Published online Jul 26, 2014. doi: 10.4330/wjc.v6.i7.539
Revised: March 25, 2014
Accepted: May 31, 2014
Published online: July 26, 2014
Obesity has become an important public health issue in Western and developing countries, with well known metabolic and cardiovascular complications. In the last decades, evidence have been growing about the active role of adipose tissue as an endocrine organ in determining these pathological consequences. As a consequence of the expansion of fat depots, in obese subjects, adipose tissue cells develope a phenotypic modification, which turns into a change of the secretory output. Adipocytokines produced by both adipocytes and adipose stromal cells are involved in the modulation of glucose and lipid handling, vascular biology and, moreover, participate to the systemic inflammatory response, which characterizes obesity and metabolic syndrome. This might represent an important pathophysiological link with atherosclerotic complications and cardiovascular events. A great number of adipocytokines have been described recently, linking inflammatory mileu and vascular pathology. The understanding of these pathways is crucial not only from a pathophysiological point of view, but also to a better cardiovascular disease risk stratification and to the identification of possible therapeutic targets. The aim of this paper is to review the role of Adipocytokines as a possible link between obesity and vascular disease.
Core tip: Our article, provide a comprehensive review of the evidence about adipose tissue and cardiovascular risk, focusing on the pathophysiological and clinical role of fat-derived mediators, the so-called adipocytokines.
- Citation: Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Russo PE, Riegler L, Bianchi R, Crisci M, Palma GD, Golino P, Russo MG, Calabrò R, Calabrò P. Adipose tissue and vascular inflammation in coronary artery disease. World J Cardiol 2014; 6(7): 539-554
- URL: https://www.wjgnet.com/1949-8462/full/v6/i7/539.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i7.539
Obesity is rapidly spreading to epidemic levels in Western and developing countries, becoming a serious health issue. It is associated with increasing morbidity and mortality[1]. Obesity shares several features with metabolic syndrome (MetS) and both are associated with well known risk factors for cardiovascular disease (CVD)[2], such as glucose and lipid metabolism impairment, endothelial dysfunction and atherosclerosis, finally leading an increased risk of cardiovascular events[2].
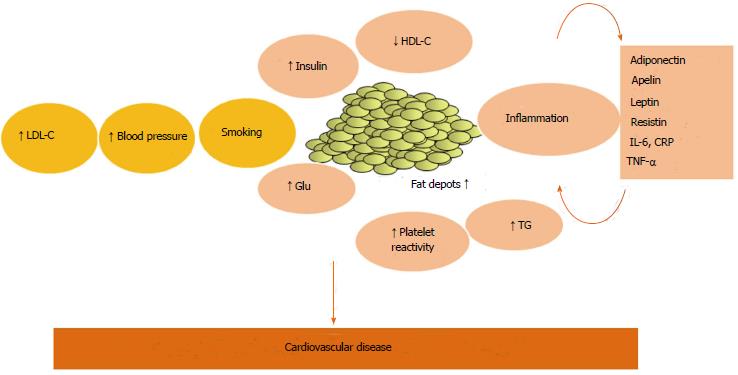
Recent evidence claimed that inflammation might represent the pathophysiological link between obesity and MetS (Figure 1), and increasing interest has been developed in the role of adipose tissue as an active trigger of this systemic inflammatory response[3].
Since adipose tissue is capable of releasing several mediators, the so-called adipocytokines, it is now considered an endocrine organ, affecting metabolism and vascular function. The understanding of these pathways is crucial not only from a pathophysiological point of view, but also to a better cardiovascular disease (CVD) risk stratification and to the identification of possible therapeutic targets.
There is consistent epidemiologic evidence for an independent association between obesity and CVD[4]. In several large, prospective, long-term studies, obesity was independently associated with all-cause mortality and death from CVD in both women and men. The Nurses’ Health Study evaluated the relationship between body mass index (BMI) and mortality in 115195 women[5]. A significant trend for increasing risk of death with increasing BMI and an an association between BMI and cardiovascular mortality were found.
In the Framingham Heart Study, obesity was an independent risk factor for all-cause mortality among male participants, followed up for 30 years[6]. However, recent data have demonstrated an “obesity paradox”, with obese patients suffering frome CVD showing a better short-and long-term prognosis than leaner matched subjects[7]. In particular, in a large metanalysis[8], the overall obesity population, defined by BMI, showed an increased risk of mortality compared with normal BMI. However, overweight patients (BMI 25-30 kg/m2) had a significantly 6% lower mortality than patients with normal BMI. This finding was more pronounced in older cohorts[9]. Interestingly, several studies[7] suggested an influence of body fitness and on the relationship between adiposity and CVD prognosis. Thus obesity paradox seemed evident in patients with low fitness.
The so-called visceral obesity is now well-known to be associated to a higher mortality than the peripheral obesity, since it is linked to a higher prevalence of dysmetabolism and hypertension and endothelial dysfunction[3]. This evidence highlighted the importance of adipose tissue location in determining its unfavourable effects.
Obesity is characterized by an expanded adipose tissue mass[10] and, interestingly, as noted above, in overweight subjects a typical pattern of adipokines is present, which negatively affect metabolic situation and cardiovascular function[11].
Typically, the source of these substances are organs (the liver), and immune cells[12-14]. Most notably, several studies indicate that fat could either regulate the synthesis of these molecules or could be itself an immediate source.
The complexity of adipose tissue as an endocrine organ has been highlighted in several studies which have reported important differences among adipocytes from different depots. These characteristics may account for a differential contribution to obesity-related disorders[15].
Adipocytes frome brown adipose tissue (BAT) are mainly represented in fetuses and newborn and are implicated in thermogenesis[16,17]. A small amount of BAT persists in adults human AT[17].
On the other hand, white adipose tissue (WAT) represents a main kind of adipose tissue in adults[15], largely present in the subcutaneous region (SAT) or close and within the abdominal viscera (VAT).
Vague[18] first described a link between increased VAT and atherosclerosis, diabetes, and other diseases. This association might be explained by the increased production of mediators, acting with endocrine, autocrine and paracrine mechanisms[3]. Bigger VAT adipocytes in obese subjects are related to a higher mediators release, comparing to SAT[19,20]. These factors, adipocytokines-include molecules like tumor necrosis factor-α (TNF-α), leptin, adiponectin, resistin, plasminogen activator inhibitor-1 (PAI-1), apelin, interleukin-6 (IL-6), resistin, angiotensinogen, serum amyloid A (SAA), and C-reactive protein (CRP)[21].
Moreover, several inflammatory cells[19] are largely represented in the WAT stroma. These cells play an important role in tissue homeostasis, such as the clearance of necrotic adipocytes[18], increased in obesity. In particular, macrophages produce the majority of TNF-α and increase IL-6 and inducible-Nitric Oxide synthase expression. Then they were thought to be the primary source of the cytokines in adipose tissue[19,22,23]. However, we have demonstrated with an in vitro model, that the mature adipocytes fraction isolated from human adipose tissue is directly involved in both CRP and SAA release[24].
Interestingly, among visceral fat depots, another specific local fat depot has been studied in the last decade for its relationship with MetS and coronary artery disease (CAD), i.e., epicardial adipose tissue (EAT). EAT surrounds the heart, within the pericardium, it is in contact with coronary vessels (i.e., perivascular) and the surface of ventricles. It shows a higher rate of lipolysis and lipogenesis comparing to other fat depots[25]. EAT is involved in myocardial energy supply, thermoregulation, and protection of the cardiac autonomic nervous system as well as in the regulation of coronary vessel motion and lumen diameter[26].
In post-mortem series, epicardial fat have been reported to account for 20% of total heart weight[27]. Obesity is associated with an increase in the amount of EAT. Both total weight of epicardial fat and epicardial fat cells size correlate with body weight[28]. Moreover, epicardial fat assessed by echocardiography correlated significantly in multivariable analysis with key components of the MetS, including waist circumference, which is known to parallel the increase in VAT seen in MetS[29,30]. Then, the cardioprotective features of EAT might be somewhat blunted by the increase in cardiovascular risk carried by its pathological enlargement in obesity[31].
From a clinical point of view, several clinical and epidemiological studies have found an association between EAT and cardiometabolic risk factors and early stages of atherogenesis. The echocardiographic EAT evaluation was found to be associated with arterial stiffness and intima-media thickness (IMT), two indexes of subclinical atherosclerosis, in hypertensive patients in a study from our group[32]. Among the 459 patients examined, subjects with epicardial fat > 7 mm were older, had higher systolic, diastolic, and pulse pressure, increased left ventricular mass index, carotid IMT, diastolic and stiffness parameters compared with those with epicardial fat < 7 mm. Age, carotid IMT, and stiffness parameters were independently related to epicardial fat. These findings have been confirmed in a more recent study by Choi et al[33].
Importantly, the Framingham Heart Study[34] and Multi-Ethnic Study of Atherosclerosis[35] identified EAT volume as independent risk markers for CVD. Moreover, recently results of the Heinz Nixdorf Recall study, a population-based prospective cohort study, have been published[36]. Among the 4093 participants incidence of coronary events increased by quartile of EAT. Doubling of the EAT, measured by computed tomography (CT) scan, carried a 1.5-fold risk of coronary events after adjusting for cardiovascular risk factors and coronary artery calcium score.
Comparing to subcutaneous fat, EAT shows a more dense inflammatory cell infiltrate, predominantly represented by macrophages[37], and it produces highly atherogenic and inflammatory adipocytokines in patients with CAD[38].
EAT might then participate also in the inflammatory process within the atherosclerotic plaque. The incidence and severity of CAD and coronary calcification have been in fact associated with EAT thickness and volume[39].
A recent study assessed the relationship between EAT volume and plaque vulnerability in significant coronary stenosis using intravascular ultrasound. Authors found a positive correlation between EAT volume, measured by CT scan and the percentage of necrotic plaque tissue, and an inverse correlation between with the percentage of fibrous tissue[40]. Low-density lipoprotein (LDL) cholesterol level and EAT volume were independently associated with the percentage of necrotic plaque tissue. These findings are consistent with previous reports[41].
Moreover, EAT is associated also with microvascular dysfunction in the absence of obstructive CAD. In a recent study, patients underwent Rb-82 positron emission tomography to obtain standard myocardial perfusion index and myocardial flow reserve (MFR). EAT thickness, EAT volume and coronary calcium score values were higher in patients with impaired flow reserve, with EAT thickness showing the strongest negative correlation with MFR[42].
In last decades, it became clear that atherosclerosis is other than a cholesterol storage disease. A large body of literature highlighted the possible role of inflammation as causal factor in atherogenesis, from endothelial dysfunction to clinical events[43]. One of the first recognized stages of the atherosclerotic process consists of LDL intimal deposition and endothelial dysfunction.This is caused by the imbalance between nitric oxide (NO) and prostacyclin (PGI2)-mediated vasorelaxation and the increase in endogenous vasoconstrictors, such as endothelin-1 (ET-1)[44]. LDL become then oxidized (ox-LDL) by local reactive oxygen species (ROS) and subsequently induce endothelial cells to express adhesion molecules, such as vascular cell adhesion molecule-1, intercellular adhesion molecule 1, and selectins[45]. This together with the secretion of chemoattractant mediators, such as complement factors, IL-8, monocyte chemoattractant protein-1, determines mononuclear cells recruitment into the vascular wall. Thus, macrophages recruited to intima become “foam cells”, via ox-LDL phagocytosis[44]. Subsequent stages include the transition of the atherosclerotic plaque from the “fatty streak” to a more fibrous lesion. Main actors in this stage are vascular smooth muscle cells, which accumulate in the intima and produce extracellular matrix (ECM)[44].
Inflammation plays a key role in plaque destabilization and rupture which cause acute vascular events. High rate of vascular inflammation interferes with fibrous cap formation, induces apoptosis and degradation of the ECM, via an upregulation of metalloproteinase. The subsequent activation of the coagulation cascade leads to intravascular thrombus formation and the acute clinical events. In this setting, tissue factor (TF) plays a pivotal role in the pathophysiology of acute coronary syndromes by triggering the formation of intracoronary thrombi following endothelial injury[45].
As noted above, from a pathophysiological point of view, adipose tissue is not an innocent bystander in this process since it is found to be capable of producing enzyme, cytokines, growth factors and hormones which might affect each one of the stages described above. Moreover, even if the majority of patients suffering of cardiovascular diseases had at least one traditional risk factor[46,47], almost 25% of subjects did not show any of those[48]. In this setting, identifying new risk factors might increase our ability to discover and take care of high-risk patients. Within novel prediction factors, adipocytokines have been studied.
Leptin has been the first adipocytokine identified as the product of the ob gene in obese ob/ob mice[49], which participate in the signalling of fat stores[50].
Then, early studies on leptin focused on its role in obesity and potential therapies to control it. However, soon it became evident a broader biological role for leptin, including its potential implication in leading to cardiovascular complications in obese patients (Table 1). However, conflicting results on leptin role in CVD have been reported.
| Adipokine | Modulation of inflammation | Association with CVD |
| Leptin | ↑ T cell activation and Th lymphocyte response ↑ cytokine release ↑ NK cell activation ↑ macrophages’ cytokine release Activates neutrophils ↑ chemotaxis ↑ oxidative stress ↑ CRP production from endothelium | ↑ blood pressure ↑ atherothrombosis: ↑ cholesterol accumulation in vessel wall ↑ adhesion molecules (ICAM, VCAM) expression ↑ endothelial dysfunction (increasing eNOS production, ↓ NO, ↑ET-1) ↑ proliferation and migration of EC and VSMC ↑ ROS accumulation ↑ VSMC apoptosis ↓ angiogenesis ↑ platelet activity ↓ fibrinolysis ↑ PAI-1 ↑TF release from mononuclear cells Induce insulin resistance |
| Adiponectin | ↓ T cell activation and proliferation ↓ NF-κB Increases IL-10 Inhibits CRP and IL-6 release | Associated with HDL-C and inversely with LDL-C ↓ atherothrombosis: ↓ ICAM-1, VCAM-1, and E-selectin ↓ Transformation of macrophages to foam cells ↓ vascular muscular smooth cells proliferation and migration ↑ TIMP, through the increase in IL-10 ↑ oxidation of free fatty acids in several tissue ↑ insulin sensitivity |
In animal and in vitro models, leptin promotes atherogenesis, through an increase in oxidative stress in endothelial cells[51], an increased platelet reactivity and thrombosis[52]. On the other hand, several studies reported opposite effects such as induction of nitric oxide production, with anti atherogenic properties[53].
Most in vivo studies in humans demonstrated that leptin levels are linked with cardiovascular risk factors, like hyperlipidemia or hypertension[54-57], and markers of endothelial dysfunction, thrombophilia and inflammation. Finally, several prospective trials described a link of leptin levels with atherosclerosis and myocardial infarction (MI)[58-61].
In the West of Scotland Coronary Prevention Study, including 377 male participants and 738 controls[59], a 20% increase in the incidence of CAD was associated with each standard deviation increase in leptin levels, even after adjustment for potential confouders. Sattar et al[54] found a moderate association, although non significant, between leptin levels and CAD risk. This association was attenuated by BMI, but continued to be significant with other validated risk factors, including several markers of inflammation. Also in female population no significant link between leptin plasma levels and CAD was noted[61]. On the other hand, Kappelle et al[62], in healthy men, demonstrated a positive association of leptinemia and leptin/adiponectin ratio with incident CVD, even after additional adjustment for several potential confounders, such as clinical risk factors, lipid and microalbuminuria.
In patients with known CAD, leptin might predict future cardiovascular events independent of other risk factors, including lipid status and CRP, according to several reports. Wolk et al[60] followed up 504 patients who had undergone coronary angiography for both stable angina (SA) and ACS for up to 4 years. In the field of ACS, admission plasma leptin levels is associated with the success of thrombolytic therapy in patients with STEMI presenting < 6 h[63]. In particular, authors found higher rates of thrombolysis failure in patients with basal plasma leptin levels ≥ 14 ng/mL, in comparison with patients with levels less than 14 ng/mL.
The association between plasma leptin and adiponectin levels and recurrent cardiovascular events after an ACS has been investigate in The Long-Term Intervention with Pravastatin in Ischaemic Disease study[64]. Leptin was a significant and independent predictor of recurrent cardiovascular events.
Consistent with this report, Rajendran et al[65] measured leptin levels, high-sensitivity C Reactive protein (hs-CRP) and IL-6 levels in patients with acute myocardial infarction (AMI), showing higher levels of leptin than control subjects.
The relationship between serial serum leptin levels measurement after thrombolysis for AMI and the degree of coronary atherosclerosis, coronary reperfusion, echocardiographic findings, and clinical outcome was investigated by Khafaji et al[66] in a small study. Leptin concentrations peaked 36 h after admission. Significant correlation of mean serum leptin with reduced ejection fraction and a trend for an increase in the mean serum leptin levels with increasing number of diseased vessels were found. However, there was no correlation between serum leptin levels and outcome or myocardial reperfusion.
Karakas et al[67] in a population-based case-cohort study within the MONICA/KORA studies. After adjustment for various confounding factors, neither increased leptin levels or low adiponectin were associated with the incidence of coronary events in healthy subjects. Moreover, the leptin/adiponectin ratio didn’t improve the ability of the single adipocytokine to predict incident CAD. In another study conducted among patients with ACS and controls, lipid profiles, leptin, pregnancy associated plamsa protein A (PAPP-A) and CRP levels were assessed as markers of plaque vulnerability[68]. Significantly higher levels of leptin, PAPP-A and high-sensitivity CRP (hs-CRP) were observed in cases. At the multivariable analysis, leptin was not independently associated with ACS, while a positive correlation between CRP and leptin concentrations was noted.
Higher adiponectin and lower leptin levels were found to be associated with a high incidence of adverse events also in a Japanese cohort after successful emergency percutaneous coronary intervetion for AMI[69]. A low leptin to adiponectin ratio remained a significant independent predictor of adverse events during long-term follow-up at the multivariable analysis.
Similiar observations came earlier from Ku et al[70] They found that subjects with low baseline leptin levels had higher subsequent CV events and death. Interestingly, although subjects with low leptin had fewer co-morbidities and more favorable metabolic and inflammatory profiles, they showed a worse prognosis than subjects with higher leptin levels. This could be an example of “reverse epidemiology”[71,72], whereby a predictor of disease becomes inversely associated with prognosis once the disease has developed. According with this idea, a second paper described an association between low leptin levels and cardiovascular death in patients with chronic kidney disease[73]. Moreover, Leptin is elevated in chronic CAD. Multiple reports have shown that leptin causes coronary vasodilatation, activates endothelial progenitor cells, prevents lipid accumulation, and protects against ischemia-reperfusion injury[53,60,74,75]. Then a relative leptin deficiency might explain poorer prognosis seen in subjects with established CAD. Finally, the lack of association between leptin and mortality, especially in patients with higher BMI, could be otherwise explained by leptin resistance[76].
Adiponectin is a well-described adipocytokine, traditionally reported as a protective factor with an anti inflammatory effect (Table 1)[11]. It is clear that its circulating levels decrease with weight gain and are inversely correlated to the amount of VAT, as illustrated in CT scan studies[11]. Interestingly, decreased adiponectin was associated with enhanced pro-inflammatory phenotype in EAT in patients with CAD[77]. As notet above about leptin, growing conflicting data on adiponectin levels are emerging, suggesting higher complexity of its role, than previously thought. This is particularly evident in the balance between obesity, cardiovascular effects, and prognosis.
Consistent with a putative protective role, Ouchi et al[78] first detected lower adiponectin levels in subjects with established CAD. Following this experience, several cross-sectional and prospective studies have confirmed an inverse correlation between plasma adiponectin levels and incidence, severity and outcome of CAD[79-82].
Recent studies, however, failed to demonstrate this correlation[83,84], or even showed a paradoxical link between higher adiponectin levels and negative events, especially in patients with known CAD or at high cardiovascular risk[85]. Moreover, Zhang et al[86] demonstrated higher adiponectin levels inpatients with stable CAD and inducible ischemia.
In the Pravastatin Or atorVastatin Evaluation and Infection Trial-Thrombolysis in Myocardial Infarction 22[87], plasma adiponectin was measured in 3931 subjects with Acute Coronary Syndrome. Adiponectin was negatively associated with age, diabetes, BMI, and triglycerides, while a positive link was noted with the risk of death, MI, and heart failure.
Seven hundred and thirty-five consecutive patients with STEMI treated with primary percutaneous coronary intervention were included in a report by Lindberg et al[88]. Plasma adiponectin was measured immediately before the procedure. Patients with highest adiponectin quartile had increased mortality compared to patients with low adiponectin. After adjustment for conventional risk factors high adiponectin concentration remained an independent predictor of all-cause mortality.
Also in a cohort from the Jackson Heart Study, conducted among African Americans, Adiponectin was associated with a higher risk of incident stroke in women[89].
This conflicting evidence has highlighted the complex role of adiponectin in the pathogenesis of CVD. Interestingly, the paradoxical association of adiponectin with a worse outcome was found in a population with a more advanced disease status, which might trigger a compensatory increase in adiponectin levels[71]. Otherwise, the worse long-term outcome could be the result of the more advanced disease status per se. Another possible explanation of these findings might be a condition of relative resistance to adiponectin, as suggested by animal models[85].
In two recent metanalysis of prospective studies, it has been shown that plasma adiponectin levels are not related to the risk of CAD or stroke in apparently healthy[90] and diabetic patients[91]. Another reason why this association could not be found might lie in adiponectin isoforms. In particular, several studies now consider the high molecular weight (HMW) form to be biologically active[92]. However, only few cohort studies have prospectively evaluated the association of HMW adiponectin with CAD[92-94].
Among 30111 women from the Nurses’ Health Study, high levels of total and HMW adiponectin, and HMW/total adiponectin ratio were associated with a lower risk of CAD. These associations were largely mediated by parameters related to glucose and lipid metabolism and inflammation, especially HDL-cholesterol levels[95]. In a study by Kunita et al[96], in 394 patients referred for computed tomography angiography (CTA), levels of plasma HMW adiponectin were evaluated. In patients with obstructive CAD HMW adiponectin was significantly lower than that in patients without. Furthermore, it was significantly associated with the disease extent and with characteristics of plaque instability, such as positive remodeling, low CT density and adjacent spotty calcium. In a recent nested case-control study conducted among 15566 free of CVD subjects, baseline total and HMW adiponectin and their ratio were examined[97]. After adjustment for matched variables and traditional risk factors, total and HMW adiponectin and their ratio were not associated with overall risk of CVD. However, the highest quartile for HMW adiponectin and HMW/total adiponectin ratio decreased risk of CVD compared with the lowest quartile among middle-aged individuals with high blood glucose, while this association was not seen among the elderly.
Resistin was discovered as a fat-derived molecule in obese mice, for its capacity of inducing insulin resistance[98,99]. The main source in animals were adipocytes, in particular, from abdominal depots[98]. The substantial lack of homology between human and mouse resistin genes made difficult to confirm these observation in humans. In particular, in humans resistin is produced mainly by stromal vascular cells, rather than adipocytes[100]. However, resistin expression has been reported in human WAT by preadipocytes[101]. Even the relationship between resistin and insulin resistance, overweight and DM type 2 was extensively reported in human with non consistent conslcusions[99,102-104].
Considering that macrophages are its main source in humans, a main pro-inflammatory role has been hypothesized for Resistin. Thus, several in vitro studies was conducted (Table 2), which illustrated that Resistin levels increased in response to endotoxin and proinflammatory cytokines administration[104]. Moreover, Chen et al[105] recently reported that ox-LDL induced resistin mRNA expression in cultured adipocytes. In a recent study from our group[106], resistin induced prothrombotic phenotype of human coronary artery endothelial cells (HCAECs). HCAECs incubated with resistin showed an upregulation of TF expression, and its activity was induced in a dose-dependent manner through the activation of NF-κB pathway.
| Adipokine | Modulation of inflammation | Association with CVD |
| Resistin | ↑NFκB dependent cytokine release and adhesion molecule expression (including TNF-α/IL-6) on endothelial cells ↑ proliferation of vascular smooth muscle cells through ERK and PI3K pathways | Endothelial dysfunction: ↓ NO and EDHF ↑ ET-1 release ↑ VEGF and MMP ↑ expression of adhesion molecules and chemokines ↓ TRAF-3 Controversial effects in humans on insulin resistance and type 2 diabetes |
| CRP | ↑ expression of ICAM, VCAM, E selectin recruitment of mononuclear cells through MCP 1 VSMC proliferation, migration ↑ CD4+ LymphocitesT ↑ gamma – INF production ↑ ET-1 release ↑ CD40/CD40L on endothelium ↑ complement activation | ↑ endothelial dysfunction through ↓ NO vasodilatation ↑ oxidized LDL opsonization and macrophages uptake with subsequent foam cells formation ↑ in vessel wall oxidative stress ↑ TF and PAI 1 ↑ MMP |
| Apelin | ↓ superoxide radicals ↓ NADPHO ↓ oxidative injury ↑ NO ↑ vascular progenitor cells mobilization | ↑ inotropism ↑ neoangiogenesis ↓ endothelial dysfunction↓ Ang II and BP ↓ myocardial damage after infarction ↑ cholesterol efflux from macrophages |
In light of these evidences, resistin has been studied for its implications in CAD.
In apparently healty individuals from the European Investigation into Cancer and Nutrition-Potsdam Study, individuals in the highest quartile of resistin levels, compared with the lowest quartile, had a relevant increased risk of myocardial infarction but not of ischemic stroke[107]. This association persisted even after adjustment for CRP levels. In contrast, subsequent study described the independent link between resistin and the incidence of ischemic stroke within post menopausal women[108].
In a recent study of 6636 adults recruited from general population, after 3.5 years of follow-up, the group in the highest quintile of resistin plasma levels had a higher incidence of AMI[109]. The serum resistin concentrations were higher in women, and the associated increase in the risk of AMI based on the resistin level was also higher in women than in men.
In patients with known CAD, some cross-sectional and case-control studies showed higher plasma resistin levels than controls. Subject referring angina which had CAD at the coronary angiography had higher Resistin levels than patients without CAD[110]. Besides, resistin was also associated with coronary artery calcification at the CT scan[111]. Pischon et al[112] documented higher resistin levels in women with CAD compared with healty subjects from the CORA study. This link remained significant even after adjustment for several risk factors except the hs-CRP levels.
In contrast, no relationship between Resistin levels and CAD was found among 1161 subjects in the LURIC study[113]. Moreover, Resistin was not associated with cardiovascular mortality. Then, high resistin levels could simply mirror the presence of other established cardiovascular risk factors. However in the same study, an enhanced expression of adhesion molecules was found in association with increased resistin levels, highlighting a pathophysiological role in atherogenesis.
In a perspective study[114] comparing subjects with stable angina and subjects with unstable angina (UA), NSTEMI, and STEMI, higher resistin levels were found within subjects with ACS. Interestingly, an early rise in resistin levels was reported, at 3-6 h after symptoms onset. This increase lasted 6 and 12 h after.
CRP is an acute phase protein, member of the pentraxin family. Since it is a well-known marker of systemic inflammation[45], CRP was one of the first studied protein from its potential role in both pathogenesis and risk prediction of atherosclerosis. Subsequent studies showed that CRP is other than an innocent bystander of the inflammatory response associated with atherogenesis[45,115] (Table 2). In particular, together with the Adipocytokines, CRP charachterizes the chronic inflammatory status associated with obesity and MetS.
Interestingly, the adipose tissue has been described as producer of CRP[23,116]. In particular, we found that mature adipocytes are able to produce CRP, under inflammatory stimuli[23]. This finding was confirmed later in the experience by Anty et al[117], which demonstrated the expression of the CRP gene in adipocytes of obese subjects.
From a clinical point of view, high sensitivity assays are available to detect even low CRP concentration. Then high-sensitivity (hs) CRP has been largely evaluated as a suitable candidate for cardiovascular risk prediction. This idea was first supported by pioneering studies by Ridker et al[118], which demonstrated higher hs-CRP levels in apparently healthy subject who developed CV events during follow-up. In light of these results, Ridker et al[119] evaluated several risk prediction algorithms to improve the cardiovascular risk classification in apparently healthy American women. In particular, a simplified score, including hs-CRP (Reynolds risk score), was validated in this study, and subsequently in a male population[120].
Then the American Heart Association (AHA) and the CDC Consensus incorporated hs-CRP into the risk prediction strategy of cardiovascular diseases[121]. Measurement of hs-CRP is considered reasonable in the assessment of absolute risk for CAD in intermediate-risk individuals, with a Framingham risk score of 10% to 20%. This recommendation was confirmed in ACCF/AHA Guidelines in 2010[122] and in the recently published European Society of Cardiology Guidelines on Prevention[123].
Moreover, in a metanalysis[124] confirmed a role of hs-CRP for a better risk stratification of subjects at intermediate risk for CVD. In particular, for every 400 to 500 people screened for hs-CRP or fibrinogen level, one additional cardiovascular event could be prevented over a period of 10 years.
Results from the Justification for the Use of Statins in Primary Prevention: an International Trial Evaluating Rosuvastatin (JUPITER)[125] provided robust evidence of the association between inflammation and cardiovascular risk. subjects with LDL cholesterol below 130 mg/dL were treated with rosuvastatin vs placebo; patients at higher cardiovascular risk were identified by a hs-CRP level of 2.0 mg/L or higher. The Steering Committee stopped the trial after a median follow-up of 1.9 years due to striking benefit in patients treated with rosuvastatin (44% relative risk reduction of the primary end point and of hard outcomes).
Tehrani et al[126], recently investigate wheter inflammatory markers had an impact on the association of high density lipoprotein (HDL) cholesterol with CVD. In 3888 older adults without known CVD, authors evaluated CRP, IL-6, and lipoprotein-associated phospholipase A2 levels. CAD incidence was higher for higher levels of CRP, IL-6, and lower for higher levels of HDL-C. Compared to high HDL-C/low-inflammation categories, incident CAD was increased for those with high HDL-C and high CRP or highest IL-6 tertile. Then the protective relation of high HDL-C for incident CAD appears to be attenuated by greater inflammation.
Hs-CRP has also been studied for its potential role in the prediction of adverse outcome in patients with established CVD. Several studies clearly demonstrated an associationbetween hs-CRP and future acute coronary events in patients with SA[42].
However, conflicting report exsists about the additive benefit of measurement of hs-CRP. While data from Sinning et al[127] suggest that, in patients with established CVD, traditional risk factors are the most powerful predictors with only little information added by inflammatory markers (including CRP), on the other hand several studies[45,128] showed that hs-CRP independently predicts cardiac events in patients with ACS[45].
Moreover, patients with higher hs-CRP on admission for ACS had higher rate of impaired myocardial perfusion[129] and death.
Nakachi et al[130] reported that an hs-CRP elevation at admission and increase independently predicted 30-d events. In contrast, Bogaty et al[131] found that serial measurements of hs-CRP in ACS patients have only a modest predictive ability, which disappeared after adjustment for common clinical variables. However authors did not exclude patients with acute or chronic inflammatory diseases.
Among ACS patients of the FAST-MI, authors found that low fetuin-A and high hs-CRP concentrations were associated with cardiovascular death, even after adjustment for GRACE risk score[132]. In another study by Schaub et al[133] in 398 consecutive patients presenting with acute chest pain novel biomarkers like myeloperoxidase, MRP-8/14 and hs-CRP, provided incremental value in the risk stratification of these patients.
Apelin was first discovered in 1998[134], as the ligand of the so-called APJ receptor, a G-protein-coupled receptor (GPCR) identified in 1993 from a human genomic library. It is produced as preproapelin, then cleaved by an AT-converting enzyme to form several shorter C-terminal active peptides, i.e., apelin-13, -16, -17, -19, -36 and a pyroglutamate form (Pyr1 apelin-13)[135]. Since the absence of an immediately apparent ligand, APJ was first classified as an orphan GPCR. It shares 31% sequence homology with the human angiotensin II (AT II) type 1 receptor, which led to further characterization of the Apelin-APJ system.
Overall, the apelin system has several physiological roles, most notably in the cardiovascular system, hypothalamus and the adipo-insular axis[136], such as fluid homoeostasis, glucose homeostasis, feeding behaviour, regulation of vascular tone, cardiac inotropism and immunity. First studies about apelin-APJ system found both similar and opposite functions to those of the AT system[137]. The distribution of both receptors and peptides overlaps in the hypothalamus and vasculature[138]. Moreover, Apelin has been detected in adipose tissue[139] and it was found that it was both produced and secreted by adipocytes. Apelin has been then considered as a novel adipokine. Also APJ is present in human and mouse adipose tissue, both in isolated adipocytes and in the stromal vascular fraction[140].
Apelin expression in adipose tissue is regulated by nutritional status. In obese subjects, APJ-apelin expression is increased and this up-regulation could be reversed after diet or surgery-induced weight loss[141]. Moreover, changes in insulin levels might be involved for both apelin and APJ regulations in adipose tissue, according to the severity of insulin resistance[140]. A close relationship between apelin and insulin has been demonstrated both in vivo and in vitro. In cultured adipocytes, insulin treatment increased expression and secretion of apelin. Apelin expression in adipocytes is increased in various mouse models of obesity associated with hyperinsulinemia[139,142]. Interestingly, in highly insulin-resistant mice, such as db/db ones, APJ expression isn’t increased[143] and in studies conducted in type 2 diabetic subjects, the effect of insulin resulted completely blunted in adipose tissue[140].
Moreover, Apelin expression in adipose tissue is regulated also by TNF-α, gastro-intestinal inflammation, peroxisome proliferator-activated receptor gamma (PPARc) coactivator-1 a (PGC1a), Eicosapentaenoic acid-a polyunsaturated fatty acid from the omega-3 family, which all increase the apelin expression and secretion[142,144-146]. ATII exerts different effects on the expression of apelin, depending on the receptor involved: type 1 AT receptor mediates an increase of the apelin secretion, while type 2 receptor may reduce its production[147]. Interestingly, glucocorticoids modulate the production of apelin and its secretion from fat cells, simultaneously increasing ATII and suppressing apelin expression, suggesting a possible pathogenetic mechanism in obesity-related hypertension[148].
Since the increase in vascular density is essential for adipose tissue expansion, with endothelial cells actively promoting the development of preadipocytes and growth of mature adipocytes, apelin has been proposed to contribute to the development of new vasculature in expanding fat depot[149]. Several studies have demonstrated that apelin is a potent angiogenic factor[149], grossly equivalent to vascular endothelial cell growth factor and, like other angiogenic factors, its gene is upregulated under hypoxia condition[150]. Hypoxia induces expression and secretion of apelin in both human and murine adipocytes, through the hypoxia-inducible transcriptor factor 1a.
The first report in humans of plasma apelin concentrations was shown in obese and hyperinsulinemic subjects[139,141] where plasma apelin levels are increased. In morbidly obese patients with or without diabetes, apelin levels were only higher in the diabetic morbidly obese subjects[151]. However, reduced plasma apelin levels were described in obese subjects with untreated type 2 diabetes, compared to non-diabetic subjects and anti-diabetic treatment (rosiglitazone and metformin) was found to increase apelin concentration, with the improvement of glycemic profile[152,153].
Changes in apelin levels after weight loss or bariatric surgery in obese individuals were also investigated. Diet-induced weight loss decreases apelin levels in moderate obese women[154] but not significantly in patients with the MetS[155] or in obese children[156]. Bariatric surgery resulted in a significant decrease in apelin levels only in morbidly obese patients exhibiting impaired fasting glucose or type 2 diabetes before surgery[151]. Probably, obesity per se is not the main determinant of increased plasma apelin concentrations since circulating apelin levels are not necessary significantly correlated to the BMI in all the published studies[140].
The possible role of Apelin in the atherosclerotic process has been investigated. In studies by Liu et al[157], Apelin-13, the predominant circulating apelin isoform, significantly promoted intracellular cholesterol efflux and reduces macrophage foam cell formation, indicating a potential antiatherogenic function. Moreover, Kadoglou et al[158] have demonstrated lower apelin levels in patients with carotid atherosclerosis as compared to healthy controls, and that apelin increment is independently associated with atorvastatin-related carotid plaque stabilization. The same group reported considerably lower apelin concentrations in CAD patients in comparison with healthy controls[159]. This finding was confirmed in other studies conducted among subjects with stable angina (SA), and the plasma apelin levels were found to be negatively correlated with the severity of the disease[160].
A decrease of Apelin levels early after AMI has also been reported, with a progressive elevation over time, however reaching values lower than control subjects at 24 wk[161]. These observation were confirmed in patients with a first STEMI, where the reduction in apelin levels was independent from left ventricular dysfunction and outcome[162]. In comparison to asymptomatic CAD patients, plasmatic apelin were lower in ACS patients on admission, with a negative correlation with the severity of CAD[163].
A myocardial protective effect have been suggested from studies on the possible therapeutic use of apelin in CAD in animal models. Azizi et al[164] demonstrated in rat models of MI that post-infarct treatment with [Pyr1]-apelin-13 significantly attenuates myocardial damage, via the reduction of oxidative injury and enhancement of NO production. In addition, apelin-13 has been found to promote angiogenesis and ameliorate cardiac repair after AMI by a mechanism involving vascular progenitor cells[165].
However from a pathophysiological point of view, some conflicting data have been reported. For example, Rittig et al[166] observed that plasma apelin levels are not associated with early stages of atherosclerosis in young subjects prone to atherosclerosis and type 2 diabetes. Interestingly, other studies in animal models suggested a role of apelin-APJ system in vasculature oxidative stress. Furthermore, Apelin is upregulated in human atherosclerotic coronary artery and potently constricts human coronary artery[167]. Data by Jin et al[168] show that genetic defects in apelin/APJ pathway may confer a potential risk for CAD in Chinese hypertensive patients. These evidences underline the complex role of Apelin and its receptor in atherosclerosis.
Vascular inflammation represent a fundamental link between obesity, MetS and their detrimental complications. Conflicting evidence about the in vitro and in vivo effects of Adipocytokines suggests the high complexity of these mediators interplay in the pathogenesis of atherosclerosis and, moreover, in the risk stratification of CAD patients. Even large evidence about the use of hs-CRP for primary and secondary prevention of CVD has been questioning for its real additive value.
However, the involvement of adipocytokines in the pathogenesis of atherothrombosis and dysmetabolism remains clear, although it appears to be way more complex than previously thought. The understanding of these pathways may lead to the development of targeted treatment of obesity-related disorders. In this setting, the JUPITER trial provided some clue about the association between inflammation and the risk of CVD, even though it was not designed to evaluate the role of the pharmacological modulation of inflammation[124]. In this context, only two trials are ongoing, the Cardiovascular Inflammation Reduction Trial[169] and The canakinumab anti-inflammatory thrombosis outcomes study (CANTOS)[170]. The first is investigating the role of low-dose methotrexate on incident heart attacks, strokes, or death in people with type 2 diabetes or MetS that have had a heart attack or multiple coronary stenoses. CANTOS is studying the effect of Canakinumab, a human monoclonal antibody that neutralizes interleukin-1beta, in secondary prevention[170].
P- Reviewer: Lazzeri C, Sabate M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [PubMed] [Cited in This Article: ] |
| 3. | Calabrò P, Limongelli G, Pacileo G, Di Salvo G, Golino P, Calabrò R. The role of adiposity as a determinant of an inflammatory milieu. J Cardiovasc Med (Hagerstown). 2008;9:450-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898-918. [PubMed] [Cited in This Article: ] |
| 5. | Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677-685. [PubMed] [Cited in This Article: ] |
| 6. | Garrison RJ, Castelli WP. Weight and thirty-year mortality of men in the Framingham Study. Ann Intern Med. 1985;103:1006-1009. [PubMed] [Cited in This Article: ] |
| 7. | Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 8. | Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2536] [Cited by in F6Publishing: 2485] [Article Influence: 225.9] [Reference Citation Analysis (0)] |
| 9. | Childers DK, Allison DB. The ‘obesity paradox’: a parsimonious explanation for relations among obesity, mortality rate and aging? Int J Obes (Lond). 2010;34:1231-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959-2971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 943] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 11. | Sharma AM. Adipose tissue: a mediator of cardiovascular risk. Int J Obes Relat Metab Disord. 2002;26 Suppl 4:S5-S7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Calabró P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930-1932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 352] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1218] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 14. | Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S25-S28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001;60:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4296] [Cited by in F6Publishing: 4460] [Article Influence: 223.0] [Reference Citation Analysis (0)] |
| 17. | Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444-E452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1260] [Cited by in F6Publishing: 1257] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 18. | Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20-34. [PubMed] [Cited in This Article: ] |
| 19. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [PubMed] [Cited in This Article: ] |
| 20. | Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621-2637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 958] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 21. | Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 22. | Calabrò P, Golia E, Maddaloni V, Malvezzi M, Casillo B, Marotta C, Calabrò R, Golino P. Adipose tissue-mediated inflammation: the missing link between obesity and cardiovascular disease? Intern Emerg Med. 2009;4:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-1830. [PubMed] [Cited in This Article: ] |
| 24. | Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112-1113. [PubMed] [Cited in This Article: ] |
| 25. | Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14:1013-1022. [PubMed] [Cited in This Article: ] |
| 26. | Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf). 2012;205:194-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | REINER L, MAZZOLENI A, RODRIGUEZ FL. Statistical analysis of the epicardial fat weight in human hearts. AMA Arch Pathol. 1955;60:369-373. [PubMed] [Cited in This Article: ] |
| 28. | Womack HC. The relationship between human body weight, subcutaneous fat, heart weight, and epicardial fat. Hum Biol. 1983;55:667-676. [PubMed] [Cited in This Article: ] |
| 29. | Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311-1319; quiz 1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 30. | Wang TD, Lee WJ, Shih FY, Huang CH, Chang YC, Chen WJ, Lee YT, Chen MF. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab. 2009;94:662-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460-2466. [PubMed] [Cited in This Article: ] |
| 32. | Natale F, Tedesco MA, Mocerino R, de Simone V, Di Marco GM, Aronne L, Credendino M, Siniscalchi C, Calabrò P, Cotrufo M. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Choi TY, Ahmadi N, Sourayanezhad S, Zeb I, Budoff MJ. Relation of vascular stiffness with epicardial and pericardial adipose tissues, and coronary atherosclerosis. Atherosclerosis. 2013;229:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 34. | Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 462] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 35. | Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, Dragano N, Moebus S, Jöckel KH, Erbel R. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 37. | Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, Nishio C, Higashida M, Nakaya Y, Kitagawa T. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011;52:139-142. [PubMed] [Cited in This Article: ] |
| 38. | Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. [PubMed] [Cited in This Article: ] |
| 39. | Huang G, Wang D, Zeb I, Budoff MJ, Harman SM, Miller V, Brinton EA, El Khoudary SR, Manson JE, Sowers MR. Intra-thoracic fat, cardiometabolic risk factors, and subclinical cardiovascular disease in healthy, recently menopausal women screened for the Kronos Early Estrogen Prevention Study (KEEPS). Atherosclerosis. 2012;221:198-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Yamashita K, Yamamoto MH, Ebara S, Okabe T, Saito S, Hoshimoto K, Yakushiji T, Isomura N, Araki H, Obara C. Association between increased epicardial adipose tissue volume and coronary plaque composition. Heart Vessels. 2013;Aug 28; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 41. | Echavarría-Pinto M, Hernando L, Alfonso F. From the epicardial adipose tissue to vulnerable coronary plaques. World J Cardiol. 2013;5:68-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Alam MS, Green R, de Kemp R, Beanlands RS, Chow BJ. Epicardial adipose tissue thickness as a predictor of impaired microvascular function in patients with non-obstructive coronary artery disease. J Nucl Cardiol. 2013;20:804-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4640] [Cited by in F6Publishing: 4615] [Article Influence: 209.8] [Reference Citation Analysis (0)] |
| 44. | Bisoendial RJ, Kastelein JJ, Stroes ES. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195:e10-e18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Calabrò P, Golia E, Yeh ET. CRP and the risk of atherosclerotic events. Semin Immunopathol. 2009;31:79-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 977] [Cited by in F6Publishing: 927] [Article Influence: 44.1] [Reference Citation Analysis (1)] |
| 47. | Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, Wilson PW. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 695] [Cited by in F6Publishing: 656] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 48. | Canto JG, Iskandrian AE. Major risk factors for cardiovascular disease: debunking the “only 50%” myth. JAMA. 2003;290:947-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9119] [Cited by in F6Publishing: 8625] [Article Influence: 287.5] [Reference Citation Analysis (0)] |
| 50. | Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406-4413. [PubMed] [Cited in This Article: ] |
| 51. | Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231-1238. [PubMed] [Cited in This Article: ] |
| 52. | Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d’Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 54. | Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor DA, Kelly A, Wallace AM, Danesh J, Whincup PH. Leptin and coronary heart disease: prospective study and systematic review. J Am Coll Cardiol. 2009;53:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 55. | Chu NF, Spiegelman D, Hotamisligil GS, Rifai N, Stampfer M, Rimm EB. Plasma insulin, leptin, and soluble TNF receptors levels in relation to obesity-related atherogenic and thrombogenic cardiovascular disease risk factors among men. Atherosclerosis. 2001;157:495-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Zimmet PZ, Collins VR, de Courten MP, Hodge AM, Collier GR, Dowse GK, Alberti KG, Tuomilehto J, Hemraj F, Gareeboo H. Is there a relationship between leptin and insulin sensitivity independent of obesity? A population-based study in the Indian Ocean nation of Mauritius. Mauritius NCD Study Group. Int J Obes Relat Metab Disord. 1998;22:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Leyva F, Godsland IF, Ghatei M, Proudler AJ, Aldis S, Walton C, Bloom S, Stevenson JC. Hyperleptinemia as a component of a metabolic syndrome of cardiovascular risk. Arterioscler Thromb Vasc Biol. 1998;18:928-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Söderberg S, Ahrén B, Jansson JH, Johnson O, Hallmans G, Asplund K, Olsson T. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246:409-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 261] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 59. | Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001;104:3052-3056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 560] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 60. | Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819-1824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Lawlor DA, Smith GD, Kelly A, Sattar N, Ebrahim S. Leptin and coronary heart disease risk: prospective case control study of British women. Obesity (Silver Spring). 2007;15:1694-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Kappelle PJ, Dullaart RP, van Beek AP, Hillege HL, Wolffenbuttel BH. The plasma leptin/adiponectin ratio predicts first cardiovascular event in men: a prospective nested case-control study. Eur J Intern Med. 2012;23:755-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Amasyali B, Aytemir K, Kose S, Kilic A, Abali G, Iyisoy A, Kursaklioglu H, Turan M, Bingol N, Isik E. Admission plasma leptin level strongly correlates with the success of thrombolytic therapy in patients with acute myocardial infarction. Angiology. 2006;57:671-680. [PubMed] [Cited in This Article: ] |
| 64. | Söderberg S, Colquhoun D, Keech A, Yallop J, Barnes EH, Pollicino C, Simes J, Tonkin AM, Nestel P. Leptin, but not adiponectin, is a predictor of recurrent cardiovascular events in men: results from the LIPID study. Int J Obes (Lond). 2009;33:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Rajendran K, Devarajan N, Ganesan M, Ragunathan M. Obesity, Inflammation and Acute Myocardial Infarction - Expression of leptin, IL-6 and high sensitivity-CRP in Chennai based population. Thromb J. 2012;10:13. [PubMed] [Cited in This Article: ] |
| 66. | Khafaji HA, Bener AB, Rizk NM, Al Suwaidi J. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res Notes. 2012;5:262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 67. | Karakas M, Zierer A, Herder C, Baumert J, Meisinger C, Koenig W, Thorand B. Leptin, adiponectin, their ratio and risk of Coronary Heart Disease: results from the MONICA/KORA Augsburg Study 1984-2002. Atherosclerosis. 2010;209:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Lodh M, Goswami B, Parida A, Patra S, Saxena A. Assessment of serum leptin, pregnancy-associated plasma protein A and CRP levels as indicators of plaque vulnerability in patients with acute coronary syndrome. Cardiovasc J Afr. 2012;23:330-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 69. | Morita Y, Maeda K, Kondo T, Ishii H, Matsudaira K, Okumura N, Mitsuhashi H, Shibata R, Murohara T. Impact of adiponectin and leptin on long-term adverse events in Japanese patients with acute myocardial infarction. Results from the Nagoya Acute Myocardial Infarction Study (NAMIS). Circ J. 2013;77:2778-2785. [PubMed] [Cited in This Article: ] |
| 70. | Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011;217:503-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for “reverse epidemiology”. Horm Metab Res. 2007;39:1-2. [PubMed] [Cited in This Article: ] |
| 72. | Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925-1932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1345] [Cited by in F6Publishing: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 73. | Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring). 2007;15:1617-1622. [PubMed] [Cited in This Article: ] |
| 74. | Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5-13. [PubMed] [Cited in This Article: ] |
| 75. | Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168-173. [PubMed] [Cited in This Article: ] |
| 76. | Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247-E1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 77. | Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, Dong N, Chen X. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473-2476. [PubMed] [Cited in This Article: ] |
| 79. | Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730-1737. [PubMed] [Cited in This Article: ] |
| 80. | Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747-753. [PubMed] [Cited in This Article: ] |
| 81. | Wolk R, Berger P, Lennon RJ, Brilakis ES, Davison DE, Somers VK. Association between plasma adiponectin levels and unstable coronary syndromes. Eur Heart J. 2007;28:292-298. [PubMed] [Cited in This Article: ] |
| 82. | Liang KW, Sheu WH, Lee WL, Liu TJ, Ting CT, Hsieh YC, Wang KY, Chen YT, Lee WJ. Decreased circulating protective adiponectin level is associated with angiographic coronary disease progression in patients with angina pectoris. Int J Cardiol. 2008;129:76-80. [PubMed] [Cited in This Article: ] |
| 83. | Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623-629. [PubMed] [Cited in This Article: ] |
| 84. | Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677-5683. [PubMed] [Cited in This Article: ] |
| 85. | Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009;10:269-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Zhang MH, Spies C, Ali S, Kanaya AM, Schiller NB, Whooley MA. Adiponectin and inducible ischemia in patients with stable coronary heart disease: data from the Heart and Soul study. Atherosclerosis. 2009;205:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Wilson SR, Sabatine MS, Wiviott SD, Ray KK, De Lemos JA, Zhou S, Rifai N, Cannon CP, Morrow DA. Assessment of adiponectin and the risk of recurrent cardiovascular events in patients presenting with an acute coronary syndrome: observations from the Pravastatin Or atorVastatin Evaluation and Infection Trial-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22). Am Heart J. 2011;161:1147-55.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Lindberg S, Pedersen SH, Møgelvang R, Bjerre M, Frystyk J, Flyvbjerg A, Galatius S, Jensen JS. Usefulness of adiponectin as a predictor of all cause mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2012;109:492-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Bidulescu A, Liu J, Chen Z, Hickson DA, Musani SK, Samdarshi TE, Fox ER, Taylor HA, Gibbons GH. Associations of adiponectin and leptin with incident coronary heart disease and ischemic stroke in african americans: the jackson heart study. Front Public Health. 2013;1:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Kanhai DA, Kranendonk ME, Uiterwaal CS, van der Graaf Y, Kappelle LJ, Visseren FL. Adiponectin and incident coronary heart disease and stroke. A systematic review and meta-analysis of prospective studies. Obes Rev. 2013;14:555-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Wu Z, Cheng Y, Aung LH, Li B. Association between adiponectin concentrations and cardiovascular disease in diabetic patients: a systematic review and meta-analysis. PLoS One. 2013;8:e78485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Sattar N, Watt P, Cherry L, Ebrahim S, Davey Smith G, Lawlor DA. High molecular weight adiponectin is not associated with incident coronary heart disease in older women: a nested prospective case-control study. J Clin Endocrinol Metab. 2008;93:1846-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Baessler A, Schlossbauer S, Stark K, Strack C, Riegger G, Schunkert H, Hengstenberg C, Fischer M. Adiponectin multimeric forms but not total adiponectin levels are associated with myocardial infarction in non-diabetic men. J Atheroscler Thromb. 2011;18:616-627. [PubMed] [Cited in This Article: ] |
| 94. | Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27-e31. [PubMed] [Cited in This Article: ] |
| 95. | Pischon T, Hu FB, Girman CJ, Rifai N, Manson JE, Rexrode KM, Rimm EB. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis. 2011;219:322-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Oka T, Horiguchi J, Awai K, Kihara Y. Association between plasma high-molecular-weight adiponectin and coronary plaque characteristics assessed by computed tomography angiography in conditions of visceral adipose accumulation. Circ J. 2012;76:1687-1696. [PubMed] [Cited in This Article: ] |
| 97. | Saito I, Yamagishi K, Chei CL, Cui R, Ohira T, Kitamura A, Kiyama M, Imano H, Okada T, Kato T. Total and high molecular weight adiponectin levels and risk of cardiovascular disease in individuals with high blood glucose levels. Atherosclerosis. 2013;229:222-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [PubMed] [Cited in This Article: ] |
| 99. | Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452-5455. [PubMed] [Cited in This Article: ] |
| 100. | Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472-476. [PubMed] [Cited in This Article: ] |
| 101. | McTernan PG, McTernan CL, Chetty R, Jenner K, Fisher FM, Lauer MN, Crocker J, Barnett AH, Kumar S. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab. 2002;87:2407. [PubMed] [Cited in This Article: ] |
| 102. | Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848-4856. [PubMed] [Cited in This Article: ] |
| 103. | Utzschneider KM, Carr DB, Tong J, Wallace TM, Hull RL, Zraika S, Xiao Q, Mistry JS, Retzlaff BM, Knopp RH. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330-2333. [PubMed] [Cited in This Article: ] |
| 104. | Bala M, Kopp A, Wurm S, Büchler C, Schölmerich J, Schäffler A. Type 2 diabetes and lipoprotein metabolism affect LPS-induced cytokine and chemokine release in primary human monocytes. Exp Clin Endocrinol Diabetes. 2011;119:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Chen Y, Chen M, Wu Z, Zhao S. Ox-LDL induces ER stress and promotes the adipokines secretion in 3T3-L1 adipocytes. PLoS One. 2013;8:e81379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Calabrò P, Cirillo P, Limongelli G, Maddaloni V, Riegler L, Palmieri R, Pacileo G, De Rosa S, Pacileo M, De Palma R. Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-ĸB-dependent pathway. J Vasc Res. 2011;48:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Weikert C, Westphal S, Berger K, Dierkes J, Möhlig M, Spranger J, Rimm EB, Willich SN, Boeing H, Pischon T. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647-2653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 108. | Rajpathak SN, Kaplan RC, Wassertheil-Smoller S, Cushman M, Rohan TE, McGinn AP, Wang T, Strickler HD, Scherer PE, Mackey R. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women’s Health Initiative. Stroke. 2011;42:1813-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Cabrera de León A, Almeida González D, González Hernández A, Juan Alemán Sánchez J, Brito Díaz B, Domínguez Coello S, Marcelino Rodríguez I, Gregorio Oliva García J, Aguirre Jaime A, Rodríguez Pérez Mdel C. The association of resistin with coronary disease in the general population. J Atheroscler Thromb. 2014;21:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M, Nakamura H, Ohsuzu F. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 111. | Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 625] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 112. | Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764-1771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 113. | Pilz S, Weihrauch G, Seelhorst U, Wellnitz B, Winkelmann BR, Boehm BO, März W. Implications of resistin plasma levels in subjects undergoing coronary angiography. Clin Endocrinol (Oxf). 2007;66:380-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Lubos E, Messow CM, Schnabel R, Rupprecht HJ, Espinola-Klein C, Bickel C, Peetz D, Post F, Lackner KJ, Tiret L. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Atherosclerosis. 2007;193:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | Memoli B, Procino A, Calabrò P, Esposito P, Grandaliano G, Pertosa G, Prete MD, Andreucci M, Lillo SD, Ferulano G. Inflammation may modulate IL-6 and C-reactive protein gene expression in the adipose tissue: the role of IL-6 cell membrane receptor. Am J Physiol Endocrinol Metab. 2007;293:E1030-E1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 555] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 117. | Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A, Amor IB, Staccini-Myx A, Huet PM, Gugenheim J. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101:1824-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 118. | Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3798] [Cited by in F6Publishing: 3699] [Article Influence: 137.0] [Reference Citation Analysis (1)] |
| 119. | Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1280] [Cited by in F6Publishing: 1216] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 120. | Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243-251, 4p following 2251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 588] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 121. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [PubMed] [Cited in This Article: ] |
| 122. | Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 935] [Cited by in F6Publishing: 996] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 123. | Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2587] [Cited by in F6Publishing: 2583] [Article Influence: 215.3] [Reference Citation Analysis (0)] |
| 124. | Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 775] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 125. | Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-2207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4679] [Cited by in F6Publishing: 4528] [Article Influence: 283.0] [Reference Citation Analysis (0)] |
| 126. | Tehrani DM, Gardin JM, Yanez D, Hirsch CH, Lloyd-Jones DM, Stein PK, Wong ND. Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: cardiovascular Health Study. Atherosclerosis. 2013;231:246-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Sinning JM, Bickel C, Messow CM, Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Lackner KJ, Tiret L, Münzel T. Impact of C-reactive protein and fibrinogen on cardiovascular prognosis in patients with stable angina pectoris: the AtheroGene study. Eur Heart J. 2006;27:2962-2968. [PubMed] [Cited in This Article: ] |
| 128. | Arroyo-Espliguero R, Avanzas P, Quiles J, Kaski JC. Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis. 2009;204:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | Hoffmann R, Suliman H, Haager P, Christott P, Lepper W, Radke PW, Ortlepp J, Blindt R, Hanrath P, Weber C. Association of C-reactive protein and myocardial perfusion in patients with ST-elevation acute myocardial infarction. Atherosclerosis. 2006;186:177-183. [PubMed] [Cited in This Article: ] |
| 130. | Nakachi T, Kosuge M, Hibi K, Ebina T, Hashiba K, Mitsuhashi T, Endo M, Umemura S, Kimura K. C-reactive protein elevation and rapid angiographic progression of nonculprit lesion in patients with non-ST-segment elevation acute coronary syndrome. Circ J. 2008;72:1953-1959. [PubMed] [Cited in This Article: ] |
| 131. | Bogaty P, Boyer L, Simard S, Dauwe F, Dupuis R, Verret B, Huynh T, Bertrand F, Dagenais GR, Brophy JM. Clinical utility of C-reactive protein measured at admission, hospital discharge, and 1 month later to predict outcome in patients with acute coronary disease. The RISCA (recurrence and inflammation in the acute coronary syndromes) study. J Am Coll Cardiol. 2008;51:2339-2346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 132. | Lim P, Moutereau S, Simon T, Gallet R, Probst V, Ferrieres J, Gueret P, Danchin N. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am J Cardiol. 2013;111:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 133. | Schaub N, Reichlin T, Meune C, Twerenbold R, Haaf P, Hochholzer W, Niederhauser N, Bosshard P, Stelzig C, Freese M. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 134. | O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355-360. [PubMed] [Cited in This Article: ] |
| 135. | Cheng B, Chen J, Bai B, Xin Q. Neuroprotection of apelin and its signaling pathway. Peptides. 2012;37:171-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 136. | Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198-211. [PubMed] [Cited in This Article: ] |
| 137. | Lee DK, George SR, O’Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190-194. [PubMed] [Cited in This Article: ] |
| 138. | Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162-171. [PubMed] [Cited in This Article: ] |
| 139. | Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpéné C. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764-1771. [PubMed] [Cited in This Article: ] |
| 140. | Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 141. | Krist J, Wieder K, Klöting N, Oberbach A, Kralisch S, Wiesner T, Schön MR, Gärtner D, Dietrich A, Shang E. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes Facts. 2013;6:57-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 142. | Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache JS. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20:1528-1530. [PubMed] [Cited in This Article: ] |
| 143. | Dray C, Debard C, Jager J, Disse E, Daviaud D, Martin P, Attané C, Wanecq E, Guigné C, Bost F. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab. 2010;298:E1161-E1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 144. | Han S, Wang G, Qi X, Englander EW, Greeley GH. Involvement of a Stat3 binding site in inflammation-induced enteric apelin expression. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1068-G1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H1329-H1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 146. | Mazzucotelli A, Ribet C, Castan-Laurell I, Daviaud D, Guigné C, Langin D, Valet P. The transcriptional co-activator PGC-1alpha up regulates apelin in human and mouse adipocytes. Regul Pept. 2008;150:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 147. | Than A, Tee WT, Chen P. Apelin secretion and expression of apelin receptors in 3T3-L1 adipocytes are differentially regulated by angiotensin type 1 and type 2 receptors. Mol Cell Endocrinol. 2012;351:296-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132:27-32. [PubMed] [Cited in This Article: ] |
| 149. | Rayalam S, Della-Fera MA, Krieg PA, Cox CM, Robins A, Baile CA. A putative role for apelin in the etiology of obesity. Biochem Biophys Res Commun. 2008;368:815-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300:E877-E885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 151. | Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, Garcia-Almeida JM, Garcia-Arnes J, Tinahones FJ, Garcia-Fuentes E. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19:1574-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 152. | Erdem G, Dogru T, Tasci I, Sonmez A, Tapan S. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:289-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 153. | Kadoglou NP, Tsanikidis H, Kapelouzou A, Vrabas I, Vitta I, Karayannacos PE, Liapis CD, Sailer N. Effects of rosiglitazone and metformin treatment on apelin, visfatin, and ghrelin levels in patients with type 2 diabetes mellitus. Metabolism. 2010;59:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 154. | Castan-Laurell I, Vítkova M, Daviaud D, Dray C, Kováciková M, Kovacova Z, Hejnova J, Stich V, Valet P. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158:905-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 155. | Heinonen MV, Laaksonen DE, Karhu T, Karhunen L, Laitinen T, Kainulainen S, Rissanen A, Niskanen L, Herzig KH. Effect of diet-induced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19:626-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 156. | Reinehr T, Woelfle J, Roth CL. Lack of association between apelin, insulin resistance, cardiovascular risk factors, and obesity in children: a longitudinal analysis. Metabolism. 2011;60:1349-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 157. | Liu XY, Lu Q, Ouyang XP, Tang SL, Zhao GJ, Lv YC, He PP, Kuang HJ, Tang YY, Fu Y. Apelin-13 increases expression of ATP-binding cassette transporter A1 via activating protein kinase C α signaling in THP-1 macrophage-derived foam cells. Atherosclerosis. 2013;226:398-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 158. | Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Gerasimidis T, Kostakis A, Liapis CD. Adipokines: a novel link between adiposity and carotid plaque vulnerability. Eur J Clin Invest. 2012;42:1278-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 159. | Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med. 2013;30:e41-e50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | Li Z, Bai Y, Hu J. Reduced apelin levels in stable angina. Intern Med. 2008;47:1951-1955. [PubMed] [Cited in This Article: ] |
| 161. | Weir RA, Chong KS, Dalzell JR, Petrie CJ, Murphy CA, Steedman T, Mark PB, McDonagh TA, Dargie HJ, McMurray JJ. Plasma apelin concentration is depressed following acute myocardial infarction in man. Eur J Heart Fail. 2009;11:551-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 162. | Tycinska AM, Sobkowicz B, Mroczko B, Sawicki R, Musial WJ, Dobrzycki S, Waszkiewicz E, Knapp MA, Szmitkowski M. The value of apelin-36 and brain natriuretic peptide measurements in patients with first ST-elevation myocardial infarction. Clin Chim Acta. 2010;411:2014-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 163. | Kadoglou NP, Lampropoulos S, Kapelouzou A, Gkontopoulos A, Theofilogiannakos EK, Fotiadis G, Kottas G. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease--KOZANI STUDY. Transl Res. 2010;155:238-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 164. | Azizi Y, Faghihi M, Imani A, Roghani M, Nazari A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides. 2013;46:76-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 165. | Li L, Zeng H, Chen JX. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2012;303:H605-H618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 166. | Rittig K, Hildebrandt U, Thamer C, Staiger H, Peter A, Stefan N, Fritsche A, Häring HU, Balletshofer BM, Siegel-Axel D. Apelin serum levels are not associated with early atherosclerosis or fat distribution in young subjects with increased risk for type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119:358-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 167. | Pitkin SL, Maguire JJ, Kuc RE, Davenport AP. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br J Pharmacol. 2010;160:1785-1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 168. | Jin W, Su X, Xu M, Liu Y, Shi J, Lu L, Niu W. Interactive association of five candidate polymorphisms in Apelin/APJ pathway with coronary artery disease among Chinese hypertensive patients. PLoS One. 2012;7:e51123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 169. | Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 170. | Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T; CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: aphase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739-48. [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 393] [Article Influence: 32.8] [Reference Citation Analysis (0)] |