Published online Mar 27, 2020. doi: 10.4254/wjh.v12.i3.84
Peer-review started: July 16, 2019
First decision: November 2, 2019
Revised: December 24, 2019
Accepted: January 14, 2020
Article in press: January 14, 2020
Published online: March 27, 2020
Non-alcoholic fatty liver disease (NAFLD) is a common cause of liver disease worldwide and is a growing epidemic. A high ratio of omega-6 fatty acids to omega-3 fatty acids in the diet has been implicated in the development of NAFLD. However, the inflicted cellular pathology remains unknown. A high ratio may promote lipogenic pathways and contribute to reactive oxygen species (ROS)-mediated damage, perhaps leading to mitochondrial dysfunction. Therefore, these parameters were investigated to understand their contribution to NAFLD development.
To examine the effect of increasing ratios of omega-6:3 fatty acids on mitochondrial function and lipid metabolism mediators.
HepG2-derived VL-17A cells were treated with normal (1:1, 4:1) and high (15:1, 25:1) ratios of omega-6: omega-3 fatty acids [arachidonic acid (AA): docosahexaenoic acid (DHA)] at various time points. Mitochondrial activity and function were examined via MTT assay and Seahorse XF24 analyzer, respectively. Triglyceride accumulation was determined by using EnzyChrom™ and levels of ROS were measured by fluorescence intensity. Protein expression of the mediators of lipogenic, lipolytic and endocannabinoid pathways was assessed by Western blotting.
High AA:DHA ratio decreased mitochondrial activity (P < 0.01; up to 80%) and promoted intracellular triglyceride accumulation (P < 0.05; 40%-70%). Mechanistically, it altered the mediators of lipid metabolism; increased the expression of stearoyl-CoA desaturase (P < 0.05; 22%-35%), decreased the expression of peroxisome proliferator-activated receptor-alpha (P < 0.05; 30%-40%) and increased the expression of cannabinoid receptor 1 (P < 0.05; 31%). Furthermore, the high ratio increased ROS production (P < 0.01; 74%-115%) and reduced mitochondrial respiratory functions such as basal and maximal respiration, ATP production, spare respiratory capacity and proton leak (P < 0.01; 35%-68%).
High AA:DHA ratio induced triglyceride accumulation, increased oxidative stress and disrupted mitochondrial functions. Stimulation of lipogenic and steroidal transcription factors may partly mediate these effects and contribute to NAFLD development.
Core tip: A high ratio of omega 6:3 fatty acids in the diet has been implicated in the development of non-alcoholic fatty liver disease, a growing epidemic of major concern. The cellular pathology induced by such high ratios remains unknown. Here, we observed that in human hepatoma HepG2 (VL-17A) cells, high omega-6:omega-3 ratio reduced mitochondrial activity, increased triglyceride accumulation, elevated reactive oxygen species levels and interrupted several mitochondrial functions. Moreover, the increased expression of stearoyl-CoA desaturase, decreased expression of peroxisome proliferator-activated receptor alpha and elevation in cannabinoid receptor-1 expression collectively lead to lipogenesis and lipotoxicity, which are key features of non-alcoholic fatty liver disease development.
- Citation: Ghazali R, Mehta KJ, Bligh SA, Tewfik I, Clemens D, Patel VB. High omega arachidonic acid/docosahexaenoic acid ratio induces mitochondrial dysfunction and altered lipid metabolism in human hepatoma cells. World J Hepatol 2020; 12(3): 84-98
- URL: https://www.wjgnet.com/1948-5182/full/v12/i3/84.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i3.84
Non-alcoholic fatty liver disease (NAFLD) overarches all chronic liver conditions resulting from high liver fat content that are not caused by alcohol consumption, steatogenic medication or monogenic disorders. It encompasses a broad spectrum of pathological states ranging from the initial steatosis (fatty liver), which may progress to non-alcoholic steatohepatitis (NASH), followed by fibrosis and cirrhosis, and may result in liver failure or hepatocellular carcinoma[1]. NAFLD is believed to arise in a multi/repeated hit stage process. In the first stage, lipids (triglycerides) accumulate in the hepatocytes due to factors such as increased consumption of lipids and/or carbohydrates, insulin resistance-induced release of free fatty acids from the adipose tissue, elevated lipogenesis, reduced fatty acid oxidation, and reduced lipoprotein secretion from the liver. Subsequent stages are triggered by inflammation, oxidative stress, gut dysbiosis and mitochondrial dysfunction, which causes cellular injury eventually leading to liver fibrosis[2,3].
The current global prevalence of NAFLD is approximately 25%[4] and a common cause of liver cancer in United States[1]. With the growing epidemic of obesity in the Western world and the Middle East, NAFLD is predicted to be the most frequent indication for liver transplantation by 2030[5]. It is also associated with the metabolic syndrome, increasing the risk for cardiovascular disease, insulin resistance and type 2 diabetes[6]. Thus, effective preventive and prophylactic measures are urgently required.
A high omega 6: omega-3 fatty acid dietary ratio has been correlated with NAFLD pathogenesis[7]. A normal omega 6:3 ratio is around 1-4:1, where omega-3 fatty acids regulate hepatic lipogenesis and are anti-inflammatory and anti-thrombotic[8,9]. However, Western diets contain higher omega 6:3 ratios ranging from 15-20:1[8,10] that disrupt the regulation of intrahepatic lipids, and promote a prothrombotic and proinflammatory environment[10]. In NAFLD/NASH patients, omega-3 levels are significantly low, which correlates with hepatic steatosis and increased proinflammatory arachidonic acid (AA) metabolic products[11,12]. The therapeutic effects of low dietary omega 6:3 ratio have been observed in animal models and in clinical trials with NAFLD/NASH patients[13-15]. In this scenario, it is pivotal to understand the yet unknown underlying cellular mechanisms that prevail under states of high omega-6: omega-3 ratio. This may identify cellular and molecular targets that may help in formulating therapeutic interventions to effectively prevent and treat NAFLD.
Accordingly, this study examined the effects of high ratio of omega-6: omega-3 fatty acids on mitochondrial functions, and several parameters and regulators of lipid metabolism to understand the underlying mechanisms and extrapolate their contribution to fatty liver development and NAFLD pathogenesis. Here, the effect of high AA (omega-6): docosahexaenoic acid (DHA) (omega-3) ratio was studied on intracellular triglyceride accumulation, mitochondrial activity, oxidative stress and mitochondrial functions. Moreover, to understand the responses of the mechanistic mediators, we examined the expressions of stearoyl-CoA desaturase (SCD1) - the lipogenic enzyme involved in fatty acid synthesis, peroxisome proliferator-activated receptor alpha (PPAR-α)- a transcription factor that mediates fatty acid oxidation, sterol regulatory element-binding protein 1 (SREBP1c) - a transcription factor that induces genes involved in fatty acid synthesis, and cannabinoid receptors 1 and 2 (CB1, CB2), as their activation has been implicated in fatty liver development[16,17].
VL-17A, human hepatoma cell line (overexpress ADH and CYP2E1)[18] were maintained at 37 ºC in 5% CO2 in a high-glucose (25 mmol) Dulbecco’s Modified Eagle Medium (DMEM) (Lonza Ltd, United Kingdom) supplemented with L-Glutamine (2 mmol) (Lonza Ltd, United Kingdom), penicillin (100 U/mL) (Lonza Ltd, United Kingdom), streptomycin (100 mg/mL) (Lonza Ltd, United Kingdom), sodium pyruvate (1 mmol) and 10% foetal calf serum (FCS) (Biosera, United Kingdom)[19,20]. Prior to maintenance, cells were grown in Plasmocin Prophylactic (5 µg/mL in DMEM) for four weeks.
Cells were treated with different ratios of omega-6 AA: omega-3 DHA (1:1, 4:1, 15:1 and 25:1) for the required period. AA and DHA were of > 98% purity and acquired from Sigma-Aldrich (Gillingham, United Kingdom). Fatty acids were prepared as 8 mmol stock in DMSO and then diluted in 1% FCS low-glucose (1 g/L) DMEM to obtain the required treatment concentrations[20]. The fatty acids were in free form with a concentration of 0.02% bovine serum albumin (BSA) in the FCS[20].
Following the treatments, various parameters were examined such as mitochondrial activity, triglyceride accumulation, reactive oxygen species (ROS) levels and mitochondrial functions (basal and maximal respiration, ATP production, protein leak and spare respiratory capacity). Also, protein expression of the mediators of lipogenic, lipolytic and endocannabinoid pathways, SCD1, PPAR-α, SREBP1c, CB1 and CB2 were studied. Data were expressed relative to the experimental control i.e., cells not treated with omega fatty acids but treated with 0.5 % DMSO in maintenance medium (referred to as untreated control). Comparisons were drawn between the three groups: (1) Untreated control; (2) Moderate omega-6: omega-3 ratios of 1:1 and 4:1; and (3) High ratios of 15:1 and 25:1.
Mitochondrial activity, and thereby lipotoxicity, was assessed by using the MTT assay, adapted from previous studies[21,22]. Briefly, cells (2.5 × 104 cells/200 µL DMEM/well) were incubated overnight in 96-well plates and then treated with different ratios of omega fatty acids for 24, 48 and 72 h. After the treatment, MTT (5 mg/mL) (Sigma-Aldrich, United Kingdom) was added and the cells were incubated for 2 h at 37 ºC. Cells were then washed with PBS, treated with DMSO (100 µL/well) and incubated for 15 min at room temperature. Absorbance was measured at 550 nm using a VersaMax microplate reader (Molecular Devices, United Kingdom).
Cells (1 × 105 cell/mL) were incubated overnight in a 24-well plate and then treated with different ratios of omega fatty acids for 24, 48 and 72 h. Following the treatments, intracellular triglyceride content was determined by using EnzyChrom™ triglyceride assay kit (BioAssay Systems, United States), as per manufacturer’s instructions. Triglyceride content was normalised to protein content, as determined by using Bio-Rad protein assay kit according to manufacturer’s instructions. Data were expressed as mg triglyceride/mg protein.
Cells (1 × 104 cells/ 200-µL DMEM) were incubated overnight in a 96-well plate. Then, the cells were treated with different ratios of omega fatty acids for 30 min, 1 h, 2 h, 3 h, 6 h and 24 h. Following this, the cells were treated with 1 µmol 2′,7′-dichlorofluorescein diacetate (Sigma-Aldrich, United Kingdom) (in PBS) and incubated at 37 ºC for 45 min. Fluorescence intensity was measured at an excitation of 485 nm and an emission of 535 nm by using FLUOstar OPTIMA (Jencons-PLS, United Kingdom). Fluorescence levels were expressed as percentage of the control.
The mitochondrial oxygen consumption rate (OCR) was evaluated using the XF Cell Mito Stress Test Kit as per manufacturer’s instructions (Agilent Technologies, Craven Arms, United Kingdom). Plates were analysed using the Seahorse XF24 (Agilent Technologies, United Kingdom)., Essentially, cells (1.5 × 104 cells/100 µL DMEM) were seeded in a SeaHorse 24-well culture microplate. After incubation for 2 h at 37 ºC and 5% CO2, 150 µL of DMEM (10% FCS) was added to the cells. The cells were re-incubated overnight at 37 ºC and 5% CO2. The following day, cells were treated with various AA:DHA ratios in 10% FCS DMEM and plates were incubated at 37 ºC and 5% CO2 for 24 h. The next day, Seahorse XF Assay Medium without glucose was supplemented with sodium pyruvate (1 mmol) and glucose (25 mmol) and the pH was adjusted to 7.4. Cells were washed twice with this Seahorse medium and incubated at 37 ºC and 0% CO2 for 45 min. To monitor the OCR of the cells, SeaHorse XF analyser was calibrated with the sensor cartridge containing Mito stress drugs FCCP (1 µmol), an uncoupling agent; oligomycin (1 µmol) for ATP synthase inhibition and an antimycin/Rotenone mixture (0.5 µmol) for inhibition of oxidative phosphorylation and electron transfer, respectively. Following the measurement of OCR, 1% Triton X-100 were used to lyse the cells and the protein content was determined by using Bio-Rad protein assay kit. Seahorse cell Mito stress parameters were calculated following manufacture’s protocol, normalized to protein content and the data were expressed relative to control.
Cells were treated with different ratios of omega fatty acids for 24 h. Then, total cellular protein was extracted using 1% Triton X-100 in PBS containing a protease inhibitor cocktail tablet and the protein concentration was determined by using Bio-Rad protein assay kit, as per manufacturer's instructions (Bio-Rad Laboratories, United Kingdom). Protein (20-40 µg) was separated on 10% SDS-PAGE gels (Thermo Scientific Pierce, United Kingdom) by electrophoresis for 90 min at 120 V and transferred onto a nitrocellulose membrane (0.45 µm) for 1 h at 350 A. Membranes were blocked with 1% BSA, except when confirming β-actin where 5% BSA was used. The proteins examined were SCD1 (1:4000) (Abcam, United Kingdom), PPAR-α (1:2000) (Abcam, United Kingdom), CB1, (1:4000) (Santa Cruz Biotechnology, United Kingdom) CB2 (1:2000) (Abcam, United Kingdom), and β-actin (1:4000) (Abcam, United Kingdom). West PICO Chemiluminescent Substrate (ThermoFisher, United Kingdom) was used for detection and the X-ray films were scanned on a Bio-Rad GS-800 Calibrated Densitometer (Bio-Rad, United Kingdom)[23,24]. During post-densitometry analysis, data were expressed relative to corresponding β-actin levels and compared to the experimental control.
The statistical methods of this study were reviewed by Dr Claire Robertson, Senior Lecturer in Nutritional Epidemiology, University of Westminster.
As variances were equal between groups, a one-way ANOVA followed by post-hoc analysis by Tukey's test was used to assess differences between omega fatty acid ratios and control treated groups. Data analysis was conducted using SPSS version 23.0 Data were expressed as the mean ± SEM, n = 3-5, and where P ≤ 0.05 was considered significant.
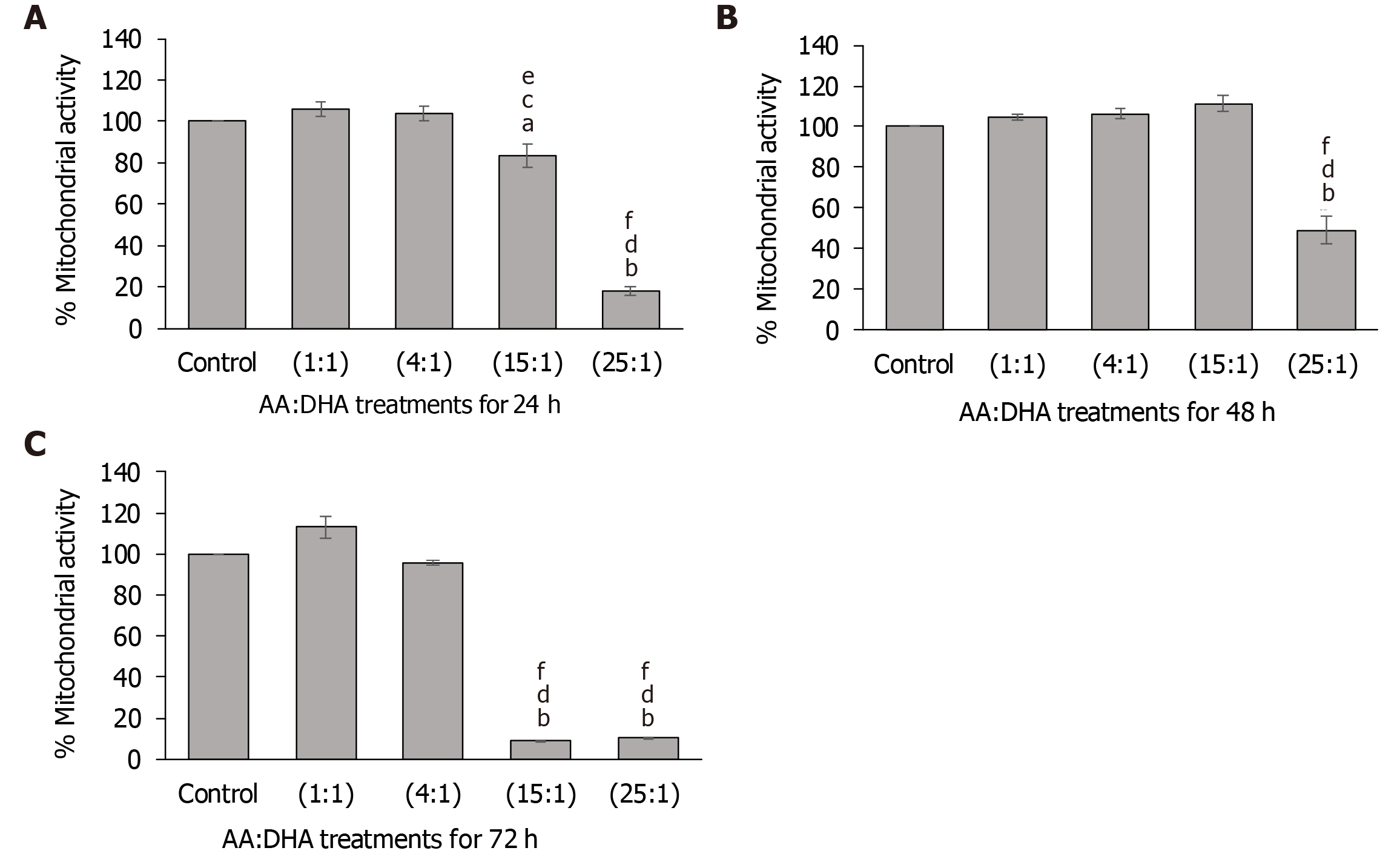
After 24 h, high AA:DHA ratios reduced mitochondrial activity, when compared to the untreated control [15:1 by 17% (P < 0.05) and 25:1 by 82% (P < 0.01)] (Figure 1A). Furthermore, high AA:DHA ratio (15:1) induced reduction in activity when compared to the moderate ratios of 1:1 (by 22%, P < 0.05) and 4:1 (by 20%, P < 0.01) (Figure 1A). A similar pattern was induced by the high 25:1 ratio, which showed reduced activity in comparison to 1:1 (by 83%, P < 0.05) and 4:1 (by 83%, P < 0.01) ratios (Figure 1A).
After 48 h, only the 25:1 ratio caused a significant reduction in activity compared to the control, 1:1, and 4:1 by 51%, 54%, 56%, respectively (P < 0.01) (Figure 1B). At 72 h, both the high ratios (15:1 and 25:1) reduced mitochondrial activity. The 15:1 ratio markedly reduced mitochondrial activity in comparison to the control (by 91%, P < 0.01), 1:1 (by 92%, P < 0.01) and 4:1 (by 91, P < 0.01) ratios (Figure 1C). Similarly, the 25:1 ratio reduced mitochondrial activity in comparison to the control (by 90%, P < 0.01), 1:1 (by 91%, P < 0.01) and 4:1 (by 89%, P < 0.01) (Figure 1C).
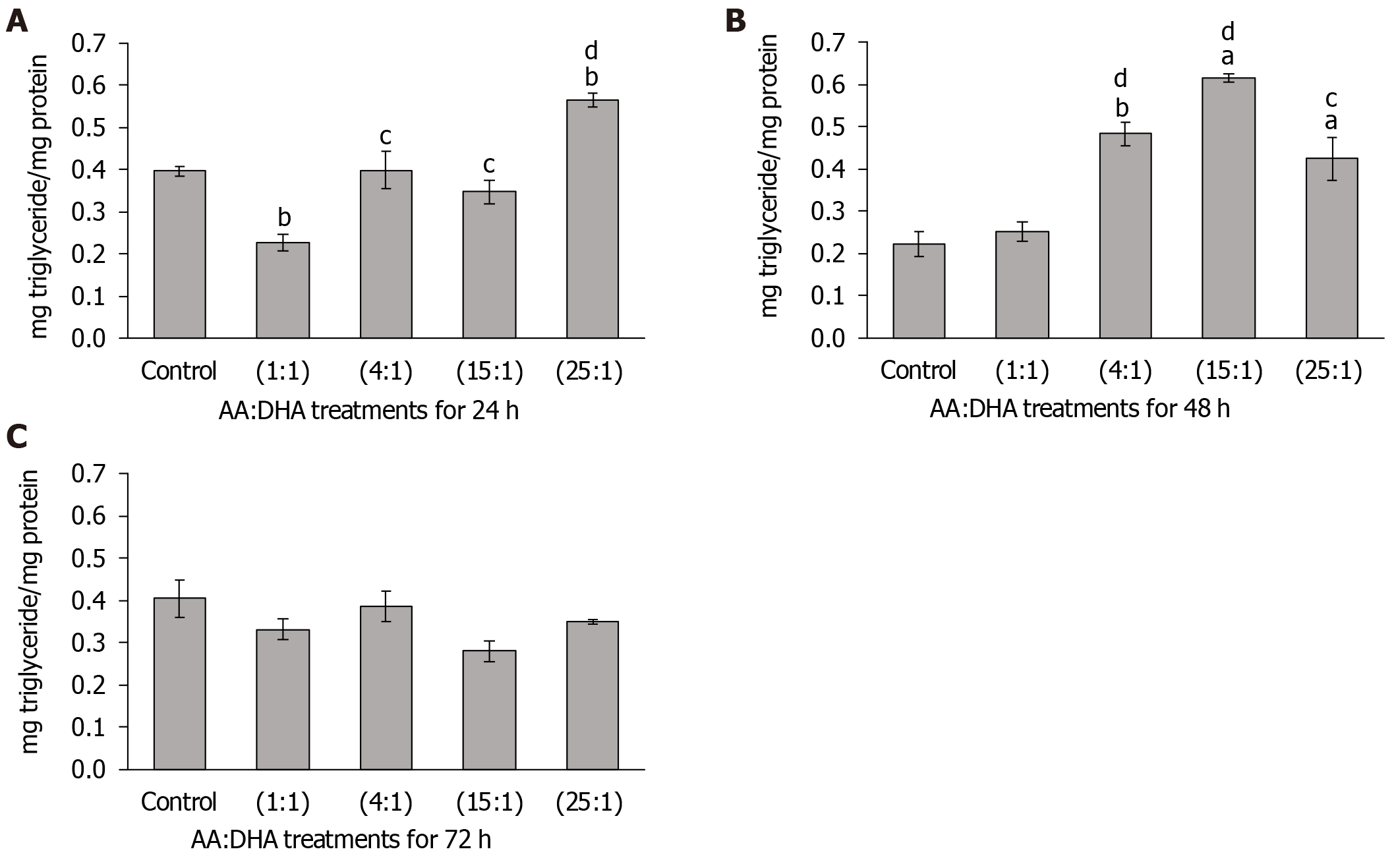
Significant changes in cellular lipid were observed after 24 h. Interestingly, in comparison to the untreated control, the 1:1 ratio reduced triglyceride accumulation (by 43%, P = 0.01), whereas a high 25:1 ratio elevated intracellular triglyceride levels (by 42%, P < 0.01) (Figure 2A). Importantly, in comparison to treatment with 1:1 ratio, lipid concentration was elevated with 4:1 ratio (by 75%, P < 0.05), 15:1 ratio (by 53%, P < 0.05), and with 25:1 ratio (by 41%, P < 0.01) (Figure 2A).
Similarly, after 48 h, treatments with 4:1, 15:1 and 25:1 ratios increased triglyceride accumulation in comparison to the control (by 117%, P = 0.01; 177%, P < 0.05; and 91%, P < 0.05, respectively), and also when compared to treatment with 1:1 ratio (by 91%, P < 0.01; 143%, P < 0.01 and 68%, P < 0.05, respectively) (Figure 2B). No significant alterations in triglyceride accumulation were observed after 72 h (Figure 2C).
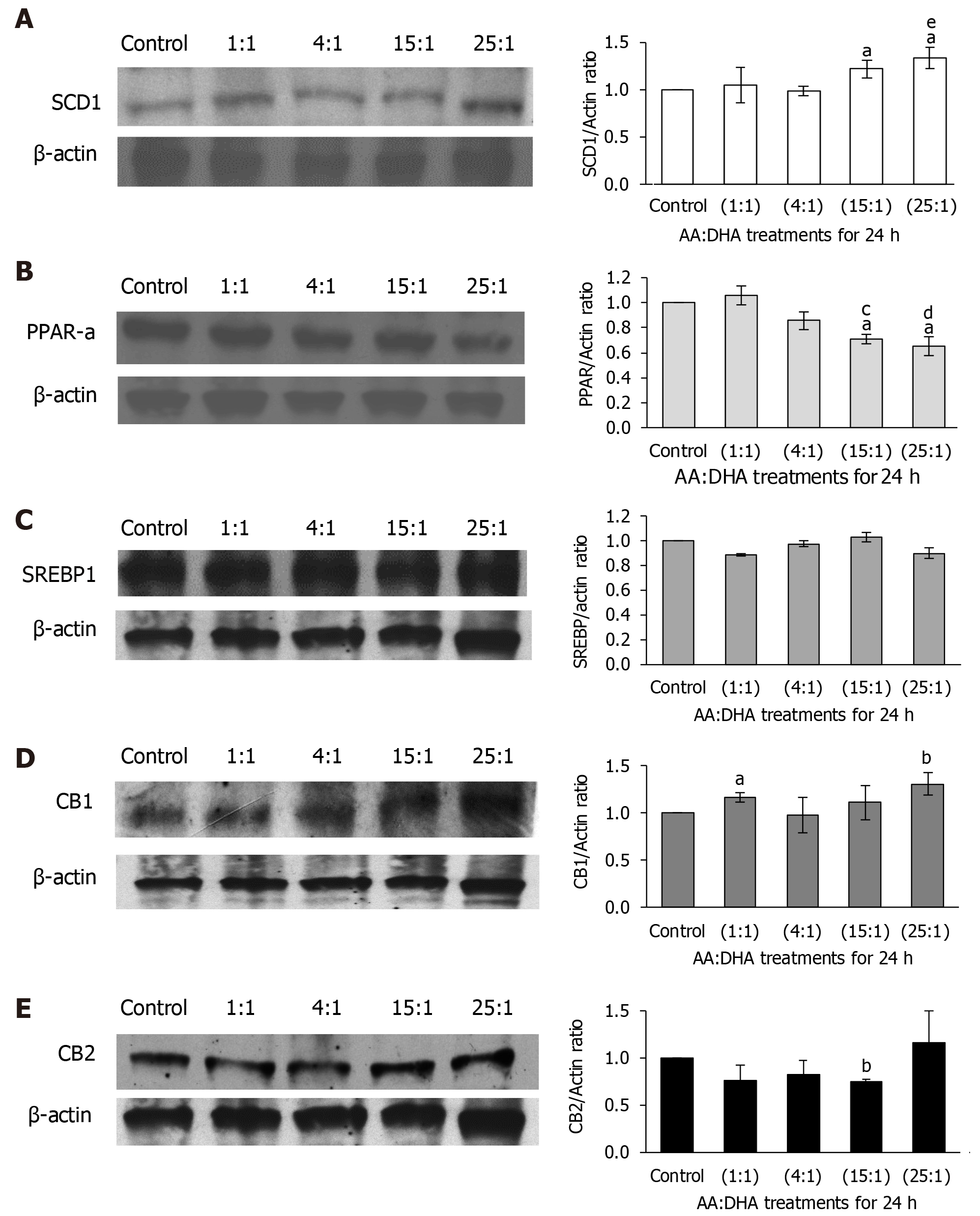
Molecular mediators of lipid metabolism were examined. When compared to the untreated control, SCD1 expression increased significantly following treatments with 15:1 and 25:1 ratios [by 22% and 33%, (P < 0.05), respectively] (Figure 3A). Furthermore, treatment with 25:1 ratio increased its expression compared to the moderate 1:1 ratio treatment (by 35%, P < 0.05) (Figure 3A).
PPAR-α expression significantly decreased with 15:1 ratio when compared with the control and 1:1 treatments (by 29% and 35%; P < 0.05, respectively) (Figure 3B). This trend of decreasing expression was more evident following treatment with the 25:1 ratio, when compared to control and 1:1 treatments (by 35%, P < 0.05 and 40%, P < 0.01, respectively) (Figure 3B). No changes in SREBP1-c expression was observed at all ratios (Figure 3C).
Following treatment with 1:1 ratio, CB1 expression increased only subtly compared to the control (by 17%, P < 0.05), but the increment was significantly greater at 25:1 ratio (by 31%, P < 0.01) (Figure 3D). CB2 expression showed no significant alterations, except with 15:1 ratio, which reduced CB2 expression compared to the control (P < 0.01) (Figure 3E), although the 25:1 ratio which showed a general increased expression when compared to all ratios.
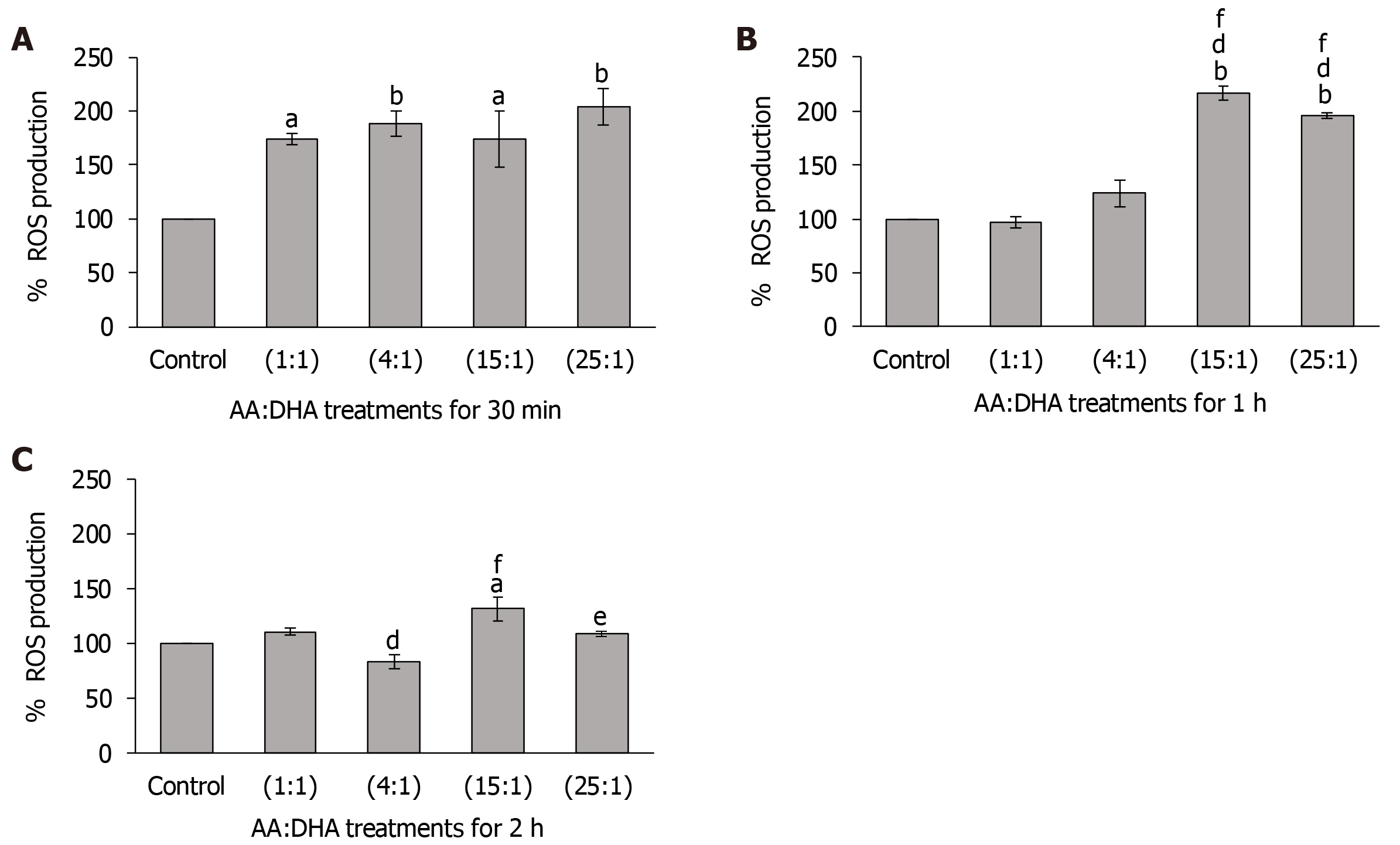
In comparison to the untreated control, ROS levels significantly increased with all ratios after 30 min (1:1 and 15:1 by 74%, P < 0.05; 4:1 and 25:1 by 88% and 104%, respectively, P < 0.01) (Figure 4A). No major differences were observed in ROS levels between the moderate ratios (1:1 and 4:1) and high ratios (15:1 and 25:1).
Similarly, after 1 h, while ROS levels increased following treatments with 15:1 ratio (by 117%; P < 0.01) and 25:1 ratio (by 96%; P < 0.01), treatments with 1:1 and 4:1 ratios did not cause any significant change compared to the control (Figure 4B). Interestingly, treatment with high ratios (15:1 and 25:1) significantly elevated ROS levels compared to the moderate ratios of 1:1 and 4:1. Specifically, when compared with the 1:1 and 4:1 ratio, the 15:1 ratio increased ROS levels by 123% (P < 0.01) and 75% (P < 0.01) respectively, and 25:1 ratio increased ROS levels by 102% (P < 0.01) and 58% (P < 0.01), respectively (Figure 4B).
After 2 h, in comparison to 1:1 ratio, ROS levels decreased with the 4:1 ratio (by 17% P < 0.01) (Figure 4C). However, in comparison to the control and 4:1 ratio, ROS levels were significantly elevated with the high 15:1 ratio (by 31%, P < 0.05 and 58% P < 0.01, respectively). These were also high with the 25:1 treatment when compared with the 4:1 treatment (by 30% P < 0.05) (Figure 4C). No effect was observed after 3 h, 6 h or 24 h for all ratios (data not shown for brevity).
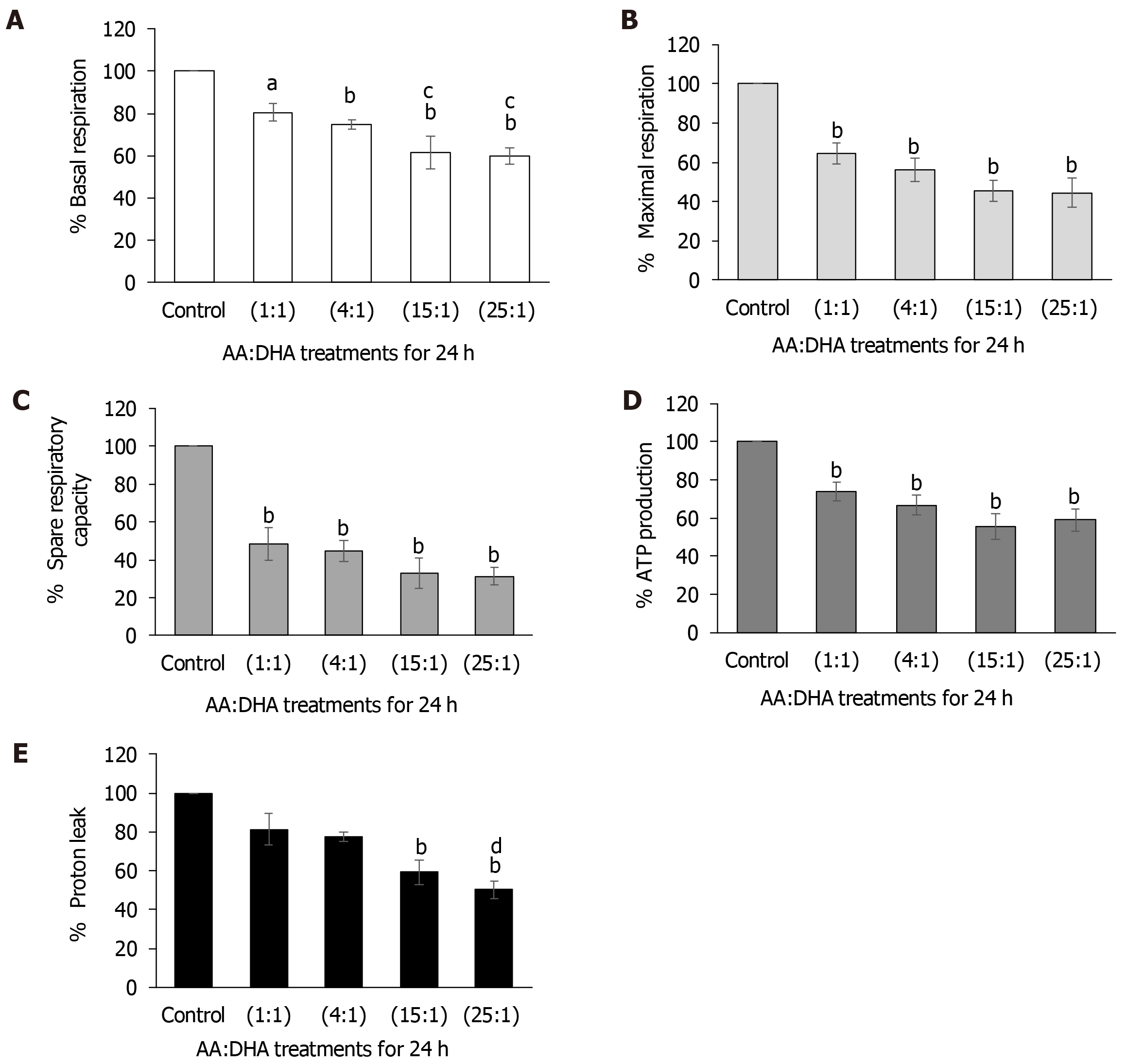
Elevation in AA:DHA ratios caused a gradual and significant decrease in basal respiration when compared to control [1:1 (by 20%, P < 0.05); 4:1 (by 25%, P < 0.01); 15:1 (by 38%, P < 0.01); and 25:1 (by 40%, P < 0.01)] (Figure 5A). Similar results were observed when compared to treatment with 1:1 ratio, whereby basal respiration significantly reduced with 15:1 and 25:1 ratios (by 19% and 21%, respectively, P < 0.05) (Figure 5A).
Maximal respiration decreased gradually with 1:1, 4:1, 15:1 and 25:1 ratios (by 35%, 44%, 54%, and 56% (P < 0.01), respectively) (Figure 5B). Spare respiratory capacity showed a similar pattern of significant reductions (by 52%, 56%, 67%, and 68% (P < 0.01) over the increasing AA/DHA ratios (Figure 5C). ATP production significantly reduced with elevation in AA:DHA 1:1, 4:1, 15:1, 25:1 ratios (by 25%, 33%, 44% and 41%, respectively, (P < 0.01)) (Figure 5D). Proton leak decreased significantly with high omega ratio of 15:1 (by 41% P < 0.01) compared to control, and with 25:1 by 50% and 31% (P < 0.01), when compared to control and 1:1 ratio, respectively (Figure 5E).
NAFLD is recognized as a growing global epidemic affecting 10%-35% of adult population in the world[25], with highest prevalence in the Middle East and South America[26]. In the US, it affects at least 18 % of the adult population and 90 % of obese people with an overall prevalence of 35 %[26]. Moreover, a recent epidemiological study demonstrated an exponential increase in NAFLD burden in the United States[27]. Predicted to “emerge as the leading cause of end-stage liver disease”[4], NAFLD calls for a thorough understanding of the underlying mechanisms.
High dietary intake of omega-6 fatty acids (mainly in processed foods) and/or lower intake of omega-3 fatty acids has been implicated in the development of fatty liver[7,28,29]. In vivo and in vitro studies have demonstrated the significance of omega-3 fatty acids in regulating lipid accumulation, fatty acid oxidation and reversing steatosis-induced mitochondrial dysfunction[30,31]. The significance of balanced omega-6:3 ratio was more evident in recent trials where treatment of NAFLD patients with omega-3 fatty acids such as eicosapentaenoic acid (EPA) or DHA reduced steatosis and liver enzymes[32,33]. However, the underlying pathological mechanisms under high omega-6: omega-3 states remain unclear. It is pivotal to understand these mechanisms to identify biochemical pathways and molecular targets that may help in formulating preventative and therapeutic interventions to halt, decelerate or reverse NAFLD progression.
Accordingly, here, we investigated the effect of increasing ratios of omega-6 (AA): omega-3 (DHA) fatty acids on various cellular parameters in VL-17A cells. We compared the effects of high AA: DHA ratios with untreated control and with the effects induced by healthy ratios of 1:1 and 4:1. The aim was to understand these altered mechanisms that prevail during high omega-6: omega-3 states and relate these to NAFLD pathogenesis.
High AA:DHA ratios significantly reduced mitochondrial activity and thus cell viability (Figure 1). This indicates lipotoxicity due to high omega-6 (AA) content, as its immediate metabolites are strongly proinflammatory[7]. The majority of studies with fatty acid treatment using in vitro models of NAFLD are based on a single 12-48 h time point to enable lipid accumulation and its subsequent detection. Our data showed a marked increase in triglyceride accumulation after 24 h and also at 48 h, in comparison to the untreated control and healthy ratios (1:1 and 4:1) (Figure 2), which is similar to other models of NAFLD where steatosis was reported in primary hepatocytes after 16-24 h treatment with oleic acid[34], palmitic acid[35] or stearic acid[36].
However, after 72 h lipid concentration was normal. This suggests a biological response to high AA:DHA ratios in the short-term. Repeating the treatment again or a sustained period of omega fatty acids in the growth media, would provide further support that the response is biological rather than an acute transient effect. Our findings are generally in line with previous studies where high-fat diet with various AA:EPA and AA:DHA ratios (1:1, 5:1, 10:1 and 20:1) increased hepatic phospholipid AA:eicosapentaenoic acid and AA:DHA in a dose dependent manner, mildly influenced inflammatory signaling, as well as key lipogenic regulators[37], though lowering the ratio did not prevent lipid accumulation[37]. Despite the latter study there is substantial evidence that the omega 6:3 ratio is an important contributory factor in NAFLD development. Recently NAFLD co-twin studies showed that the hepatic omega 6:3 ratio is significantly greater when liver fat > 5% suggesting the impact of diet independent of genetics plays a role in NAFLD occurrence[38]. In other models of NASH where animals were fed a Western diet, omega 6 lipid concentrations were increased in hepatic membranes, whereas omega 3 lipid concentrations were reduced; inflammatory markers were also increased, and this effect was reversed when animals were given DHA[39]. Whereas with other models of fatty liver, lowering the omega 6:3 ratio attenuated gut and liver injury suggesting that a normal ratio is important in fatty liver reversal[40].
Under physiological conditions, free fatty acids fuel oxidative stress and produce inflammatory cytokines that induce cell injury. Therefore, their esterification and deposition in the liver as triglycerides provide a protective mechanism to prevent additional liver damage[2]. Thus, it is possible that the high AA:DHA-ratio-induced elevated triglyceride concentration observed here (Figure 2) are a manifestation of a counter-protective response against the high omega-6: omega-3 ratio. However, it is also possible that this triglyceride accumulation may contribute to fatty liver pathogenesis because excessively increased cellular triglycerides may dysregulate lipid metabolism, promote steatosis and insulin resistance, elevate gluconeogenesis and decrease glycogenesis[41].
Following the confirmation of high AA:DHA-induced cellular triglyceride accumulation (Figure 2), molecular mediators of lipid metabolism were examined. High dietary fat can increase the synthesis of endocannabinoids, which upregulate the activity of CB1 and CB2[42-44]. This activation has been implicated in the development of NAFLD, partly via upregulation of lipogenic enzymes like SCD1 that promote fatty acid synthesis, and downregulation of PPAR-α expression, which stimulates hepatic oxidation of fatty acids[7]. Whether high AA:DHA ratio alters these mediators of lipid metabolism remain unknown. Therefore, we studied the effect of high AA:DHA ratio on the expression of SCD1 to understand its lipogenic capacity and PPARα to examine its potential for fatty acid oxidation.
Here, high AA:DHA ratio increased SCD1 expression (Figure 3A), indicating elevation in lipogenic capacity. This is consistent with a previous study where mice fed a diet with high omega-6: omega-3 ratio demonstrated increased expression of SCD1[45]. The increase in SCD1 expression could be due to direct binding of liver X receptor (it’s activator) or due to activation of its transcription factor SREBP1c[30,46,47]. Conversely other studies have reported a decrease in lipid droplet levels combined with a downregulation in SCD1 & SREBP levels following DHA treatment for 12 h in primary hepatocytes[48], although here no significant alterations were observed in SREPB1c expression (Figure 3C). Finally, high AA:DHA ratio reduced PPARα expression (Figure 3B), which indicated reduced capacity of fatty acid oxidation under these conditions. Elevation in SCD1 in combination with a reduction in PPARα expression may partly explain the mechanisms underlying fatty liver development under high omega-6:3 environment.
We also examined the expression of CB1 and CB2 under conditions of high omega-6:3 ratio to evaluate its stimulatory potential for fatty acid synthesis. As hypothesized, CB1 expression significantly increased following treatment with a high 25:1 ratio (Figure 3D). Although CB1 activation via LXR is thought to upregulate SREBP1c[49], our data suggested no direct correlation. This is similar to studies in HepG2 cells where CB1 agonist did not significantly alter the expression of SREBP-1c[50]. Furthermore, liver CB1 mRNA expression negatively correlated with hepatic PPARα expression, but not SREBP1c in patients with NASH[51], which is similar to the pattern observed in this study. Furthermore, inhibition of CB1 receptors has shown to improve lipogenesis in vitro[52]. Since high omega-6:3 ratio promotes obesity via both, AA-derived eicosanoid metabolites and over-activation of the cannabinoid system[10], it is possible that cannabinoid-independent pathways may sufficiently operate for high omega-6-induced fatty acid synthesis or other regulators may predominate. For example, CB1 receptors could be regulated by SREBP-1c, ChREBP and LXRs[52]. Overall, these findings suggest a role for CB1 activation under high omega 6:3 ratios leading to enhanced steatosis.
Except for 25:1 ratio, CB2 receptors showed moderately reduced expression when compared to control (Figure 3E), which was significantly lower following treatment with 15:1 ratio (Figure 3E). While some studies suggest that CB2 activation promotes NAFLD, other studies indicate a preventative role[42,53,54]. However, other studies have also reported a strong downregulation of CB2 gene expression in human hepatocytes following fatty acid treatment[50]. Further studies using agonists/antagonists of the CB1 and 2 receptors are required to determine their exact role in NAFLD deve-lopment.
Increased oxidative stress is one of the key characteristics of NAFLD, which promotes tissue injury[55]. There is no data yet on the effect of high AA:DHA ratios on ROS production. Here, we observed that ROS levels increased with all AA:DHA ratios within 30 min and generally beyond this time when compared to the untreated control (Figure 4). Moreover, high AA:DHA ratios elevated ROS levels in comparison to the healthy ratios of 1:1 and 4:1 at 1 h and 2 h (Figure 4B, 4C), clearly indicating the impact of high AA:DHA ratio on ROS production. After 3, 6 or 24 h no increase in ROS production occurred, supporting the point that incubation time is an important aspect in this model. This is similar to other models of NAFLD, where ROS production was significantly increased after 30 min of palmitic acid treatment[56]. Alternatively, after 30 min incubation with DHA ROS levels were lower than steatotic HepG2 cells[31]. However, other studies have shown an increase in ROS levels after a single measurement at 24 h of palmitic acid treatment[57,58]. This highlights the variance in time dependent ROS effects depending on the model used, although the net effect is increased oxidative stress.
The high ROS effect could be attributed to the high AA content in the AA:DHA ratio, which acts as a strong inhibitor of complexes I and III in the respiratory chain[59]. This would hinder electron flow through the respiratory chain causing electron leakage from these complexes and reduce mitochondrial membrane potential, thereby promoting ROS production[60]. However, other sources of ROS could be via CYP2E1 metabolism of AA[61]. Interestingly the high 15:1 ratio maintained the high levels of ROS after 1 h and 2 h, whereas the lower ratios of 1:1 and 4:1 either restored ROS levels to that of control or decreased ROS production (Figure 4B, 4C). This restoration could be due to fatty acid oxidation and the capability of the respiratory chain to handle the excess electrons released when the ratio was in the recommend healthy range.
Disturbed mitochondrial function has been implicated in the pathogenesis of NAFLD[55]. A 30%-40% decrease in the respiratory rate along with mitochondrial uncoupling has been reported in obese patients with NASH[62]. Therefore, we investigated the impact of high AA:DHA ratio on several mitochondrial functions. We observed that high AA:DHA ratios (15:1 and 25:1) decreased basal and maximal respiration, spare respiratory capacity, proton leak and ATP production (Figure 5). The mechanisms responsible for this could relate to destabilization of cytochrome C affecting electron flow and ultimately ATP synthesis[63], the direct uncoupling protonophoric ability of fatty acids which impairs ATP synthesis[63] or the opening of the mitochondrial permeability transition pores causing mitochondrial membrane potential loss leading to mitochondrial swelling and cell death[64]. These findings are similar to animal studies with fatty liver, where a reduction in the respiratory rate, increased oxidative stress, and reduction in complex I activity was observed[65-67].
Further work is required to elucidate the exact damaging effect of omega 6 fatty acids, however, it is important to state that not all omega 6 fatty acids are harmful/proinflammatory or omega 3 fatty acids are protective, and thus it is important not to generalize fatty acids, but to focus on specific fatty acids.
In terms of study limitations, there are a number of caveats. Firstly, measurement of hepatic phospholipid AA: eicosapentaenoic acid and AA:DHA, the SCD activity which measures the conversion of fatty acids to lipids, and mRNA levels of fatty acid synthase and acetyl-CoA carboxylase would confirm the lipid changes following high omega 6:3 ratio supplementation in HepG2 cells. As we only observed ROS induced injury over a short period of time, other markers of oxidative stress such as lipid peroxides and protein carbonyls, combined with markers of endoplasmic reticulum stress, such as c-Jun N-terminal kinase, and measurement of AA metabolites such as leukotrienes would provide further weighting behind the observations. The precise role of CB1 and CB2 receptor activation requires further elucidation; application of receptor agonists/antagonists would provide insight into lipogenesis under high omega ratios. Finally, it will be important to examine the effect of adding DHA directly to the cells after omega treatment to ascertain whether lipid accumulation can be reversed. Nutritional interventions that focus on particular omega 3 fatty acids is of interest due to the growing body of evidence showing reversal of NAFLD upon treatment with omega fatty acids.
In conclusion, this is the first study using high to normal omega 6:3 ratios in VL17A (HepG2 cells). These cells overexpress CYP2E1 which can metabolize AA, thus they resemble metabolic changes occurring in hepatocytes. In addition, the majority of in vitro NAFLD models of fatty acid treatment are based on a single 12-48 h time point to enable lipid accumulation and its subsequent detection. As far as we are aware no studies have reported a time course effect of omega fatty acids from 24 to 72 h in HepG2 cells studying lipid accumulation or ROS production, combined with lipogenic markers.
In summary, high omega 6:3 ratios of AA:DHA stimulated lipid synthesis by reducing fatty acid oxidation (decrease in PPAR alpha expression), increased CB1 expression, and also promoting the conversion of unsaturated fatty acids to saturated fatty acids via SCD1 with the net effect of lipid accumulation in human hepatoma (VL17A) cells. High ratios also led to increased oxidative stress, which is an important feature in the inflammatory state. Lipids synthesised in the liver are thus susceptible to oxidative attack by ROS. The loss of mitochondrial function possibly due to ROS or direct uncoupling effect of AA also promotes cell death, thus compounding the inflammatory effects. These results suggest that high omega 6:3 ratios can possibly lead to key steps in the progression from fatty liver to NASH.
Fatty liver disease is due to the consumption of excess dietary calories as well as a disruption in lipid metabolism and leads to the condition non-alcoholic fatty liver disease (NAFLD). The prevalence of NAFLD in most Western societies ranges from 20%-50%. Whilst the mechanisms for NAFLD development are complex, NAFLD patients show higher levels of omega-6 fatty acids than omega 3 fatty acids. Omega 6 fatty acids are known to be damaging to the liver causing toxicity, but their precise role in the pathology of NAFLD is not understood. This was an important question since NAFLD is a major public health problem and alternative interventions are required.
The main treatment options for NAFLD are dietary and lifestyle changes. We therefore addressed the question of how diet increases the risk of NAFLD, specifically the role of omega 6 fatty acids in relation to omega 3 fatty acids. Since this is a modifiable risk factor it is important to understand how high levels of omega 6 fatty acids can damage the liver and whether omega 3 fatty acids can minimise/reduce this damage. This will lead to improved understanding of NAFLD development and new therapeutic treatment options.
This research study aimed to understand the role of high omega 6:omega 3 ratios in a hepatic cell line model of NAFLD, thus allowing its contribution to lipotoxicity, oxidative stress and mitochondrial function to be investigated. These findings may help explain the progression from fatty liver to the inflammatory state, non-alcoholic steatohepatitis.
Human hepatoma cells, named VL-17A were treated with a range of high and normal ratios of omega-6: omega-3 fatty acids [arachidonic acid (AA): docosahexaenoic acid (DHA)]. These novel experiments examined the effect of these ratios on mitochondrial function, oxidative stress and viability. Changes in lipid accumulation combined with the expression of relevant lipogenic proteins was also assessed.
High omega-6:omega-3 (AA:DHA) ratio altered several processes in VL-17A cells. It reduced mitochondrial activity indicating lipotoxicity, increased triglyceride accumulation, elevated reactive oxygen species levels and interrupted several mitochondrial functions. Moreover, our study provided mechanistic data as to which of these detrimental effects may be mediated under high omega-6: omega-3 conditions and contribute to NAFLD development. Specifically, these include increased expression of stearoyl-CoA desaturase and decreased expression of peroxisome proliferator-activated receptor alpha. Also, elevation in cannabinoid receptor CB1 expression was observed that has been positively associated with fatty liver development. The present study clearly demonstrates the potential long-term consequences of high omega-6:3 ratios in NAFLD development.
The conclusions from this study strongly suggest that high omega 6:omega 3 ratios are detrimental to liver function, promoting an oxidant environment combined with higher amounts of lipid accumulation. These features are the hallmark of NAFLD indicating that altered omega 6:3 fatty acid ratios play an important role in NAFLD development and progression. Therefore, the original hypothesis was confirmed which can form the basis for further mechanistic studies examining other omega 6 fatty acids in NAFLD pathogenesis. The study also supports various studies where clinical interventions using omega 3 fatty acids have been utilised for NAFLD treatment.
Whilst further work is required, when investigating patients with NAFLD, measurement of circulating omega fatty acids should be considered. This may lead to the possibility of treating patients with omega 3 fatty acids. Other interventions that ameliorate oxidative stress and which improve mitochondrial function also require future research as this may lead to the reversal of NAFLD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng JT, Mizuguchi T, Niu ZS, Shimizu Y, Sitkin S S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3544] [Cited by in F6Publishing: 4072] [Article Influence: 678.7] [Reference Citation Analysis (7)] |
| 2. | Sharp PA. New insights into the role of iron in the development of nonalcoholic fatty liver disease. Hepatology. 2010;52:408-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313-3327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 682] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 4. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2383] [Cited by in F6Publishing: 3045] [Article Influence: 507.5] [Reference Citation Analysis (2)] |
| 5. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1516] [Cited by in F6Publishing: 1764] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 6. | Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | El-Badry AM, Graf R, Clavien PA. Omega 3 - Omega 6: What is right for the liver? J Hepatol. 2007;47:718-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2337] [Cited by in F6Publishing: 2001] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 9. | Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 10. | Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8:128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1007] [Cited by in F6Publishing: 854] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 11. | Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2004;106:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 499] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 12. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, Sanyal AJ. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 13. | Lazic M, Inzaugarat ME, Povero D, Zhao IC, Chen M, Nalbandian M, Miller YI, Cherñavsky AC, Feldstein AE, Sears DD. Reduced dietary omega-6 to omega-3 fatty acid ratio and 12/15-lipoxygenase deficiency are protective against chronic high fat diet-induced steatohepatitis. PLoS One. 2014;9:e107658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Hodson L, Bhatia L, Scorletti E, Smith DE, Jackson NC, Shojaee-Moradie F, Umpleby M, Calder PC, Byrne CD. Docosahexaenoic acid enrichment in NAFLD is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: a pilot study. Eur J Clin Nutr. 2017;71:1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Okada T, Kawakami S, Nakamura Y, Han KH, Ohba K, Aritsuka T, Uchino H, Shimada K, Sekikawa M, Ishii H, Fukushima M. Amelioration of D-galactosamine-induced acute liver injury in rats by dietary supplementation with betaine derived from sugar beet molasses. Biosci Biotechnol Biochem. 2011;75:1335-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Kotronen A, Seppänen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepää AL, Oresic M, Yki-Järvinen H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Huang L, Quinn MA, Frampton GA, Golden LE, DeMorrow S. Recent advances in the understanding of the role of the endocannabinoid system in liver diseases. Dig Liver Dis. 2011;43:188-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Donohue TM, Osna NA, Clemens DL. Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int J Biochem Cell Biol. 2006;38:92-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Chandrasekaran K, Swaminathan K, Kumar SM, Chatterjee S, Clemens DL, Dey A. Elevated glutathione level does not protect against chronic alcohol mediated apoptosis in recombinant human hepatoma cell line VL-17A over-expressing alcohol metabolizing enzymes--alcohol dehydrogenase and Cytochrome P450 2E1. Toxicol In Vitro. 2011;25:969-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Gyamfi D, Everitt HE, Tewfik I, Clemens DL, Patel VB. Hepatic mitochondrial dysfunction induced by fatty acids and ethanol. Free Radic Biol Med. 2012;53:2131-2145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Mehta K, Busbridge M, Renshaw D, Evans RW, Farnaud S, Patel VB. Characterization of hepcidin response to holotransferrin in novel recombinant TfR1 HepG2 cells. Blood Cells Mol Dis. 2016;61:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Chuturgoon AA, Phulukdaree A, Moodley D. Fumonisin B₁ inhibits apoptosis in HepG2 cells by inducing Birc-8/ILP-2. Toxicol Lett. 2015;235:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Mehta KJ, Coombes JD, Briones-Orta M, Manka PP, Williams R, Patel VB, Syn WK. Iron Enhances Hepatic Fibrogenesis and Activates Transforming Growth Factor-β Signaling in Murine Hepatic Stellate Cells. Am J Med Sci. 2018;355:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Patel VB, Spencer CH, Young TA, Lively MO, Cunningham CC. Effects of 4-hydroxynonenal on mitochondrial 3-hydroxy-3-methylglutaryl (HMG-CoA) synthase. Free Radic Biol Med. 2007;43:1499-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8 Suppl 1:S4-S8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6285] [Article Influence: 785.6] [Reference Citation Analysis (0)] |
| 27. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in F6Publishing: 1303] [Article Influence: 217.2] [Reference Citation Analysis (0)] |
| 28. | Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 29. | Da Silva HE, Arendt BM, Noureldin SA, Therapondos G, Guindi M, Allard JP. A cross-sectional study assessing dietary intake and physical activity in Canadian patients with nonalcoholic fatty liver disease vs healthy controls. J Acad Nutr Diet. 2014;114:1181-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Pachikian BD, Essaghir A, Demoulin JB, Neyrinck AM, Catry E, De Backer FC, Dejeans N, Dewulf EM, Sohet FM, Portois L, Deldicque L, Molendi-Coste O, Leclercq IA, Francaux M, Carpentier YA, Foufelle F, Muccioli GG, Cani PD, Delzenne NM. Hepatic n-3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets. PLoS One. 2011;6:e23365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Jiang L, Hu W, Zheng Q, Xiang W. Mitochondrial dysfunction during in vitro hepatocyte steatosis is reversed by omega-3 fatty acid-induced up-regulation of mitofusin 2. Metabolism. 2011;60:767-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | He XX, Wu XL, Chen RP, Chen C, Liu XG, Wu BJ, Huang ZM. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2016;11:e0162368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Musa-Veloso K, Venditti C, Lee HY, Darch M, Floyd S, West S, Simon R. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev. 2018;76:581-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Mahli A, Seitz T, Beckröge T, Freese K, Thasler WE, Benkert M, Dietrich P, Weiskirchen R, Bosserhoff A, Hellerbrand C. Bone Morphogenetic Protein-8B Expression is Induced in Steatotic Hepatocytes and Promotes Hepatic Steatosis and Inflammation In Vitro. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Miao L, Zhang H, Wu G, Zhang Z, Lv J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Health Dis. 2018;17:270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Zhang Y, Dong L, Yang X, Shi H, Zhang L. α-Linolenic acid prevents endoplasmic reticulum stress-mediated apoptosis of stearic acid lipotoxicity on primary rat hepatocytes. Lipids Health Dis. 2011;10:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Enos RT, Velázquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model. Nutr Res. 2015;35:449-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Bogl LH, Kaprio J, Pietiläinen KH. Dietary n-6 to n-3 fatty acid ratio is related to liver fat content independent of genetic effects: Evidence from the monozygotic co-twin control design. Clin Nutr. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | García-Jaramillo M, Lytle KA, Spooner MH, Jump DB. A Lipidomic Analysis of Docosahexaenoic Acid (22:6, ω3) Mediated Attenuation of Western Diet Induced Nonalcoholic Steatohepatitis in Male Ldlr -/- Mice. Metabolites. 2019;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Warner DR, Warner JB, Hardesty JE, Song YL, King TN, Kang JX, Chen CY, Xie S, Yuan F, Prodhan MAI, Ma X, Zhang X, Rouchka EC, Maddipati KR, Whitlock J, Li EC, Wang GP, McClain CJ, Kirpich IA. Decreased ω-6:ω-3 PUFA ratio attenuates ethanol-induced alterations in intestinal homeostasis, microbiota, and liver injury. J Lipid Res. 2019;60:2034-2049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 41. | Ress C, Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J Gastroenterol. 2016;22:1664-1673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 73] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Purohit V, Rapaka R, Shurtleff D. Role of cannabinoids in the development of fatty liver (steatosis). AAPS J. 2010;12:233-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Mallat A, Teixeira-Clerc F, Deveaux V, Manin S, Lotersztajn S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol. 2011;163:1432-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Jorgačević B, Vučević D, Đuričić I, Šobajić S, Mladenović D, Vesković M, Vukićević RJ, Radosavljević T. The effect of cannabinoid receptor 1 blockade on hepatic free fatty acid profile in mice with nonalcoholic fatty liver disease. Chem Phys Lipids. 2017;204:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Sealls W, Gonzalez M, Brosnan MJ, Black PN, DiRusso CC. Dietary polyunsaturated fatty acids (C18:2 omega6 and C18:3 omega3) do not suppress hepatic lipogenesis. Biochim Biophys Acta. 2008;1781:406-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786-6798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520-9528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 505] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 48. | On S, Kim HY, Kim HS, Park J, Kang KW. Involvement of G-Protein-Coupled Receptor 40 in the Inhibitory Effects of Docosahexaenoic Acid on SREBP1-Mediated Lipogenic Enzyme Expression in Primary Hepatocytes. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Regnell SE. Cannabinoid 1 receptor in fatty liver. Hepatol Res. 2013;43:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | De Gottardi A, Spahr L, Ravier-Dall'Antonia F, Hadengue A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int. 2010;30:1482-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Auguet T, Berlanga A, Guiu-Jurado E, Terra X, Martinez S, Aguilar C, Filiu E, Alibalic A, Sabench F, Hernández M, Del Castillo D, Richart C. Endocannabinoid receptors gene expression in morbidly obese women with nonalcoholic fatty liver disease. Biomed Res Int. 2014;2014:502542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Shi D, Zhan X, Yu X, Jia M, Zhang Y, Yao J, Hu X, Bao Z. Inhibiting CB1 receptors improves lipogenesis in an in vitro non-alcoholic fatty liver disease model. Lipids Health Dis. 2014;13:173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, Manin S, Nhieu JT, Belot MP, Zimmer A, Even P, Cani PD, Knauf C, Burcelin R, Bertola A, Le Marchand-Brustel Y, Gual P, Mallat A, Lotersztajn S. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One. 2009;4:e5844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 54. | Schmitz K, Mangels N, Häussler A, Ferreirós N, Fleming I, Tegeder I. Pro-inflammatory obesity in aged cannabinoid-2 receptor-deficient mice. Int J Obes (Lond). 2016;40:366-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Yu J, Marsh S, Hu J, Feng W, Wu C. The Pathogenesis of Nonalcoholic Fatty Liver Disease: Interplay between Diet, Gut Microbiota, and Genetic Background. Gastroenterol Res Pract. 2016;2016:2862173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Imarisio C, Alchera E, Bangalore Revanna C, Valente G, Follenzi A, Trisolini E, Boldorini R, Carini R. Oxidative and ER stress-dependent ASK1 activation in steatotic hepatocytes and Kupffer cells sensitizes mice fatty liver to ischemia/reperfusion injury. Free Radic Biol Med. 2017;112:141-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Moravcová A, Červinková Z, Kučera O, Mezera V, Rychtrmoc D, Lotková H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol Res. 2015;64 Suppl 5:S627-S636. [PubMed] [Cited in This Article: ] |
| 58. | Dessirier JM, O'Mahony M, Sieffermann JM, Carstens E. Mecamylamine inhibits nicotine but not capsaicin irritation on the tongue: psychophysical evidence that nicotine and capsaicin activate separate molecular receptors. Neurosci Lett. 1998;240:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Cocco T, Di Paola M, Papa S, Lorusso M. Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic Biol Med. 1999;27:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 61. | Caro AA, Cederbaum AI. Role of intracellular calcium and phospholipase A2 in arachidonic acid-induced toxicity in liver cells overexpressing CYP2E1. Arch Biochem Biophys. 2007;457:252-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 567] [Cited by in F6Publishing: 630] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 63. | Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008;45:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 64. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 839] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 65. | Oliveira CP, Coelho AM, Barbeiro HV, Lima VM, Soriano F, Ribeiro C, Molan NA, Alves VA, Souza HP, Machado MC, Carrilho FJ. Liver mitochondrial dysfunction and oxidative stress in the pathogenesis of experimental nonalcoholic fatty liver disease. Braz J Med Biol Res. 2006;39:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Petrosillo G, Portincasa P, Grattagliano I, Casanova G, Matera M, Ruggiero FM, Ferri D, Paradies G. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochim Biophys Acta. 2007;1767:1260-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Lee K, Haddad A, Osme A, Kim C, Borzou A, Ilchenko S, Allende D, Dasarathy S, McCullough A, Sadygov RG, Kasumov T. Hepatic Mitochondrial Defects in a Nonalcoholic Fatty Liver Disease Mouse Model Are Associated with Increased Degradation of Oxidative Phosphorylation Subunits. Mol Cell Proteomics. 2018;17:2371-2386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |