Published online Oct 26, 2014. doi: 10.4330/wjc.v6.i10.1049
Revised: February 10, 2014
Accepted: August 27, 2014
Published online: October 26, 2014
Various previous studies have found a negative correlation between the risk of cardiovascular events and serum high-density lipoprotein (HDL) cholesterol levels. The reverse cholesterol transport, a pathway of cholesterol from peripheral tissue to liver which has several potent antiatherogenic properties. For instance, the particles of HDL mediate to transport cholesterol from cells in arterial tissues, particularly from atherosclerotic plaques, to the liver. Both ATP-binding cassette transporters (ABC) A1 and ABCG1 are membrane cholesterol transporters and have been implicated in mediating cholesterol effluxes from cells in the presence of HDL and apolipoprotein A-I, a major protein constituent of HDL. Previous studies demonstrated that ABCA1 and ABCG1 or the interaction between ABCA1 and ABCG1 exerted antiatherosclerotic effects. As a therapeutic approach for increasing HDL cholesterol levels, much focus has been placed on increasing HDL cholesterol levels as well as enhancing HDL biochemical functions. HDL therapies that use injections of reconstituted HDL, apoA-I mimetics, or full-length apoA-I have shown dramatic effectiveness. In particular, a novel apoA-I mimetic peptide, Fukuoka University ApoA-I Mimetic Peptide, effectively removes cholesterol via specific ABCA1 and other transporters, such as ABCG1, and has an antiatherosclerotic effect by enhancing the biological functions of HDL without changing circulating HDL cholesterol levels. Thus, HDL-targeting therapy has significant atheroprotective potential, as it uses lipid transporter-targeting agents, and may prove to be a therapeutic tool for atherosclerotic cardiovascular diseases.
Core tip: The reverse cholesterol transport pathway played with high-density lipoprotein (HDL) has several potential antiatherogenic properties. Both ATP-binding cassette (ABC) A1 and ABCG1 are lipid transporters and have been involved in mediating cholesterol effluxes from cells in the presence of HDL or apoA-I, and they exerted antiatherosclerotic effects. As a therapeutic approach for increasing HDL cholesterol levels, much focus has been placed on increasing not only HDL cholesterol levels, but also HDL-biological functions. Reconstituted HDL and apoA-I mimetics have significant atheroprotective potential, as it uses lipid transporter-targeting agents, and may prove to be a novel therapeutic tool for atherosclerotic cardiovascular diseases.
- Citation: Uehara Y, Saku K. High-density lipoprotein and atherosclerosis: Roles of lipid transporters. World J Cardiol 2014; 6(10): 1049-1059
- URL: https://www.wjgnet.com/1949-8462/full/v6/i10/1049.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i10.1049
High-density lipoprotein (HDL) cholesterol is widely known as “good cholesterol”, because various previous studies have found a negative correlation between the risk of cardiovascular events and serum HDL cholesterol levels[1]. However, this is still controversial whether the association is the cause or just only an ensuing symptom of a general atherosclerotic damage. HDL has several potential for antiatherogenic properties, for instance, cholesterol is transported from peripheral tissues such as the cells in the arterial walls to the liver by HDL particles, where it is used for a composition of lipoproteins and a synthesis of bile acids, steroid hormones, or fat-soluble vitamins[1]. Whereas, low-HDL cholesterolemia is often observed as a characterized component of metabolic syndrome, such as in people who are overweight or obese, those with glucose intolerance or have obvious diabetes, those with hypertriglyceridemia, and those with high blood pressure, each of which conditions contribute to the cause of atherosclerosis[2].
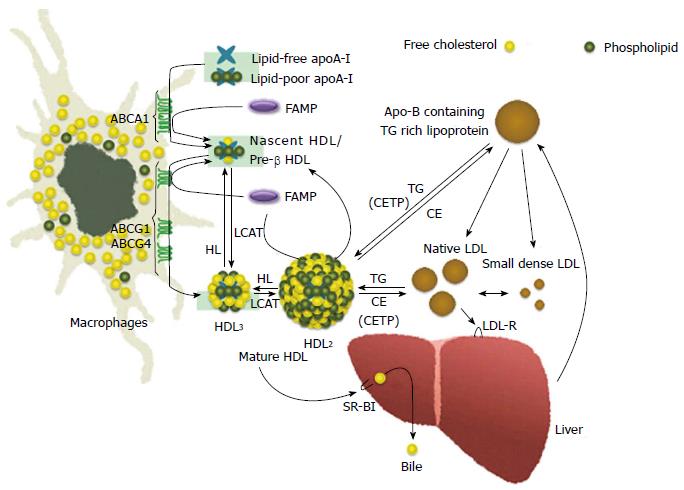
Although HDL is a lipoprotein when isolated by ultracentrifugation has a density in the range of 1.063-1.21 g/mL (HDL2, 1.063 < d < 1.125 g/mL; HDL3, 1.125 < d < 1.21 g/mL), HDL composes a heterogeneous group of particles that differ in density, size, composition of apolipoprotein (apo) or lipid, and electrophoretic mobility[3]. It is possible to separate HDL into two major subfractions on the basis of electro-mobility by electrophoresis; the major subfraction has the same mobility as alpha HDL, whereas the other subfractions migrate similar to pre-beta HDL, in addition the majority of HDL particles in human plasma are alpha HDL, and pre-beta HDL represents only 2%-14% of all apoA-I[4,5] (Figure 1).
HDL metabolism has the complicated mechanisms in association with several HDL-related genes such as various enzymes and protein, lipids, receptors, or transporters and its synthesis involves a complex pathway. The underlying genetic deficiency in many cases of primary low-HDL cholesterolemia are not clearly understood, however mutations in three pivotal genes as apoA-I, lecithin: cholesterol acyltransferase, and ATP-binding cassette transporter (ABC) A1, are associated with reducing serum HDL cholesterol levels, furthermore some of these genes’ mutations are also closely correlated with an increased risk of premature atherosclerosis and coronary artery disease (CAD)[6].
Tangier disease (TD) is the most severe form of HDL deficiency, which was first described by Fredrickson et al[7]. The biological hallmarks of TD patients’ plasma are a defect of HDL cholesterol, reduced low-density lipoprotein (LDL) cholesterol levels, and moderate increased triglyceride. The plasma apoA-I concentration in TD is markedly decreased to approximately 1%-3% of normal. TD is a very rare autosomal recessive disorder which is characterized by the almost absence serum apoA-I and HDL cholesterol levels. Furthermore, cholesteryl ester (CE) accumulates in many macrophage enriched tissues, such as tonsils, spleen, liver, lymph nodes, peripheral nerves, thymus, and also arterial walls. Clinical symptoms among homozygotes patients include hepatosplenomegaly, hyperplastic orange-yellow tonsils, corneal opacification, and premature CAD and atheosclerosis in a half of cases as well as relapsing peripheral neuropathy due to CE deposition in macrophages and Schwann cells[7-9].
In 1999, a cause of TD was found in a defect of the ABCA1 (formerly ABC1) gene[1,10,11] that is located on chromosome 9q31. This gene comprises 50 exons that span a region of approximately 149 kb[12,13]. ABCA1 has been identified as an important gene for regulating cellular cholesterol homeostasis and serum HDL cholesterol levels, which is defect in patients with TD. ABCA1 gene mutations cause gene dose-dependent decreases in serum HDL cholesterol levels and a decreased capacity of skin fibroblasts and monocyte-derived macrophages releasing cholesterol in the presence of extracellular apolipoproteins in TD patients and their heterozygous relatives[1,10,11,14,15].
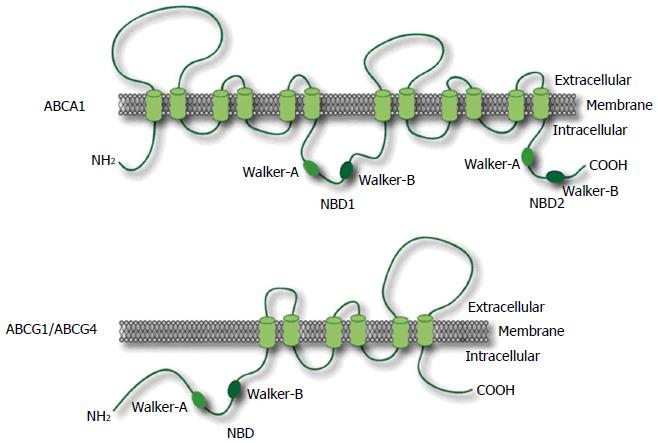
A transmembrane protein, ABC transporter facilitates to carry out the specific substrates across cell membranes in an ATP-dependent manner. ABCA1 is a member of the ABC transporter superfamily comprised 48 human transporters, and the superfamily is divided into seven subfamilies, including from half- to full-transporters, designated ABCA-ABCG. These transporters are integral membrane proteins carrying out various substrates, including lipids, ions, peptides, amino acids, carbohydrates, vitamins, glucuronides, glutathione conjugates, and xenobiotics[16,17]. ABCA1 is expressed in various organs in human, particularly the highest expression levels are existed in the placenta, liver, lung, adrenal glands, and fetal tissues[18].
ABC transporter superfamilies are defined by the presence of similar nucleotide binding domains (NBD) to interact with ATP. These domains have two conserved peptide motifs, Walker-A and Walker-B, which are found in many proteins that utilize ATP[16,19] (Figure 2).
ABCA1 proteins transport phospholipids (PLs) and cholesterol from the membranous inner leaflet to the outer leaflet, subsequently lipid-poor or lipid-free apoA-I takes up this transported cholesterol and PLs by ABCA1 to form nascent HDL[20]. ABCA1 is localized at the plasma membrane and intracellular compartments, where it can potentially facilitate lipid transport to either cell surface-bound[21] or internalized apolipoproteins[22].
HDL metabolism is composed of at least three different steps. As the first step, lipid-free or lipid-poor apoA-I removes free cholesterol from peripheral cells through ABCA1 transporter to form nascent-HDL. Second, nascent-HDL has a further lipidation, thereafter it grows to mature-HDL. Third, mature-HDL interacts with other apoB containing lipoproteins, such as intermediate density lipoprotein (IDL) and very-low-density lipoprotein (VLDL). Thus, ABCA1 is indispensable for the nascent-HDL formation, in addition it is also an important and essential molecule for the initial step of the reverse cholesterol transport (RCT).
Cultured blood monocyte-derived (mod)-macrophages from a healthy subject showed an approximately 125% increase in cholesterol efflux mediated lipid-free apoA-I, whereas it did not respond to apoA-I mediated efflux in macrophages from TD patients[23]. Although a lipid-free apoA-I showed an increase the cholesterol efflux mediated by in cultured mod-macrophages from healthy persons, the apoA-I did not elevate cholesterol efflux in mod-macrophages from TD patients. These results indicated that ABCA1 is a key molecule for apoA-I-specific cholesterol efflux pathway, but not basal efflux in macrophages.
Since ABCA1 plays an important role in mediating cholesterol and PL effluxes by lipid-free apoA-I, it is involved in a formation of discoidal HDL precursor, furthermore ABCA1 poorly interacts with HDL2 and HDL3. Patients with TD have extremely low levels of HDL cholesterol and they cannot compose nascent HDL particles due to a genetic defect in ABCA1 gene.
Disrupting the ABCA1 in mice resulted in HDL deficiency and impaired cholesterol transport similar to TD[24,25]. ABCA1 overexpression resulted in increased apoA-I-mediated cholesterol efflux in transgenic mice[26,27]. These results indicate that ABCA1 is an important gene in regulating circulating HDL cholesterol levels and cellular cholesterol homeostasis.
ABCG1, formerly ABC8 is also a member of the ABC transporter family which has been mapped on chromosome 21q22.3[19,28-32]. ABCG1 is one of half-transporter that contains only one NBD and a transmembrane domain, in contrast to ABCA1[19,31] (Figure 2). Thus, ABCG1 may require a dimeric partner to become active with ABCG1 or ABCG4.
Although ABCA1 promotes cholesterol efflux to lipid-poor or lipid-free apoA-I, it only modestly induces lipid efflux of smaller particles, such as HDL3, and does not promote a cholesterol efflux of the larger HDL2 fraction[33,34]. It has been also shown by Wang et al[35] that ABCG1 and ABCG4 contributed to HDL2- and HDL3-mediated cholesterol effluxes and had an important function related to HDL lipidation[35-37].
Administering a high-cholesterol, high-fat diet to ABCG1 knock-out mice resulted in a large amount of lipid accumulation in macrophages, whereas overexpression of human ABCG1 gene was able to protect a dietary fat-induced lipid accumulation in murine model[38]. Moreover, It was shown by Mauldin et al[39] that reduced function of ABCG1 facilitated foam cell formation in diabetes mice[39]. Transplanting bone marrow from ABCG1-deficient (ABCG1-/-) mice into LDL receptor-deficient mice, a model of familial hypercholesterolemia, produced contrasting effects on the formation of atherosclerotic lesion[40-42]. In contrast to these report, decreased lesion size and formation were observed in the absence of macrophages from ABCG1-deficient mice[41,42], and whole body ABCG1 expression protected against the development of early atherosclerotic plaque[43]. However, it remains unclear that the physiological roles of ABCG1 and its contribution to atherosclerotic progression in humans. In addition, ABC transporters such as ABCG1 and ABCG4, but not ABCA1, are not only responsible for passive and nonspecific efflux pathway but also mature HDL-mediated cholesterol efflux, which are spherical and transport almost all HDL cholesterol[35,37].
ABCG5 and ABCG8 are half-transporters as well as ABCG1 that function together as a heterodimer, and mutations in either of these genes can cause sitosterolemia which is a rare autosomal, recessively inherited disorder, characterized by premature atherosclerosis and xanthomas[44-47]. These transporters mediate the sterols efflux including cholesterol and plant sterols from enterocytes return into the intestinal lumen and their excretion into the bile[44,48]. Accordingly, they protect the lipid accumulation in the body and augment RCT system. In animal model, ABCG5 and ABCG8 deficient mice have been shown to reduce a secretion of cholesterol in the bile and elevate sterol absorption[49], on the other hand ABCG5 and ABCG8 genes-overexpressed mice promotes cholesterol secretion in the bile, decreases cholesterol absorption from diet, and increases neutral sterol excretion in the feces[50]. Liver X receptor (LXR) agonists promote the cholesterol efflux by the upregulation of ABCA1 and ABCG1, and also stimulate ABCG5 and ABCG8 which accelerate direct HDL transport of intestine into the lumen, thus these genes also play an important role in the RCT system and their enhancement by LXR agonists prevent an atherosclerotic development[51].
ABCA1 gene expression and cellular efflux of cholesterol are enhanced by cholesterol[15,18], oxysterols[52], retinoids[53], and cAMP analogs[15,54]. The ABCA1 gene promoter has been analyzed[13,52]. Both oxysterols and retinoids are ligands for the nuclear transcription factor, LXRα/β and retinoid X receptor-alpha (RXRα), respectively, which have been identified as an enhancer of ABCA1 gene expression[52,53,55,56]. It is present in dimeric form of LXR and RXR as active transcriptional heterodimers that preferentially bind to responsive elements in the ABCA1 gene promoter[13,57]. LXRα/β and RXRα bind to the specific responsive element, called direct repeat 4 (DR4) element within the ABCA1 promoter, which is characterized by two direct hexameric repeats separated by four nucleotides, thereafter they are activated by oxysterols and retinoids[58,59]. ABCA1 transcription are activated to bind either one or both ligands. Treatment with either a ligand of LXRα/β or RXRα enhances cellular ABCA1 expression, furthermore their combination treatment has a marked synergistic effect[60].
Since peroxisome proliferator activating receptor (PPAR)-α and -γ agonists such as fibrates and thiazolidine derivative (TZD) upregulate LXR mRNA expression, the activation of PPARs indirectly enhances a transcription activity of ABCA1 via LXR in cultured cells. In contrast, it is already known the zinc finger protein ZNF202 transcription factor as a major transcriptional repressor for ABCA1. In addition to the factor ZNF202, unsaturated fatty acids, but not saturated one, drastically suppress ABCA1-mediated cholesterol effluxes from macrophages by which they antagonize the binding of specific agonist, oxysterol to LXR[61,62]. Moreover, various transcription factors, such as upstream stimulatory factor (USF)1, USF2, Fra2, and Sp3, also have the potential to repress the ABCA1 transcription[63].
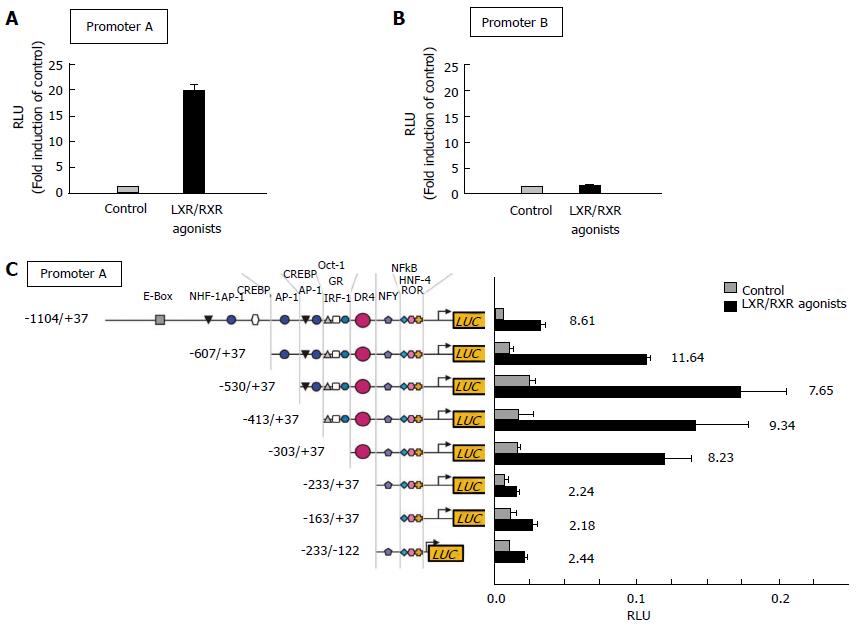
The ABCG1 gene has a promoter upstream of exon 1 and another intron promoter, which encodes several transcripts[64-66]. Our previous study demonstrated that LXR activation drastically increased the ABCG1 promoter activity (Promoter-A) located upstream of exon 1 as well as the ABCA1 gene (Figure 3A). On the other hand, the activity of ABCG1 promoter-B located within intron 4 was not changed by an activation of LXR (Figure 3B)[62]. These results indicate that the gene transcription of exon 5 and subsequent exons might be also regulated, at least in part, by the ABCG1 promoter-A.
Electrophoretic mobility shift assay was done to confirm these findings, and it showed the existence of DNA-binding nuclear receptors on extracted ABCG1 promoter-A having DR4 element. As would be expected from these finding, only the ABCG1 promoter-A contained a DR4 element, but not promoter-B, which is required for binding to LXRα/RXR. In fact, a promoter response to ligands of LXR/RXR was totally abolished in the mutated ABCG1 promoter lacked an active DR4 element[62] (Figure 3C).
It remains unclear whether ABCG1 itself contributes to circulating lipid levels, such as HDL cholesterol and arterial plaque regression in humans. There have been only five reports on ABCG1 polymorphisms. Our previous study was the first regarding an ABCG1 polymorphism, which appeared to be a potent functional ABCG1 polymorphism located in the promoter region[67-71]. The ABCG1 promoter -257T>G polymorphism, rs1378577, -394 T/G from the transcription start site (NM_207627.1:c. -394T>G), -134 T/G from exon 1 (NM_207627.1) is a single nucleotide mutation (SNP) on the ABCG1 promoter region upstream of exon 1, which was reported to be a functional promoter with an LXR-responsive element[62,67].
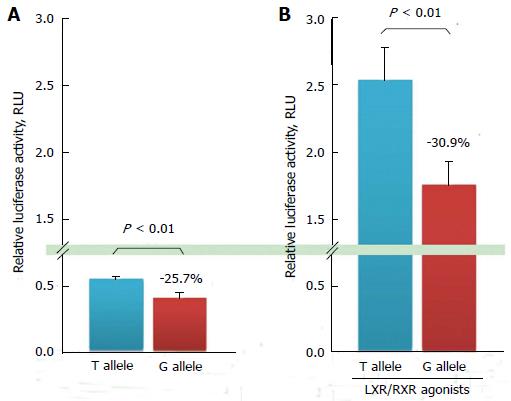
To investigate whether this promoter polymorphism influenced gene transcriptional activity, in vitro luciferase reporter gene assays were performed after transient transfection in cultured cells. In these experiments, the amount of luciferase activity was 25.7% higher in T allelic sequence containing construct than that in G allelic one on ABCG1 promoter-A; these responses were significantly different (Figure 4A). ABCG1 promoter activity induced by LXR and RXR agonists increased by 4.6-fold, and the amount of luciferase produced by the construct containing the T allelic sequence was 30.9% higher than that produced by the construct containing the G allele, which was also significantly different as well as in the absence of LXR/RXR agonists (Figure 4B). The transcription activity in the T allelic sequence was significantly higher than that in the G allelic sequence on ABCG1 promoter-A.
Furthermore, the ABCG1 promoter showed increased activity via stimulation by LXR and RXR, and a similar genotype-dependent effect on ABCG1 gene transcription under these conditions was identified. These results suggest that the ABCG1 promoter polymorphism might be an isolated regulating factor for ABCG1 gene transcription activity, independent of LXR and RXR.
We genotyped 109 Japanese male CAD patients for the ABCG1 promoter SNP. This polymorphism was associated with CAD severity in Japanese men, but not with changes in lipid levels under fasting conditions in a case control study. Logistic regression analysis showed that there was an interaction between the ABCG1 promoter genotype and CAD severity.
Genotype frequencies were grouped on the basis of whether patients had multi- or single-vessel CAD. The adjusted relative risk associated with the G allele (assuming an additive effect) in a matched-pair analysis was 2.1 for multi-vessel CAD compared with single-vessel CAD and 3.5 for the G/G and T/G genotypes compared with T/T (assuming a dominant effect of the G allele)[67]. These results were consistent with the proposition that the variations for ABCG1 gene might make a contribution to interindividual variability in susceptibility or severity of atherosclerotic changes.
ABCG1 expression levels in atherosclerotic tissues might be lower among those with the G allele and may be associated with a mechanism for an increased incidence of atherosclerosis in these individuals. These results were similar to a previous study by Baldán et al[72] of transgenic mice in whom the ABCG1 gene was deleted[73]. Furthermore, a recent study regarding ABCG1 as a candidate gene with possible important antiatherogenic properties also illustrates the current interest in this transporter.
Inhibiting scavenger receptor BI (SR-BI), CE transfer protein (CETP) or PL transfer protein, and an activating ABCA1 or apoA-I elevate HDL cholesterol levels. However, it is uncertain whether the effects of these interventions on atherosclerosis are consistence with the results of studies with animal models and inborn human HDL metabolism errors. Although it has not found a such small molecule which strongly promotes apoA-I production, one possible candidate molecule is LXR agonist which increase HDL cholesterol levels via upregulation of ABCA1 and ABCG1 expressions. Unfortunately, previous study has shown that concurrent with an activation of RCT, the agonist induces hypertriglycemia consequent on increasing hepatic VLDL production.
As a therapeutic approach for increasing HDL levels, much research has focused both increasing HDL cholesterol levels and on enhancing HDL biochemical functions. HDL therapies that used injections of reconstituted HDL, apoA-I mimetics, or full-length apoA-I are remarkably effective[74,75]. Nissen et al[75] showed that in humans, intravenous administation of ETC-216, an apoA-I-Milano complexed with phospholipids, produced a significant regression of coronary atherosclerotic plaques as determined by intravascular ultrasound (IVUS). After infusing ETC-216, regression of coronary atherosclerosis was accompanied by reverse remodeling of the external elastic membrane and with no changes in luminal dimensions as assessed by IVUS analyses[76].
Reconstituted HDL (rHDL), a complex of apoA-I or apoA-I mimetics with PL, must be shaped as disc, and it may be a suitable administration in patients with atherosclerotic plaque and TD. ABCA1 plays an important role for apoA-I-mediated cholesterol efflux in macrophages, and thereby is involved in discoidal HDL precursor formation. Mature HDL particles shaped spherical induce cholesterol effluxes by other transporters such as ABCG1 and ABCG4, rather than ABCA1[35]. We previously established a discoidal rHDL, which was a complex of human serum-derived full length of apoA-I with PL, 1-palmitoyl-2-oleoylphosphatidylcholine (POPC)[77]. Interestingly, the apoA-I complex with a PL, a POPC/apoA-I disc, could take up cholesterol from macrophages in both normal subjects and TD patients.
Although studies on the use of apoA-I mimetic peptides (e.g., 4F and L37pA) are underway[78-80], none of these agents are currently available for clinical use. To develop a physiological HDL-generating apoA-I mimetic peptide that functions with ABCA1 transporter, different candidate peptides were synthesized by focusing on the amino acid sequence alignments of human apoA-I interactions with ABCA1. We recently established a novel short apoA-I mimetic peptide that comprised 24 amino acids and without phospholipids Fukuoka University ApoA-I Mimetic Peptide (FAMP), which retained the amphipathic helical structure of the 243-amino acid apoA-I and the ability to associate with lipids[81]. This was shown to enhance HDL function and suppress aortic plaque formation in apoE-knockout mice that were fed a high-fat diet. FAMP markedly increased pre-beta HDL formation as well as increased the overall cholesterol effluxes from peripheral tissues[82].
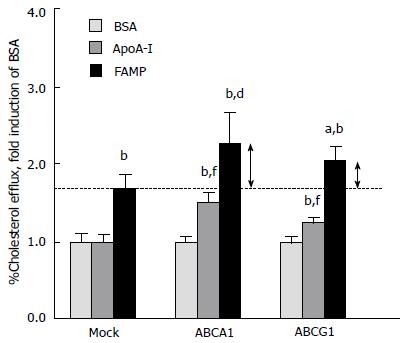
In contrast to apoA-I, FAMP-mediated cholesterol effluxes were not completely abolished under ABCA1-inactivated conditions, such as in cells treated with probucol, an ABCA1 antagonist, and Tangier macrophages. These results suggested that FAMP functioned in removing cholesterol through both the ABCA1 pathway and another specific pathway that must be dependent on ABCG1 transporters (Figure 1). In support of this, COS-7 cells that were transiently transfected with the ABCA1 and ABCG1 genes had significantly increased FAMP-mediated effluxes compared with mock transfection (Figure 5).
Injections of HDL apo-A-I mimetics, apoA-I-Milano, and full-length apoA-I are effective both in vitro and in vivo. However, it remains unclear whether apoA-I or its mimetics actually enter atherosclerotic plaque lesions and remove cholesterol. ApoA-I may generate nascent, new HDL and reverse the macrophage foam cell phenotype.
We developed a novel PET tracer that was functionalized with DOTA and labeled with 68Ga to specifically image the status of atherosclerotic plaques. Atherosclerotic plaques and aortic atherosclerotic plaques show high uptake of this tracer, and this novel tracer provides for impressive in vivo imaging of an aortic plaque using PET/CT[83]. HDL-targeting therapy, including FAMP, may have tremendous atheroprotective potential and prove to be a new therapeutic tool for atherosclerotic cardiovascular disease. While most research has focused on the therapeutic use of HDL, an apoA-I mimetic peptide may also contribute to the development of a tool for plaque diagnosis.
The RCT pathway has several potential antiatherogenic properties. Both ABCA1 and ABCG1 are lipid transporters on plasma membrane that have been contributed in mediating effluxes of cholesterol and PLs from cells in the presence of lipid-poor or lipid-free apoA-I and HDL. As a therapeutic approach for increasing HDL levels, much research has focused both on increasing HDL cholesterol levels and on enhancing HDL biochemical functions. HDL therapies with reconstituted HDL, apoA-I mimetics, or full-length apoA-I am dramatically effective. In particular, a novel apoA-I mimetic peptide, FAMP, effectively removes cholesterol via specific ABCA1 and other transporters, such as ABCG1. FAMP has an antiatherosclerotic effect by enhancing biological HDL functions without changing circulating HDL cholesterol levels. These HDL-targeting therapies have significant atheroprotective potential, as they are lipid transporter-targeting agents. Thus, HDL-targeting therapy may prove to be a therapeutic tool for atherosclerotic cardiovascular diseases.
P- Reviewer: Albacker T, Biyik I, Corciu AI, Can M, Kato M, Kobza R, Latif N, Prella F S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21:13-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 511] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 2. | Després JP, Marette A. Relation of components of insulin resistance syndrome to coronary disease risk. Curr Opin Lipidol. 1994;5:274-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6731] [Cited by in F6Publishing: 6995] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 4. | Kunitake ST, La Sala KJ, Kane JP. Apolipoprotein A-I-containing lipoproteins with pre-beta electrophoretic mobility. J Lipid Res. 1985;26:549-555. [PubMed] [Cited in This Article: ] |
| 5. | Ishida BY, Frolich J, Fielding CJ. Prebeta-migrating high density lipoprotein: quantitation in normal and hyperlipidemic plasma by solid phase radioimmunoassay following electrophoretic transfer. J Lipid Res. 1987;28:778-786. [PubMed] [Cited in This Article: ] |
| 6. | Miller M, Rhyne J, Hamlette S, Birnbaum J, Rodriguez A. Genetics of HDL regulation in humans. Curr Opin Lipidol. 2003;14:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 7. | Fredrickson DS, Altrocchi PH, Avioli LV, Goodman DS and Goodman HC. Tangier disease combined clinical staff conference at the National Institutes of Health. Ann Intern Med. 1961;55:1016-1031. [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 218] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Assman G, von Eckardstein A and Brewer HBJ. Familial high density lipoprotein deficiency: Tangier disease. The metabolic and Molecular Basis of Inherited Disease.7th ed. C. R. Scriver, A. L. Beaudet, W. S. Sly and D. Valle, editor. New York: McGraw-Hill 1995; 2053-2072. [Cited in This Article: ] |
| 9. | Hobbs HH, Rader DJ. ABC1: connecting yellow tonsils, neuropathy, and very low HDL. J Clin Invest. 1999;104:1015-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336-345. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1359] [Cited by in F6Publishing: 1298] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 11. | Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1146] [Cited by in F6Publishing: 1079] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 12. | Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C. Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original Tangier disease kindred. Proc Natl Acad Sci USA. 1999;96:12685-12690. [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 219] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Santamarina-Fojo S, Peterson K, Knapper C, Qiu Y, Freeman L, Cheng JF, Osorio J, Remaley A, Yang XP, Haudenschild C. Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci USA. 2000;97:7987-7992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1167] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 15. | Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25-R31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 576] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | Klein I, Sarkadi B, Váradi A. An inventory of the human ABC proteins. Biochim Biophys Acta. 1999;1461:237-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 446] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 439] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 18. | Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE and Schmitz G. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem-Biophys-Res-Commun. 1999;257:29-33. [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 406] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945-951. [PubMed] [Cited in This Article: ] |
| 20. | Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42:1173-1179. [PubMed] [Cited in This Article: ] |
| 21. | Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S. Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem. 2001;276:27584-27590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | von Eckardstein A, Rohrer L. Transendothelial lipoprotein transport and regulation of endothelial permeability and integrity by lipoproteins. Curr Opin Lipidol. 2009;20:197-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Uehara Y, Tsuboi Y, Zhang B, Miura S, Baba Y, Higuchi MA, Yamada T, Rye KA, Saku K. POPC/apoA-I discs as a potent lipoprotein modulator in Tangier disease. Atherosclerosis. 2008;197:283-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Orsó E, Broccardo C, Kaminski WE, Böttcher A, Liebisch G, Drobnik W, Götz A, Chambenoit O, Diederich W, Langmann T. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet. 2000;24:192-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA. 2000;97:4245-4250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 444] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Vaisman BL, Lambert G, Amar M, Joyce C, Ito T, Shamburek RD, Cain WJ, Fruchart-Najib J, Neufeld ED, Remaley AT. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Singaraja RR, Bocher V, James ER, Clee SM, Zhang LH, Leavitt BR, Tan B, Brooks-Wilson A, Kwok A, Bissada N. Human ABCA1 BAC transgenic mice show increased high density lipoprotein cholesterol and ApoAI-dependent efflux stimulated by an internal promoter containing liver X receptor response elements in intron 1. J Biol Chem. 2001;276:33969-33979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Croop JM, Tiller GE, Fletcher JA, Lux ML, Raab E, Goldenson D, Son D, Arciniegas S, Wu RL. Isolation and characterization of a mammalian homolog of the Drosophila white gene. Gene. 1997;185:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Chen H, Rossier C, Lalioti MD, Lynn A, Chakravarti A, Perrin G, Antonarakis SE. Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am J Hum Genet. 1996;59:66-75. [PubMed] [Cited in This Article: ] |
| 30. | Savary S, Denizot F, Luciani M, Mattei M, Chimini G. Molecular cloning of a mammalian ABC transporter homologous to Drosophila white gene. Mamm Genome. 1996;7:673-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1179] [Cited by in F6Publishing: 1152] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 32. | Klucken J, Buchler C, Orso E, Kaminski WE, Porsch-Ozcurumez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA. 2000;97:817-822. [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 420] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 33. | Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest. 1995;96:78-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 363] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 34. | Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053-33058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 463] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 35. | Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101:9774-9779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 803] [Cited by in F6Publishing: 775] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 36. | Smith JD. Insight into ABCG1-mediated cholesterol efflux. Arterioscler Thromb Vasc Biol. 2006;26:1198-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Uehara Y, Yamada T, Baba Y, Miura S, Abe S, Kitajima K, Higuchi MA, Iwamoto T, Saku K. ATP-binding cassette transporter G4 is highly expressed in microglia in Alzheimer’s brain. Brain Res. 2008;1217:239-246. [PubMed] [Cited in This Article: ] |
| 38. | Kennedy MA, Barrera GC, Nakamura K, Baldán A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 638] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 39. | Mauldin JP, Srinivasan S, Mulya A, Gebre A, Parks JS, Daugherty A, Hedrick CC. Reduction in ABCG1 in Type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem. 2006;281:21216-21224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, Kuipers F, Van Berkel TJ, Van Eck M. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Baldán A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA. Impaired development of atherosclerosis in hyperlipidemic Ldlr-/- and ApoE-/- mice transplanted with Abcg1-/- bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2301-2307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1-/- bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2308-2315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Out R, Hoekstra M, Meurs I, de Vos P, Kuiper J, Van Eck M, Van Berkel TJ. Total body ABCG1 expression protects against early atherosclerotic lesion development in mice. Arterioscler Thromb Vasc Biol. 2007;27:594-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275-48282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 323] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 45. | Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102:1041-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1115] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 47. | Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 480] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 48. | Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031-1043. [PubMed] [Cited in This Article: ] |
| 49. | Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237-16242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 529] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 50. | Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Calpe-Berdiel L, Rotllan N, Fiévet C, Roig R, Blanco-Vaca F, Escolà-Gil JC. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J Lipid Res. 2008;49:1904-1911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240-28245. [PubMed] [Cited in This Article: ] |
| 53. | Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1013] [Cited by in F6Publishing: 966] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 54. | Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634-28640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508-34511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 423] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 56. | Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097-12102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 784] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 57. | Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742-23747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 58. | Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 818] [Cited by in F6Publishing: 830] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 59. | Bungert S, Molday LL, Molday RS. Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters: identification of N-linked glycosylation sites. J Biol Chem. 2001;276:23539-23546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun. 2000;274:794-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 349] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 61. | Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, Langer C, Schachtrup C, Wiekowski J, Lorkowski S. Polyunsaturated fatty acids and acetoacetate downregulate the expression of the ATP-binding cassette transporter A1. Diabetes. 2002;51:2922-2928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Uehara Y, Miura S, von Eckardstein A, Abe S, Fujii A, Matsuo Y, Rust S, Lorkowski S, Assmann G, Yamada T. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Yang XP, Freeman LA, Knapper CL, Amar MJ, Remaley A, Brewer HB, Santamarina-Fojo S. The E-box motif in the proximal ABCA1 promoter mediates transcriptional repression of the ABCA1 gene. J Lipid Res. 2002;43:297-306. [PubMed] [Cited in This Article: ] |
| 64. | Lorkowski S, Rust S, Engel T, Jung E, Tegelkamp K, Galinski EA, Assmann G, Cullen P. Genomic sequence and structure of the human ABCG1 (ABC8) gene. Biochem Biophys Res Commun. 2001;280:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Langmann T, Porsch-Ozcürümez M, Unkelbach U, Klucken J, Schmitz G. Genomic organization and characterization of the promoter of the human ATP-binding cassette transporter-G1 (ABCG1) gene. Biochim Biophys Acta. 2000;1494:175-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Kennedy MA, Venkateswaran A, Tarr PT, Xenarios I, Kudoh J, Shimizu N, Edwards PA. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem. 2001;276:39438-39447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Furuyama S, Uehara Y, Zhang B, Baba Y, Abe S, Iwamoto T, Miura S, Saku K. Genotypic Effect of ABCG1 gene promoter -257T& gt; G polymorphism on coronary artery disease severity in Japanese men. J Atheroscler Thromb. 2009;16:194-200. [PubMed] [Cited in This Article: ] |
| 68. | Xu Y, Wang W, Zhang L, Qi LP, Li LY, Chen LF, Fang Q, Dang AM, Yan XW. A polymorphism in the ABCG1 promoter is functionally associated with coronary artery disease in a Chinese Han population. Atherosclerosis. 2011;219:648-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Di Martino MT, Arbitrio M, Leone E, Guzzi PH, Rotundo MS, Ciliberto D, Tomaino V, Fabiani F, Talarico D, Sperlongano P. Single nucleotide polymorphisms of ABCC5 and ABCG1 transporter genes correlate to irinotecan-associated gastrointestinal toxicity in colorectal cancer patients: a DMET microarray profiling study. Cancer Biol Ther. 2011;12:780-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Abellán R, Mansego ML, Martínez-Hervás S, Morcillo S, Pineda-Alonso M, Carmena R, Real JT, Redon J, Rojo-Martínez G, Martín-Escudero JC. Dietary polyunsaturated fatty acids may increase plasma LDL-cholesterol and plasma cholesterol concentrations in carriers of an ABCG1 gene single nucleotide polymorphism: study in two Spanish populations. Atherosclerosis. 2011;219:900-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Olivier M, Tanck MW, Out R, Villard EF, Lammers B, Bouchareychas L, Frisdal E, Superville A, Van Berkel T, Kastelein JJ. Human ATP-binding cassette G1 controls macrophage lipoprotein lipase bioavailability and promotes foam cell formation. Arterioscler Thromb Vasc Biol. 2012;32:2223-2231. [PubMed] [Cited in This Article: ] |
| 72. | Baldán A, Tarr P, Vales CS, Frank J, Shimotake TK, Hawgood S, Edwards PA. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem. 2006;281:29401-29410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circ Res. 2009;104:1142-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Iwata A, Miura S, Zhang B, Imaizumi S, Uehara Y, Shiomi M, Saku K. Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis. 2011;218:300-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1374] [Cited by in F6Publishing: 1264] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 76. | Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, Nissen SE. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006;47:992-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Rye KA, Hime NJ, Barter PJ. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J Biol Chem. 1997;272:3953-3960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 79. | Li X, Chyu KY, Faria Neto JR, Yano J, Nathwani N, Ferreira C, Dimayuga PC, Cercek B, Kaul S, Shah PK. Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;110:1701-1705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44:828-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Uehara Y, Ando S, Oniki K, Abe S, Yahiro E, Tanigawa H, Miura SI and Saku K. FAMP, a novel apoA-I mimetic peptide promotes HDL via ABCA1-dependent cholesterol efflux. Atheroscl Suppl. 2010;11:3-3. [Cited in This Article: ] |
| 82. | Uehara Y, Ando S, Yahiro E, Oniki K, Ayaori M, Abe S, Kawachi E, Zhang B, Shioi S, Tanigawa H. FAMP, a novel apoA-I mimetic peptide, suppresses aortic plaque formation through promotion of biological HDL function in ApoE-deficient mice. J Am Heart Assoc. 2013;2:e000048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Kawachi E, Uehara Y, Hasegawa K, Yahiro E, Ando S, Wada Y, Yano T, Nishikawa H, Shiomi M, Miura S. Novel molecular imaging of atherosclerosis with gallium-68-labeled apolipoprotein A-I mimetic peptide and positron emission tomography. Circ J. 2013;77:1482-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |