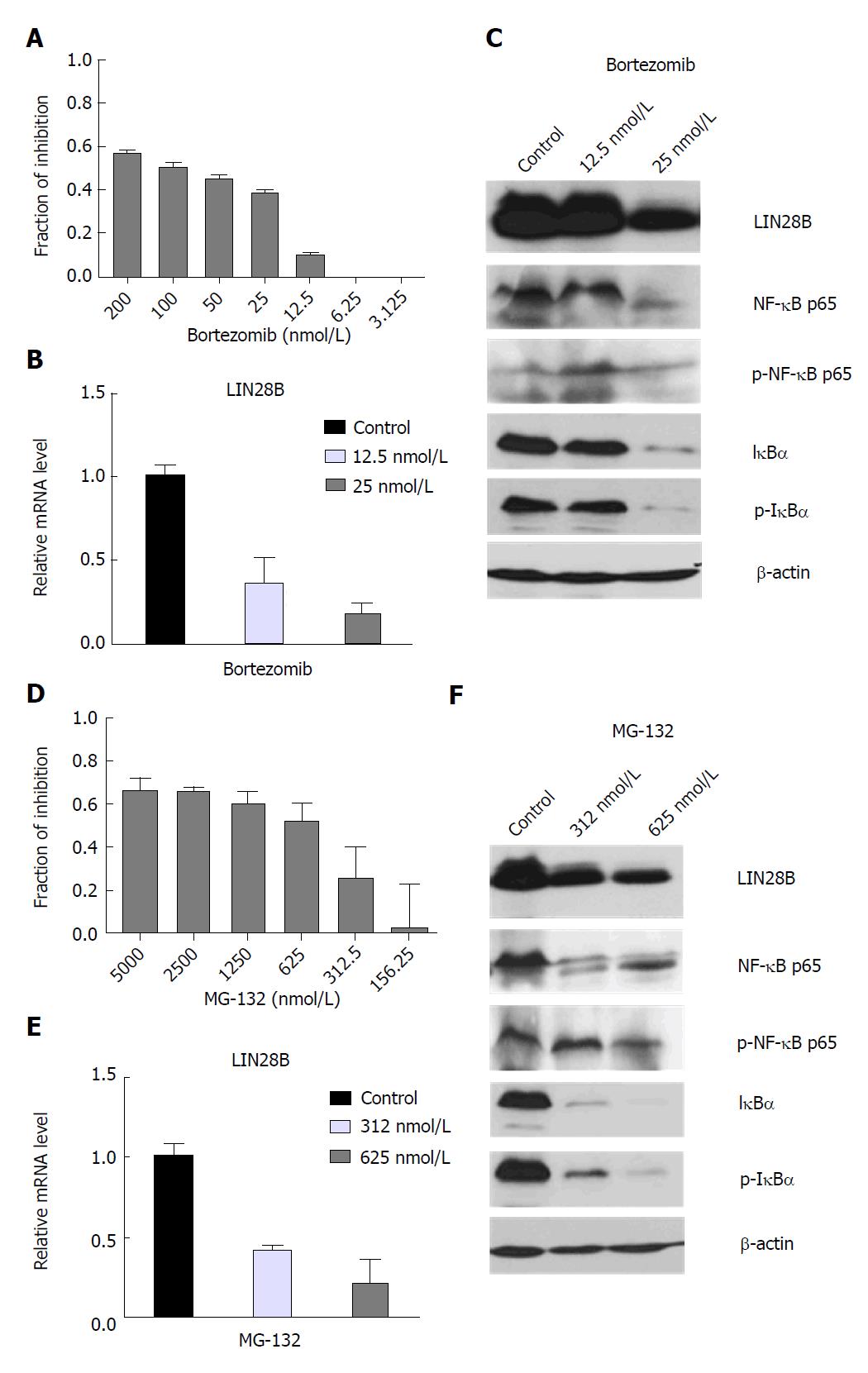
We previously reported that human AML cell line TF-1a cells express abundant endogenous LIN28B protein[19]. In order to investigate the relationship between NF-κB and LIN28B, we treated the TF-1a cells with gradual concentrations of Bortezomib and MG-132, two different NF-κB inhibitors[20].
A significant decrease in cell viability could be observed when treated with increasing dose of Bortezomib and MG-132 (Figure 1A and D) and their IC50 values were about at 50 mol/L and 625 nmol/L, respectively. qRT-PCR analysis of TF-1a cells treated with 12.5 nmol/L and 25 nmol/L of Bortezomib showed a decrease in LIN28B mRNA by about 63% and 82%, respectively, compared to the control sample (Figure 1B). The reduction of LIN28B protein level was noticeable in cells treated with 25 nmol/L of Bortezomib, which coincides with decreased NF-κB p65, I-κBα (Figure 1C). Notably, a lower dose 12.5 nmol/L of Bortezomib didn’t induce significant change of NF-κB p65, I-κBα. In concordance, LIN28B protein was not changed significantly either, indicating a specific correlation between NF-κB activity and LIN28B expression.
In agreement with the results from Bortezomib treatment, qRT-PCR analysis in MG-132-treated cells (312 and 625 nmol/L) showed significant reduction of LIN28B mRNA level (58.2% and 79%, respectively) relative to control (Figure 1E). Decline in NF-κB p65, I-κBα, as well as in LIN28B protein expression was observed in both of MG-132-treated cells as compared to control (Figure 1F). As Bortezomib and MG-132 are proteasome inhibitors, we also used an IKK2 inhibitor IV, a more specific and direct NF-κB inhibitor to treat the TF-1a cells. We observed increasing inhibition of cell proliferation starting from 0.625 µmol/L to 10 µmol/L, with 50% inhibition at around 5 µmol/L (Supplementary Figure S1A). In agreement with the data derived from Bortezomib and MG-132, IKK-2 inhibitor IV treatment significantly reduced the mRNA (Supplementary Figure S1B) and protein levels (Supplementary Figure S1C) of LIN28B. In conclusion, the regulation of LIN28B by NFκB activity is specific.
Taken together, our data suggest that inhibition of NF-κB decreases cell viability, accompanying reduction in LIN28B mRNA and protein, suggesting a possible role for NF-κB in regulation of LIN28B expression.
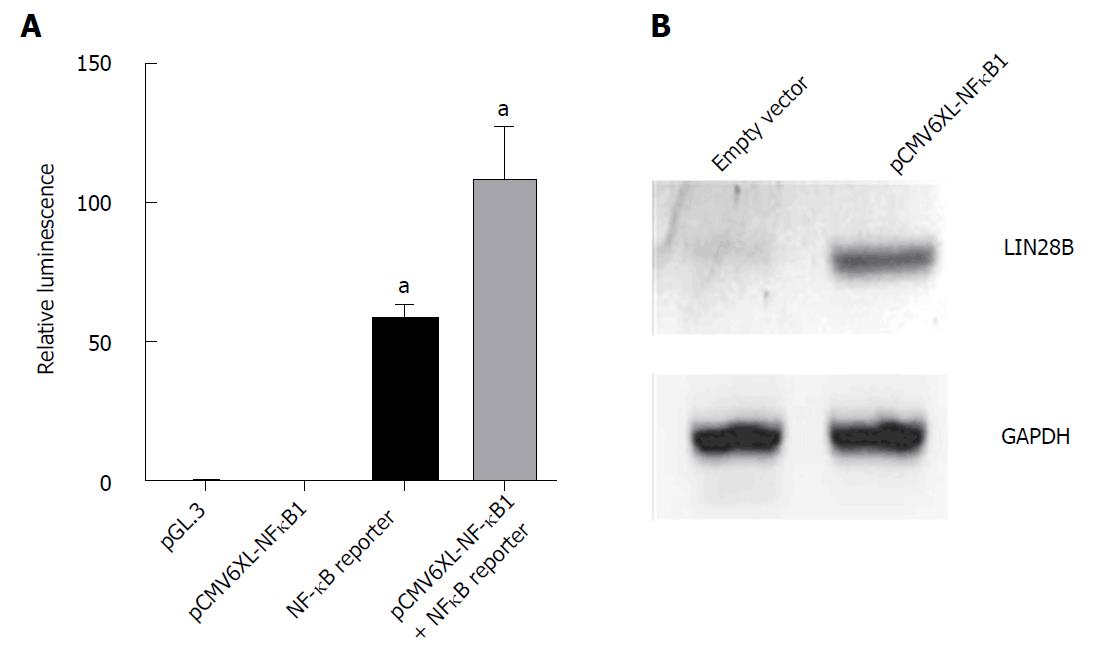
We further examined the role of NF-κB in controlling LIN28B by overexpression of NF-κB in HEK-293T. The ability of pCMV6-XL4 NF-κB1 plasmid to overexpress NF-κB protein was determined using Dual luciferase assay. pCMV6-XL4 NFκB1 that encodes p105 which can undergo proteolysis to produce p50 that interacts with p65 was overexpressed in HEK293T cells. Figure 2A showed that by overexpressing NF-κB1 could result in increase in activating NFκB reporter gene two times relative to cells with only expressed NF-κB reporter gene. This result validated the overexpression efficiency of this plasmid, which was subsequently used to overexpress in HEK293T cells for 48 h (Figure 2B). LIN28B protein was upregulated in NF-κB1 overexpression cells, suggesting the regulatory effect of NF-κB activity on LIN28B expression through the canonical pathway. Taken together, these data together with abovmentioned results derived from NF-κB inhibitors, firmly support the regulatory role of NF-κB in LIN28B expression.
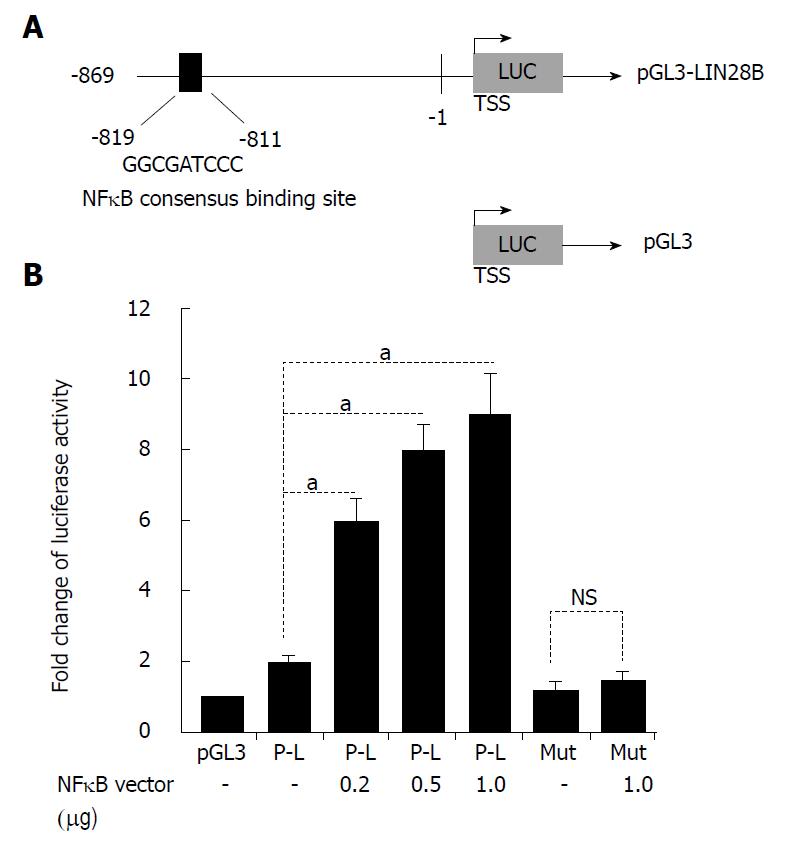
We decided to explore the possible connection between NF-κB and LIN28B promoter. A detailed search of the -2.0 kb human LIN28B promoter region discovered one putative NF-κB -consensus-binding sequences[21], as decipted in Figure 3A, located at -819 to -811 relative to TSS (sequence: GGCGATCCC). Gene reporter assays revealed that luciferase activity increased by two times in 293T cells transfected with LIN28B constructs relative to the same cells transfected with pGL3 empty vector (Figure 3B). Then, we determine whether NF-κB has the ability to activate the LIN28B promoter. To this end, we co-transfected an escalating doses of NF-κB p65 expression vector and the LIN28B promoter plasmid in 293T cells. We found that NF-κB p65 plasmid could dose-dependently increased activation of LIN28B promoter activity (Figure 3B). In order to validate that NF-κB binding motif on the LIN28B promoter is critical for the regulation of LIN28B by NF-κB, we employed site-directed mutagenesis method to mutate the consensus-binding sequence from GGCGATCCC to GGCGATTTT. These intended site mutations were validated by DNA sequencing method. As assessed by dual-luciferase reporter assays, the luciferase activity of the mutated LIN28B promoter displayed 3 times less when compared to that of the wild-type LIN28B promoter (Figure 3B). Taken together, these data provide strong evidence that LIN28B promoter activity is positively regulated by NF-κB and the consensus NF-κB-binding motif is critical for the regulation.
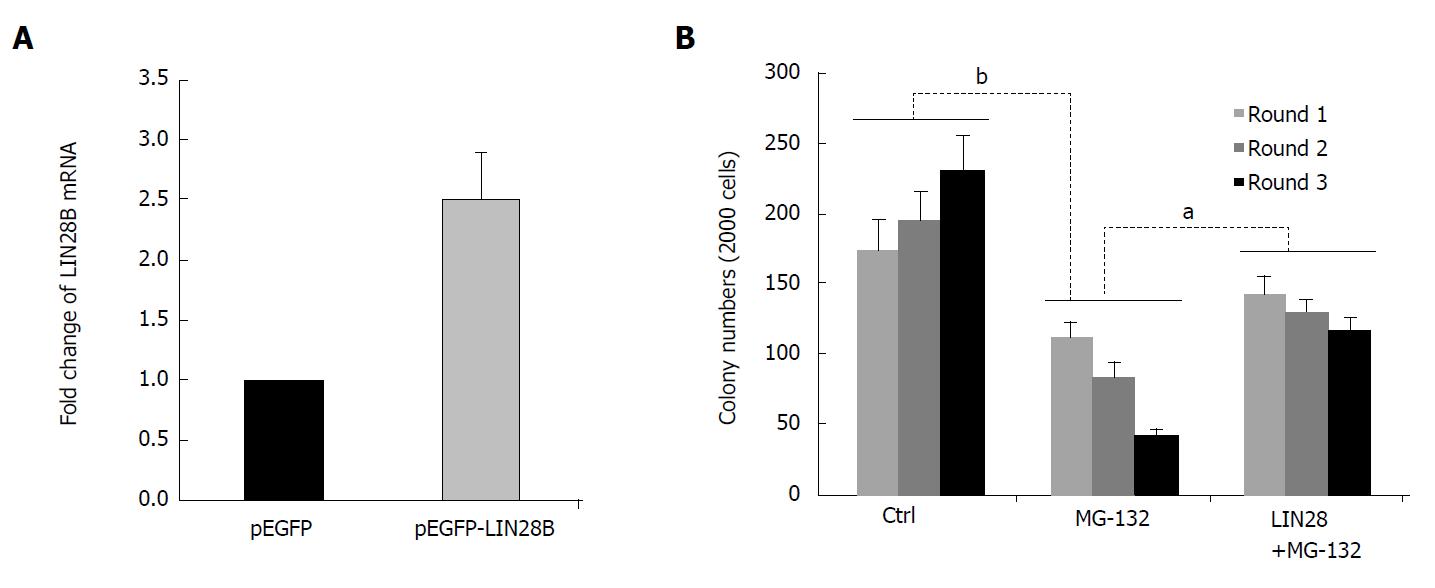
NF-κB is known to increase cell survival and growth, and promotes cells to less sensitive to drug treatment through upregulation of some important antiapoptotic molecules. Many studies have demonstrated that NF-κB is constitutively activated in human AML stem cells, but not in normal human CD34+ progenitor cells[15]. Notably, inhibition of NF-κB activity preferably kill LSC, while spare normal progenitor cells in AML patients[22,23]. To assess the functional involvement of LIN28B in NF-κB-mediated LSC property, we conducted serial replating assay to assess the self-renewal capacity, one of the intrinsic characteristic of LSC, in TF-1a cells. The increased of LIN28B mRNA by pEGFP-LIN28B was examined by qRT-PCR too. As shown in Figure 4A, the LIN28B expression was increased to 2.5-fold in TF-1a cells transfected with pEGFP-LIN28B than pEGFP transfected cells (Figure 4A). As expected, TF-1a cells were capable of being serially replated in basic methylcellulose without additional cytokines (Figure 4B), whereas the numbers of colonies were significantly reduced in TF-1a cells treated with NF-κB inhibitor, MG-132. Next, we overexpressed a GFP-tagged LIN28B gene in TF-1a cells, then followed by MG-132 treatment and serially replating assays. In fact, ectopic expression of LIN28B could partially rescue the colony formation capacity of TF-1a treated with MG-132 (Figure 4B). Overall, these data support a functional role for LIN28B in NF-κB-mediated LSC property of AML cells.
LIN28B has been associated with cancer progression metastasis. For example, overexpression of LIN28B was found to be associated with patients with colon and liver cancer who had a shorted survival and higher chance of tumor recurrence[24,25]. Increasing number of studies have shown that LIN28B plays an important role in the growth of CSCs[24,26], which could be the reason for recurrence of cancer. We previously reported that inhibition of LIN28B impairs the growth of AML cells through disrupting leukemia cell metabolism[19]. In this study, we uncovered that LIN28B is essential for NF-κB-mediated leukemic stem-cell like property of AML cells.
Constitutive activation of NFκB activity in TF-1a cells was shown to increase the expression of LIN28B, thus promoting cell proliferation. Meanwhile, it is well known that NF-κB activation could make the cancer cells resistant to apoptosis, enhanced proliferation and metastasis[27]. This study demonstrated the relationship between NF-κB signaling and LIN28B by treating TF-1a with NF-κB inhibitors, which acted on 26S proteasome to keep NFκB in an inactive state, thus reducing active NFκB p65 binding onto the promoter for LIN28B. In order to further support the result that the decrease in NF-κB level after drug treatments were responsible for LIN28B’s down regulation but not due to the effect of extensive apoptosis, an overexpression study of NF-κB1 in HEK-293T cells was carried out and showed positive increase in LIN28B expression. Together, these data further reinforces the finding that NF-κB regulates LIN28B.
One of the hallmarks of HSCs and most important characteristic of LSCs is their ability of sustainable self-renewal[28]. LSCs are regarded as the fundamental source of disease relapse and treatment failure[29]. Therefore, it is clinically desirable to targeting LSCs. In this study, we unveiled that LIN28B is transcriptionally regulated by NF-κB and LIN28B plays a key role in NF-κB-mediated leukemia stem cell-like properties. NF-κB inhibitors strongly decreases LIN28B expression and compromises LSC self-renew and abrogates its tumor-initiating capacity. This NF-κB/LIN28B regulatory axis could be the “Achilles’ heel” of LSCs in AML, and inhibiting NF-κB activity may provide an opportunity to eradicate LSCs in some subtypes of AML in which NF-κB/LIN28B is essential for the disease progression, offering a greater strategy in eradicating AML and LSCs to prevent relapse of the disease.
Acute myeloid leukemia (AML) is a common blood cancer adult. The current standard chemotherapy can’t cure the disease, as most of the patient relapse and become refractory to treatment. Leukemia stem cells (LSCs) are a small subpopulation that sustain the disease and often resistant to chemotherapy. LSCs are responsible for the disease relapse. So, a better understanding molecular biology of AML and novel therapies are urgently needed for AML patients.
The nuclear factor kappa B (NF-κB) is a pivotal transcription factor, playing different roles in all most all cellular functions. Aberrant activation of NF-κB has been found specifically in LSCs, but not in normal hematopoietic progenitor cells. LIN28 and LIN28B are RNA-binding protein and transcriptional regulators, which are used to create induced pluripotent stem cells (iPS). However, the detailed molecular basis of how NF-κB contributes to the LSC-like properties of AML cells is not well-understood.
In this study, we aim to explore the relationship between NF-κB and LIN28B expression, as well as to assess their roles in LSC-like properties. It will help us to better understand the formation of LSCs, and provide the opportunity to target LSCs.
Several NF-κB inhibitors with different mode-of-actions was used to treat leukemia cells, then followed by assessment of cell viability. Western blot and qRT-PCR was employed to examine the correlation between NF-κB and LIN28B protein and mRNA levels. Luciferase reporter was constructed and applied to explore the transcriptional regulation of LIN28B. Colony forming and serial replating assays are functional assays for LSC-like properties.
Treatment of leukemia cells with direct and indirect NF-κB inhibitors significantly decreased LIN28B protein and mRNA levels and reduced cell viability. Mechanistically, the region of -819 to -811 region on the LIN28B promoter contains specific, consensus NF-κB binding motif, and mutations in this region compromised transcription activity and LIN28B expression. On contrast, transfection of NFκB1 increased LIN28B protein. Overexpression of LIN28B partially rescued the self-renewal capacity impaired by pharmacological inhibition of NF-κB activity. The functional role of NF-κB and LIN28B regulatory axis in LSCs was confirmed.
Our data demonstrated the existing of NF-κB/LIN28B regulatory axis in AML, which plays a pivotal role in the formation of LSCs. This study provides a deep understanding of the previous finding that NF-κB is activated in CD34+CD38- AML cells. LIN28B is a critical downstream target of NF-κB pathway. This study also highlights the targeting NF-κB or LIN28B as an effective approach for eradication LSCs in AML.
In summary, we characterized the NF-κB/LIN28B regulatory axis and its functional roles in maintenance of LSC-like properties of AML cells. We proposed that targeting either NF-κB or LIN28B could be an effective way to eradication of LSCs, which are known to resist to chemotherapy. Although NF-κB inhibitors are available, their side-effects should be carefully examined as NF-κB play important roles in multiple cellular processes, like immune defense. Furthermore, specific LIN28B inhibitor is currently not available. The development of novel class of small molecular inhibitors or drug-like compounds to inhibit LIN28B should be the focus of the future research.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kan L, Liu L, Tanabe S S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14:1270-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1262] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 3. | Ryzhakov G, Teixeira A, Saliba D, Blazek K, Muta T, Ragoussis J, Udalova IA. Cross-species analysis reveals evolving and conserved features of the nuclear factor κB (NF-κB) proteins. J Biol Chem. 2013;288:11546-11554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Cildir G, Low KC, Tergaonkar V. Noncanonical NF-κB Signaling in Health and Disease. Trends Mol Med. 2016;22:414-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | Finco TS, Beg AA, Baldwin AS, Jr . Inducible phosphorylation of i kappa b alpha is not sufficient for its dissociation from nf-kappa b and is inhibited by protease inhibitors. Proc Natl Acad Sci USA. 1994;91:11884-11888. [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 245] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Wertz IE, Dixit VM. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Inoue J, Gohda J, Akiyama T, Semba K. NF-kappaB activation in development and progression of cancer. Cancer Sci. 2007;98:268-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Ching YQ, Chng WJ. Aberrant nuclear factor-kappa b activity in acute myeloid leukemia: From molecular pathogenesis to therapeutic target. Oncotarget. 2015;6:5490-5500. [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 777] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 10. | Chng WJ, Goldschmidt H, Dimopoulos MA, Moreau P, Joshua D, Palumbo A, Facon T, Ludwig H, Pour L, Niesvizky R. Carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia. 2017;31:1368-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Li F, Sethi G. Targeting transcription factor nf-kappab to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167-180. [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, Beverloo HB, Moorhouse MJ, van der Spek PJ, Löwenberg B. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1044] [Cited by in F6Publishing: 993] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 13. | Zhou J, Lu X, Tan TZ, Chng WJ. X-linked inhibitor of apoptosis inhibition sensitizes acute myeloid leukemia cell response to TRAIL and chemotherapy through potentiated induction of proapoptotic machinery. Mol Oncol. 2018;12:33-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Plesa A, Dumontet C, Mattei E, Tagoug I, Hayette S, Sujobert P, Tigaud I, Pages MP, Chelghoum Y, Baracco F. High frequency of CD34+CD38-/low immature leukemia cells is correlated with unfavorable prognosis in acute myeloid leukemia. World J Stem Cells. 2017;9:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappab is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301-2307. [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 16. | Birkenkamp KU, Geugien M, Schepers H, Westra J, Lemmink HH, Vellenga E. Constitutive NF-kappaB DNA-binding activity in AML is frequently mediated by a Ras/PI3-K/PKB-dependent pathway. Leukemia. 2004;18:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood. 2012;120:1290-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Bi C, Cheong LL, Mahara S, Liu SC, Tay KG, Koh TL, Yu Q, Chng WJ. The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood. 2011;118:2830-2839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Zhou J, Bi C, Ching YQ, Chooi JY, Lu X, Quah JY, Toh SH, Chan ZL, Tan TZ, Chong PS. Inhibition of LIN28B impairs leukemia cell growth and metabolism in acute myeloid leukemia. J Hematol Oncol. 2017;10:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799:775-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 561] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 21. | Wan F, Lenardo MJ. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring Harb Perspect Biol. 2009;1:a000067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163-4169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 488] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Siveen KS, Uddin S, Mohammad RM. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol Cancer. 2017;16:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260-4268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Cheng SW, Tsai HW, Lin YJ, Cheng PN, Chang YC, Yen CJ, Huang HP, Chuang YP, Chang TT, Lee CT. Lin28B is an oncofetal circulating cancer stem cell-like marker associated with recurrence of hepatocellular carcinoma. PLoS One. 2013;8:e80053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 27. | Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 781] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 28. | Wang X, Huang S, Chen JL. Understanding of leukemic stem cells and their clinical implications. Mol Cancer. 2017;16:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Horton SJ, Huntly BJ. Recent advances in acute myeloid leukemia stem cell biology. Haematologica. 2012;97:966-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |