SPHINGOSINE-1-PHOSPHATE SIGNALING SYSTEM IN THE VASCULATURE
Sphingosine-1-phosphate (S1P) exerts pleiotropic effects including cell migration, cell proliferation, cell survival, and differentiation in a diversity of cell types[1-4]. Most of the diverse biological activities of S1P are mediated through one of five G-protein-coupled receptors (GPCRs), S1P1-5[2,4,5]. S1P1, S1P2 and S1P3 are widely expressed in various tissues and the major receptor subtypes in blood vessels. S1P1 is coupled exclusively via Gi to Ras-mitogen activated protein kinase, phosphoinositide 3-kinase/Akt pathway, and phospholipase C pathway, whereas S1P2 and S1P3 are coupled to multiple G proteins, i.e. Gq, G12/13 and Gi to activate the phospholipase C and Rho pathways, as well as the above-mentioned Gi-dependent pathways (Figure 1). The diversity of S1P actions depends upon their subtype-specific distinct repertoire of heterotrimeric G protein coupling, in combination with tissue- and cell-type-specific receptor expression patterns.
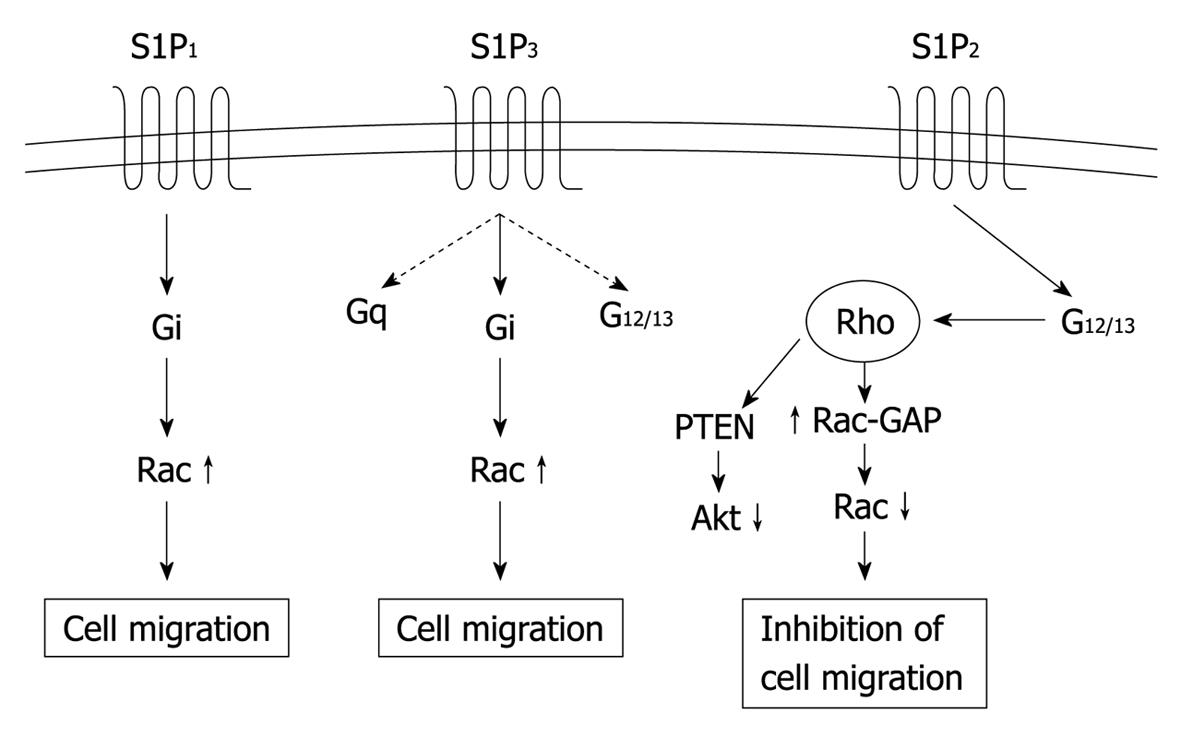
Figure 1 Receptor-subtype-specific signaling mechanisms of S1P1, S1P2 and S1P3.
These three receptors activate partially overlapping but distinct sets of signaling pathways via coupling to different sets of the heterotrimeric G proteins. S1P1 and S1P3 induce activation of Rac in a Gi-dependent manner to stimulate cell migration, whereas S1P2 inhibits Rac via G12/13-Rho to mediate inhibition of cell migration. S1P2 also stimulates 3’-specific polyphosphoinositide phosphatase “phosphatase and tensin homolog deleted from chromosome 10” (PTEN) to inhibit the serine/threonine protein kinase Akt, which probably participates in inhibition of cell migration. S1P: Sphingosine-1-phosphate.
S1P is present at a concentration of around 10-7 mol/L in the plasma, largely in forms bound to plasma proteins including high-density lipoprotein and albumin[6,7]. S1P is generated by the phosphorylation of sphingosine by sphingosine kinases (SPHKs) 1 and 2, which share a conserved catalytic domain but are expressed in a spatiotemporally distinct manner[8]. Deletion of either SPHK1 or SPHK2 is functionally fully compensated by the other isozyme, whereas SPHK1/SPHK2 double knockout mice are embryonic lethal with undetectable level of S1P, which indicates that S1P is produced exclusively by SPHKs in vivo[9]. The major source of plasma S1P is believed to be red blood cells, vascular endothelial cells (ECs), activated platelets, and other cell types[6,10-12].
ECs and vascular smooth muscle cells (SMCs) show distinct patterns of expression of S1P1, S1P2 and S1P3. Previous studies have shown that ECs largely express S1P1 and S1P3, whereas S1P2 appears to be expressed only in ECs of certain vascular beds[13,14]. S1P stimulates EC proliferation and survival, migration, and capillary-like tube formation via S1P1 and S1P3in vitro (Figure 1), which is indicative of its angiogenic activity[13,15,16]. S1P also maintains endothelial barrier function via S1P1[17-19].
S1P3 receptor is abundantly expressed in ECs and medial SMCs[13,20,21]. S1P3 stimulates endothelial nitric oxide synthase (eNOS) and nitric oxide production in ECs through Gi- and Akt-mediated phosphorylation of eNOS in concert with a Gq-mediated, Ca2+/calmodulin-dependent activation process[22]. S1P3 in SMCs mediates vasoconstriction through Gq-coupled Ca2+ mobilization and G12/13-coupled Rho-dependent myosin light chain phosphatase inhibition in certain vascular beds[20]. S1P3 knockout mice are phenotypically normal[23], however, S1P3 deletion in mice abrogates a variety of S1P effects on the cardiovascular system. These include vasopressor effects after intravenous administration of S1P and endothelium-dependent vasodilation[20,24]. Vasopressor effects of S1P suggest that direct constrictive effects of S1P on vascular smooth muscle dominate its endothelium-dependent dilatory effect in many vascular beds.
S1P2 receptor seems to be essential for functional integrity of certain vascular beds[25]; for example, S1P2 is involved in functional maintenance of the vasculature in the stria vascularis in the inner ear, in which S1P2-mediated vasoconstriction controls arterial perfusion appropriately[26]. Disruption of S1P2 in mice results in age-dependent impairment of normal function of vessels in the inner ear, which leads to abnormalities in the inner ear structure, hearing loss and ataxia. In addition, activation of S1P2 has been reported to induce disruption of adherens junctions, and as a result, vascular hyperpermeability in in vitro and in vivo[27].
In this review, we focus on the role of S1P for angiogenesis, receptor-subtype-specific stimulatory and inhibitory actions of S1P in angiogenesis and vascular maturation, and usefulness of S1P in therapeutic angiogenesis. Understanding S1P regulation of vascular formation provides further insights into molecular mechanisms of physiological and pathological vascular formation and the basis for targeting the S1P signaling system to treat cancer and ischemic diseases.
S1P IN VASCULAR FORMATION
Vessel formation occurs by two distinct mechanisms, vasculogenesis and angiogenesis (Figure 2)[28-30]. Early in development, mesoderm-derived hemangioblasts proliferate and differentiate into vascular EC precursors, and then coalesce to form a primitive vascular plexus, which is a process termed vasculogenesis. This primary vascular network is modified to form the mature vasculature with the interconnecting branching pattern through pruning and vessel enlargement (remodeling). Further expansion of the vascular plexus occurs by the process of angiogenesis, which involves the sprouting from pre-existing vessels into a previously avascular tissue. Vessels formed by vasculogenesis and angiogenesis maturate by the recruitment of mural cells (SMCs and pericytes) and development of the surrounding matrix[31,32]. Vascular endothelial cell growth factors (VEGFs) are recognized as the most crucial driver of vasculogenesis and angiogenesis. Angiopoietins, ephrins and platelet-derived growth factors (PDGFs), and transforming growth factor (TGF) are required for remodeling and maturation[28-32]. Besides these angiogenic peptides, the lipid mediators including S1P, lysophosphatidic acid and prostaglandins have emerged as the regulators of the processes of angiogenesis and maturation[33].
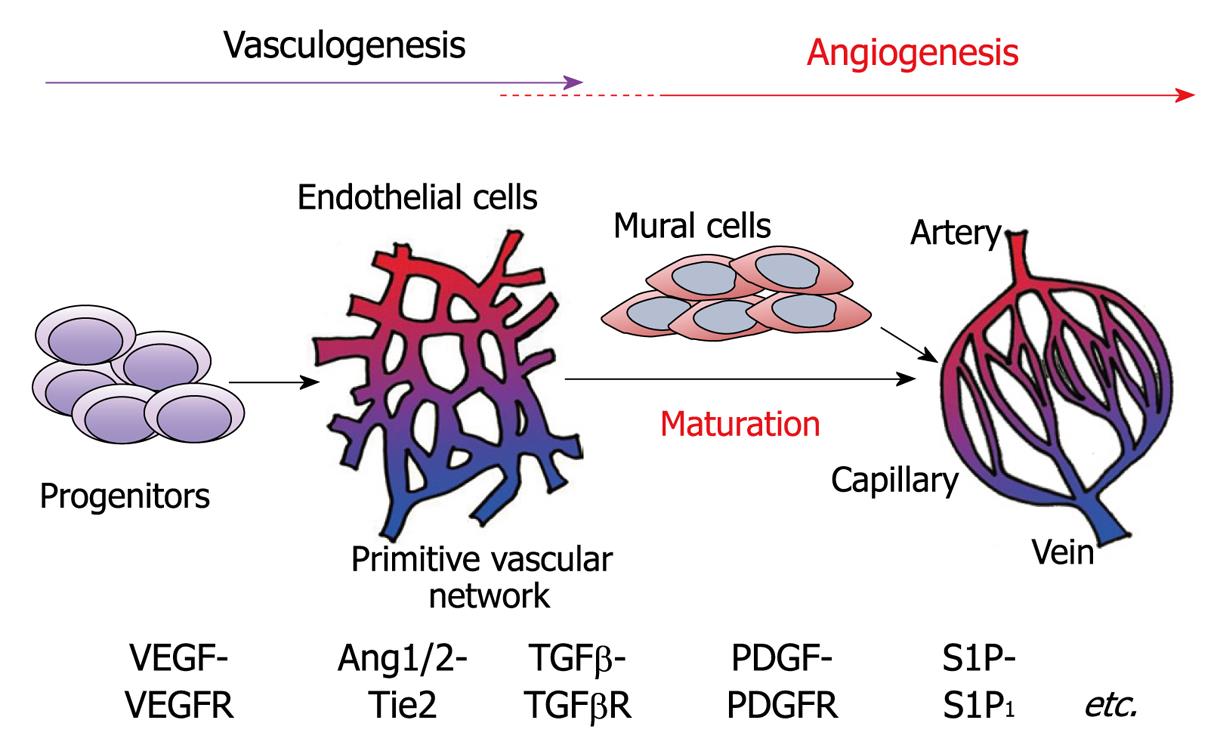
Figure 2 Schematic representation of vascular formation.
The processes include vasculogenesis and angiogenesis. During vasculogenesis and angiogenesis, various growth factors and their receptors play spaciotemporally specific roles. Newly formed vessels are stabilized by the recruitment of mural cells, smooth muscle cells and pericytes. S1P: Sphingosine-1-phosphate; VEGF: Vascular endothelial growth factor; TGF: Transforming growth factor; PDGF: Plateletderived growth factor; VEGFR: VEGF receptor; TGFβR: TGFβ receptor; PDGFR: PDGF receptor.
S1P, which directly acts on ECs[13,15,16], is involved in the regulation of angiogenesis and mural cell recruitment during development and in the adult. S1P is involved in physiological and pathological vascular formation. Importantly, S1P regulates vascular formation positively or negatively in a receptor-subtype-specific manner.
S1P stimulates angiogenesis in vivo mainly via S1P1, and to a lesser extent, S1P3. The first discovery of in vivo angiogenic activity of S1P came from the observation that S1P stimulated angiogenesis in Matrigel implants in mice[15]. This effect was inhibited by anti-sense oligonucleotide-mediated downregulation of either S1P1 or S1P3. Intensive studies have suggested that S1P1- and S1P3-mediated activation of the Rho family GTPase Rac plays a crucial role in S1P-induced angiogenesis[1,4,13,15,16]. Subsequently, it has been demonstrated that S1P1-gene ablation in mice impairs accumulation of pericytes and SMCs to vessels, i.e. vascular maturation or stabilization[34] (see below for more detail). An S1P1-selective antagonist inhibited VEGF-induced angiogenesis in a corneal model, which suggests that endogenous S1P is involved in VEGF-induced angiogenesis[35].
Recent studies have shown the contribution of bone-marrow-derived circulating endothelial precursor cells to new blood vessel formation in ischemic tissues and tumors. CD34+ vascular endothelial progenitors express S1P3 receptor[36]. Stimulation of progenitor S1P3 receptor with S1P or synthetic analog FTY720 activates the CXCR4 chemokine receptor to enhance the effectiveness of progenitor cell therapy for angiogenesis. More recent studies[37] have shown that SPHK1 exerts inhibitory effects on mobilization of endothelial progenitor cells (EPCs) from the bone marrow and the differentiation of circulating EPCs into mature ECs. These observations suggest that S1P has a modulatory role in EPC-dependent vascular formation.
In contrast to S1P1, S1P2, which is also expressed in ECs, inhibits Rac and thereby cell migration via a G12/13/Rho-dependent mechanism in several cell types[38-40]. S1P2 in ECs mediates S1P-induced inhibition of growth-factor-induced Rac activation, cell migration and capillary-like tube formation[14] (Figure 1). Additionally, the overexpression of S1P2 exaggerates these S1P-induced responses in ECs. In senescent ECs, S1P2 is upregulated, which leads to inhibition of migration and tube formation in vitro[41]. Therefore, it is an intriguing possibility that S1P2 might play an inhibitory role in angiogenesis, which contrasts with S1P1. Consistent with this notion, the S1P2-selective antagonist JTE-013 enhances S1P-induced angiogenesis in Matrigel plugs in mice[14]. More recently, in a murine neonatal retinal angiogenesis model, S1P2 expressed on the endothelium in the retinal vessels was shown to exert an inhibitory effect on angiogenesis in avascular areas of the retina[42].
In pathological angiogenesis, the above-mentioned S1P1 antagonist inhibits angiogenesis in a collagen-antibody-induced arthritis model[35]. In a mouse model of hypoxia-induced retinal angiogenesis, S1P2 expressed in newly formed, abnormal vessels invading the vitreal body rather than promoted pathological neo-angiogenesis, which contrasts with the inhibitory role of S1P2 in physiological neonatal angiogenesis in avascular areas of the retina[42]. S1P also plays a crucial role in tumor angiogenesis and is emerging as a target for anticancer treatment, and might be beneficial for therapeutic angiogenesis in ischemic diseases (see below). The relative expression of S1P1, S1P2 and S1P3 in ECs is probably different between vascular beds, in vivo and in vitro, and physiological and pathological angiogenesis. The efficiency of these endothelial S1P receptors in coupling to the key signaling molecules including Rho, Rac and Akt might also be different among EC types under various conditions as mentioned above. Therefore, the role and effect of endogenous and exogenous S1P and its analogues in angiogenesis could differ in a context-dependent manner. In any event, accumulated evidence suggests that S1P is one of the key regulators that promote or inhibit angiogenesis under both physiological and pathological conditions.
Developmental vascular formation
Liu et al[34] have demonstrated that homozygous deletion of S1P1 gene in mice results in intrauterine death between E12.5 and E14.5. S1P1-null embryos exhibit widespread hemorrhage, severe edema throughout the body, pericardial effusion, and poor limb development with bleeding in the edematous yolk sac. Immunohistochemical staining using antibodies against EC-specific markers such as anti-CD31 and anti-CD34 has revealed a nearly normally arborized vascular network with capillary sprouting in S1P1-null embryos, which suggests that the processes of vasculogenesis and angiogenesis have occurred. Consistent with this, the mRNAs of VEGF, its receptor VEGFR2, angiopoietin-1 and -2, and their receptor Tie-2 are expressed in S1P1-null embryos at levels similar to those in wild-type embryos. In S1P1-null embryos, however, the coverage of vessels by SMCs is abnormal; normally, the dorsal aorta is completely covered with SMCs by E11.5, whereas SMCs are present only on the ventral surface of the entire length of the dorsal aorta, but deficient on the dorsal surface of the aorta in S1P1-null embryos at E12.5. Similarly, the substantial fraction of medium-sized arteries displays a discontinuous and patchy covering by SMCs in S1P1-null embryos. Bleeding into the surrounding tissue space from defective aorta and arteries has been observed. In addition, capillaries in S1P1-null embryos are not associated with pericytes. Although S1P has been shown to be involved in the formation of EC junctions in vitro, S1P1 deficiency in mice does not appear to affect cell junctions in the vasculature. The expression of PDGF-B chain and PDGF β receptor, which are essential for vascular mural cell recruitment, is normal in S1P1-null embryos. S1P1-null-embryo-derived fibroblasts are defective in chemotaxis toward S1P. Based on these observations, S1P1 deficiency has been suggested to result directly or indirectly in defects in SMC recruitment.
The same group has addressed the question of whether S1P1 controls SMC coverage of vessels in a cell-autonomous fashion by acting on SMCs or indirectly through its activity in ECs. They have studied EC-specific conditional S1P1 knockout (EC-KO) mice and have found that the S1P1 EC-KO mice exhibit phenotypes similar to those of the global S1P1 KO mice, namely massive bleeding, pericardial effusion and hypoplastic limbs with intrauterine death between E12.5 and E14.5[43]. Vessels in S1P1 conditional EC-KO embryos are incompletely surrounded by SMCs. Thus, vessel coverage by SMCs is directed by S1P1 in ECs. Hla and colleagues have shown that, mechanistically, S1P1 in ECs directs the proper delivery of the cell adhesion molecule N-cadherin to the contact site between ECs and SMCs, to strengthen N-cadherin-dependent cell-cell adhesion through the Gi-Rac pathway[44].
Different from S1P1-null mice, either S1P2- or S1P3-single null mice are alive without a vascular formation defect. It has been shown that, compared with mice null for S1P1 alone, embryos null for S1P1 and S1P2, null for S1P1 and S1P3, and null for S1P1, S1P2 and S1P3 exhibit more severe S1P1-null phenotypes, including a vascular maturation defect and hemorrhage with earlier intrauterine death[45]. Mice null for S1P2 and S1P3 show partial embryonic lethality (50% death), probably due to bleeding, but without a defect in the SMC coverage of vessels. Additionally, vessels in the S1P2 and S1P3-double null mice have a very thin, discontinuous EC layer. These observations indicate that S1P1 is the most important receptor for vascular development, whereas S1P2 and S1P3 possess partially redundant and cooperative functions in S1P regulation of vascular formation. It remains to be clarified whether the supporting actions of S1P2 and S1P3 in developmental vascular formation are mediated by these receptors in ECs or non-EC cells, and how deletion of S1P2 and S1P3 results in aggravation of vascular formation defects in the compound mutant mice.
SPHK1/SPHK2 double-null embryos also show bleeding, defects in SMC coverage in large vessels, and vascular dilation and aberrant vascular network formation in the brain with abnormal EC morphology, which results in embryonic lethality at E11.5-12.5[46]. This phenotype is similar to those observed in mice with global and EC-specific disruption of the S1P1 gene[34,43], which underlines the importance of S1P-S1P1 signaling in vascular formation during development.
Tumor angiogenesis
In growing tumors, ECs in pre-existing blood vessels located in the vicinity of tumors are induced to migrate toward a tumor, proliferate and develop morphogenesis to form networks of microvessels in a tumor, i.e. angiogenesis occurs[28,29,47]. Recruitment of pericytes and SMCs to the newly formed microvessel wall stabilizes them, and establishes functional tumor vessels that facilitate oxygen and nutrient supply for rapidly proliferating tumor cells[30-32]. These processes are regulated by multiple extracellular signaling molecules including VEGFs, angiopoietin-1, fibroblast growth factors (FGFs) and PDGFs, which are produced by hypoxic tumor cells and stromal cells including infiltrating bone-marrow-derived cells (BMDCs), which are also recruited by the tumor itself[48,49]. A subpopulation of BMDCs might also contribute to tumor angiogenesis through their differentiation into ECs and mural cells.
The S1P signaling system is involved in tumor neovascularization[50,51]. A recent report[52] has shown that repeated administration of monoclonal anti-S1P antibody inhibits tumor growth in several tumor models, including orthotopic injections of MDA MB-231 and MDA MB-468 breast carcinoma cells and SKOV3 ovarian cancer cells, and subcutaneous injection of A549 lung adenocarcinoma cells. The extent of tumor growth inhibition by anti-S1P antibody is substantial and more than that obtained with monoclonal anti-VEGF antibody. This antitumor effect has been attributed to inhibition of tumor angiogenesis and motility, survival and proliferation in tumor cells. Anti-S1P antibody suppresses VEGF- and FGF-induced angiogenesis in Matrigel plugs in mice, which suggests that endogenous S1P plays a permissive role in angiogenesis or function downstream of VEGF and FGF[51]. S1P1 in vessels at sites of tumor implantation has been shown to be upregulated[50]. S1P1 silencing by repeated local injections of S1P1-specific siRNA has been shown to inhibit S1P1 expression in tumor neovessels in an animal model of subcutaneous tumor implantation. Concomitantly, siRNA-mediated silencing suppresses tumor angiogenesis and vascular maturation, which results in inhibition of tumor growth. These observations suggest that S1P1 is a crucial receptor to stimulate angiogenesis and vascular maturation in tumors.
Compared with accumulation of evidence for the involvement of S1P1 in tumor angiogenesis, the role of S1P2 in tumor angiogenesis is little understood. We originally isolated S1P2 as an orphan GPCR from vascular SMCs[53], which was later cloned from the brain by another group[54]. Although S1P2 mRNA has been detected in a wide variety of organs and tissues, it is unknown which cell types express S1P2 transcripts. We have shown, by analyzing the expression of β-galactosidase (LacZ)-knockin mice (S1P2LacZ/+ mice) in which LacZ gene expression is driven by endogenous S1P2 promoter, that LacZ (namely S1P2) is expressed mainly in blood vessels, ECs and vascular SMCs, in a variety of normal organs including the brain, heart, lung liver, kidney, skeletal muscle and bone marrow[55]. In these organs, only a minor fraction of cells besides vascular cells express S1P2. In murine tumor implantation models, tumor vasculature also expresses S1P2. Thus, the antimigratory receptor S1P2, as well as the migratory receptor S1P1, is expressed in ECs, which suggests that S1P2 exerts a distinct effect from S1P1 on tumor angiogenesis. It remains to be defined whether the expression of S1P2 in tumor vasculature differs from that in normal vessels.
S1P2-null mice show increased growth of implanted LLC lung carcinoma cells and B16BL6 melanoma cells, compared with wild-type mice[55]. Tumor growth is 60%-100% enhanced in S1P2-null mice compared with wild-type mice. The microvessel density is greater in LLC and B16BL6 tumors of S1P2-null mice, compared with those in wild-type mice. Higher vascularity in tumors in S1P2-null mice is associated with increased tumor cell proliferation. In S1P2-null mice, the coverage of tumor neovessels with pericytes and SMCs is enhanced compared with that in wild-type mice. Infusion of fluorescent (FITC-labeled) dextran has shown that the density of functional, perfused tumor microvessels is more abundant in tumors in S1P2-null mice. These observations demonstrate that the absence of S1P2 increases functional neovessels and stabilizes them in murine tumors. In Matrigel plugs, S1P2-null mice also exhibit enhancement of VEGF- and FGF2-induced microvascular formation and stimulate vascular maturation, which suggests that this growth-factor-induced angiogenesis is dependent on endogenous S1P. In tumors, S1P2 is expressed in hypervascular regions that are located at the tumor periphery, and blood vessels of a larger size in the peritumoral host tissue. The S1P2-positive cells include ECs in microvessels and ECs and mural cells in larger vessels. In addition, S1P2 expression has been detected in the scattered host-derived non-vascular cells in the tumor stroma and the tumor-surrounding host tissue, many of which appear to be BMDCs. Deletion of S1P2 does not affect expression of the angiogenic S1P1 receptor in tumor vessels.
The ECs isolated from the lung of S1P2LacZ/+ mice are essentially all positive for LacZ, which indicates that lung microvascular ECs express S1P2[55]. S1P2-null microvascular ECs display altered phenotypes compared with wild-type ECs; S1P2-null ECs show higher rates of cell proliferation in response to serum plus VEGF compared with wild-type mice. S1P2-null ECs also show augmented cell migration in wound healing assays in the presence of serum and VEGF. Moreover, S1P2-null murine lung-derived ECs (MLECs) show greater activity to form tube-like structures than do wild-type MLECs. In S1P2-null MLECs, two major changes in the intracellular signals have been noted. Both the basal and S1P-stimulated activities of Rac, which is a molecular switch of cell motility, are greater in S1P2-null ECs compared with wild-type ECs. Second, S1P inhibits Akt, a serine/threonine protein kinase that is activated downstream of phosphoinositide 3-kianse and mediates cell proliferation and survival, in wild-type but not S1P2-null MLECs. S1P2 stimulates PTEN, a 3’-specific phosphatase of polyphosphoinositides, in a Rho- and Rho kinase-dependent manner[56]. Therefore, it is likely that, in S1P2-null ECs, abolition of S1P-induced PTEN stimulation leads to abolition of Akt inhibition. Thus, S1P2 negatively regulates angiogenesis activity through mechanisms that involve the G12/13-Rho-Rac/PTEN signaling pathway in an EC-autonomous manner[14,38,39]. This notion was supported by the finding that hypodermal co-inoculation of tumor cells and S1P2-null ECs accelerate tumor growth compared with co-inoculation of tumor cells and wild-type ECs[55].
Increasing evidence has shown that myeloid cells participate in tumor angiogenesis through multiple mechanisms[48,49,57]. Infiltrating myeloid cells in tumors release pro-angiogenic factors including VEGFs, FGF-2, PDGFs and matrix metalloproteinases (MMPs), the enzymes that contribute to angiogenesis through degradation of the extracellular matrix proteins and resultant release of VEGFs and TGFβ that have been deposited in the matrix[57,58]. Recent studies[58,59] also have suggested that a subpopulation of BMDCs is capable of transdifferentiating into vascular ECs and becoming incorporated into new blood vessels in tumors. In particular, BMDCs that are positive for the myelomonocytic cell marker CD11b are implicated in tumor neovascularization[49,57]. S1P2-null mice have shown a nearly twofold increase in the number of CD11b+ cells that infiltrate tumors compared with wild-type mice[55]. These BMDCs are probably recruited by chemoattractants including chemokines, VEGFs and other mediators that are secreted by tumor cells and tumor stromal cells. The antimigratory S1P2 that is expressed in BMDCs probably mediates inhibition of their migration into tumors. S1P2 expressed in BMDCs exerts an inhibitory effect on tumor growth and neovessel formation; wild-type mice that have been transplanted with S1P2-null bone marrow cells display acceleration of tumor growth and angiogenesis compared with wild-type mice that have received wild-type bone marrow. In reciprocal experiments, when S1P2-null mice are given wild-type or S1P2-null bone marrow, tumor growth is suppressed in mice with wild-type marrow.
In S1P2-null mice, it has been noted that recruitment of SMCs and pericytes to tumor blood vessels is facilitated, thus, tumor vessels are more stabilized. This might be explained by stimulated local production of angiogenic factors with a vascular stabilizing activity[55], which include TGFβ and PDGFs, the loss of chemorepulsion mediated by S1P2 on mural precursor cells[13,38] in the tumor microenvironment, in which the concentration of S1P in the blood is presumed to be higher than in the perivascular tumor stroma[11], and the greater contribution of BMDCs as mural cell precursors in S1P2-null mice. Concerning the latter possibility, S1P2-expressing mural cells are not readily detected in the vascular wall in tumors of mice that have received transplantation of S1P2LacZ/+ bone marrow, which suggests that the incorporation of S1P2-expressing bone-marrow-derived mural cell precursors into the vascular wall is unlikely[55].
S1P2 exerts inhibitory effects on tumor angiogenesis through EC autonomous and myeloid-cell-dependent actions. These S1P2 actions open the possibility of a novel anti-angiogenic therapy to target S1P2. It is an interesting possibility that S1P-receptor-subtype-selective pharmacological targeting strategies, i.e. S1P1 inhibition in combination with S1P2 activation, could lead to more effective inhibition of tumor angiogenesis. In addition to an expected anti-angiogenic action of S1P2-selective agonist, which is mediated through S1P2 expressed on ECs and BMDCs, selective activation of S1P2 expressed on tumor cells directly inhibits tumor cell migration in vivo[40], which leads to inhibition of invasion and metastasis, as previously demonstrated in an animal model[60].
APPLICATION OF S1P TO ANGIOGENIC THERAPY IN ISCHEMIC DISEASES
Therapeutic angiogenesis is an attractive strategy for treating patients with ischemia[61,62]. Current attempts to develop therapeutic angiogenesis are categorized into angiogenic factor therapy to supply angiogenic growth factors by direct administration or gene transduction of the expression vectors, and cell therapy to supply bone-marrow-derived vascular precursor cells and/or angiogenic-molecule-producing cells. To date, the therapeutic efficacy of angiogenic peptide growth factors, including VEGFs, FGF-2 and hepatocyte growth factor (HGF), and their expression plasmids, has been tested by topical and systemic administration[61-63]. However, the trials in human patients have failed to show unequivocal efficacy of the tested agents; different from the beneficial effects that have been demonstrated in animal experiments. This is partly due to insufficient gene transduction or rapid washout of proteins. Some of these therapies also are accompanied by several drawbacks including tissue edema, proteinuria and promotion of atherosclerosis.
As stated above, S1P is a growth factor, chemoattractant and morphogen for ECs. S1P also promotes the coverage of nascent vessels with mural cells to stabilize them. This maturating action of S1P, together with its barrier-protecting action[13,15-19], inhibits tissue edema and can produce a synergistic therapeutic effect with other angiogenic factors including VEGFs and FGFs. In addition, local administration of S1P into ischemic sites, which is expected not to elevate the circulating S1P level, probably does not cause serious systemic side effects. Recently, we have demonstrated for the first time that daily intramuscular injections of S1P solutions over 28 d promoted blood flow in a murine hindlimb that was rendered ischemic by femoral artery ligation, which is one of the best characterized animal models for ischemia-induced angiogenesis to evaluate the potential of angiogenic factors as therapeutic agents[64]. The stimulatory effect on the blood flow of an optimal dose of S1P is similar or a little stronger in magnitude to that induced by FGF-2. S1P injections increase the microvascular density in muscle of ischemic limbs. We have observed that transgenic mice that overexpress the S1P-synthesizing enzyme SPHK1 and have an elevated S1P level in muscle exhibit enhancement of blood flow recovery and a microvascular increase after femoral artery ligation, compared with wild-type mice. S1P injections or transgenic SPHK1 overexpression do not increase vascular permeability in ischemic limb muscle, as determined by extravasation of Evans blue. These studies have provided evidence that S1P is an effective angiogenesis stimulator in post-ischemic condition in vivo, which accelerates blood flow recovery and neo-vessel formation without local edema or any other adverse effects, including bradycardia and lymphopenia, and with a relatively low cost.
The necessity of daily injections of an S1P solution seems to be a drawback in the clinical setting, therefore, development of a sustained release formulation of S1P is desirable. If S1P release from a sustained release formulation is controllable, the drug delivery system could be more favorable for therapeutic angiogenesis[63]. Microspheres made from poly(lactic-co-glycolic acid) (PLGA) are biocompatible, bioabsorbable and non-toxic, and have been successfully employed as a controlled drug delivery system for sustained release of various drugs[65,66]. We have developed new sustained release preparations of S1P by using PLGA-based microparticles[67]. The half-life of biodegradation of PLGA particles differs, depending on the lactic acid/glycolic acid ratio and the extent of their polymerization. These S1P-containing PLGA (PLGA-S1P) microparticles have exhibited continuous release of S1P into a physiological solution with different kinetics in vitro. We have tested the effects of injections of a single bolus dose or divided doses (constant total dose) of PLGA-S1P microparticles on blood flow in ischemic limb muscle over 28 d, and have found that repeated injections of divided amounts, rather than a single bolus injection, of PLGA-S1P microparticles conferred the optimal stimulatory effect on blood flow[67]. Also, local injections of PLGA-S1P microparticles inhibit limb necrosis and improve limb movement. The particle size of PLGA microspheres could affect release profile of drugs encapsulated in microspheres, and thereby, dose-response relationships and optimal inter-injection time intervals.
PLGA-S1P injections increase anti-CD31+ microvessel density in ischemic limb muscle. PLGA-S1P also increases α-smooth muscle actin+ blood vessels in ischemic muscle, which indicates that S1P facilitates smooth muscle coverage of blood vessels, i.e. arteriogenesis. Arteriogenesis generally involves growth and remodeling of pre-existing collateral vessels or reflects de novo formation of mature vessels[68]. The maturation of blood vessels into multilayer structures is essential for their persistence. PLGA-S1P also promotes association of pericytes with capillaries, and stabilizes newly formed vessels. The stabilization or maturation of capillaries inhibits regression of newly formed vessels and vascular permeability. Thus, S1P seems to stabilize neovessels in ischemic tissues, similarly to developmental angiogenesis and tumor angiogenesis[34,45,50,55]. Injections of PLGA-S1P suppress VEGF-induced edema in ischemic limbs, which is consistent with its vascular stabilization (see above) and barrier-protective actions[17-19].
S1P directly acts on ECs to stimulate migration and cell-cell adhesion, in which S1P1-Gi-Rac pathway is implicated as mentioned above[15,18]. In addition to this pathway, injections of PLGA-S1P stimulates Akt and extracellular signal-regulated kinase (ERK), which is presumably mediated by S1P1 and S1P3via Gi, in ischemic limb muscle[67]. Akt and ERK are key signaling molecules that are implicated in cell proliferation, survival, migration, and activation of eNOS in ECs, and thereby angiogenesis[69-71]. In fact, PLGA-S1P increases phosphorylation level of eNOS at Ser1177, which is the Akt and ERK phosphorylation site that is crucial for its activation. Administration of the NOS inhibitor Nω-nitro-L-arginine methyl ester reduces blood flow in PLGA-S1P-treated mice, which suggests that PLGA-S1P-induced neovascularization is at least in part dependent on eNOS/NO. In addition, S1P-induced stimulation of Akt and eNOS/NO could be involved in blood flow increase through NO-induced vasodilation.
Previous studies[72,73] have shown that BMDCs, which release angiogenic growth factors and MMPs, are recruited to ischemic sites and contribute to neovascularization in the murine hindlimb ischemic model. Injections of PLGA-S1P increase BMDCs positive for the pan-myeloid cell marker CD45, the myelomonocytic lineage cell marker CD11b, or the neutrophil marker Gr-1, which indicates that S1P stimulates recruitment of BMDCs into ischemic limb muscle. S1P has been suggested to activate and recruit BMDCs to an ischemic site through S1P3-dependent sensitization of the SDF-1/CXCR4 signaling pathway[36]. These observations collectively suggest that exogenous S1P stimulates blood flow by promoting angiogenesis, vascular maturation, and arteriogenesis through both the EC-autonomous and non-autonomous mechanisms.
S1P2, which shows anti-angiogenic activity in tumor angiogenesis models and in implanted Matrigel, is expressed in muscle blood vessels[55], and probably counteracts the stimulatory actions of S1P1 and S1P3 on angiogenesis, although regulation of the expression of these S1P receptor subtypes in the vascular endothelium in ischemic tissues is not well understood. The observation that injections of S1P, a non-selective activator of S1P1, S1P2 and S1P3, stimulate the Akt/eNOS pathway in vascular ECs of limb muscle suggests that the angiogenic activity mediated by S1P1 and S1P3 dominates over the anti-angiogenic activity mediated by S1P2in situ in ECs of limb muscle, which is different from isolated MLECs in which S1P induces Akt inhibition via S1P2[55]. Hence, the combination of the selective stimulation of S1P1 and S1P3 and the blockade of S1P2 might be a better strategy for angiogenic therapy to target S1P receptors (Figure 3).
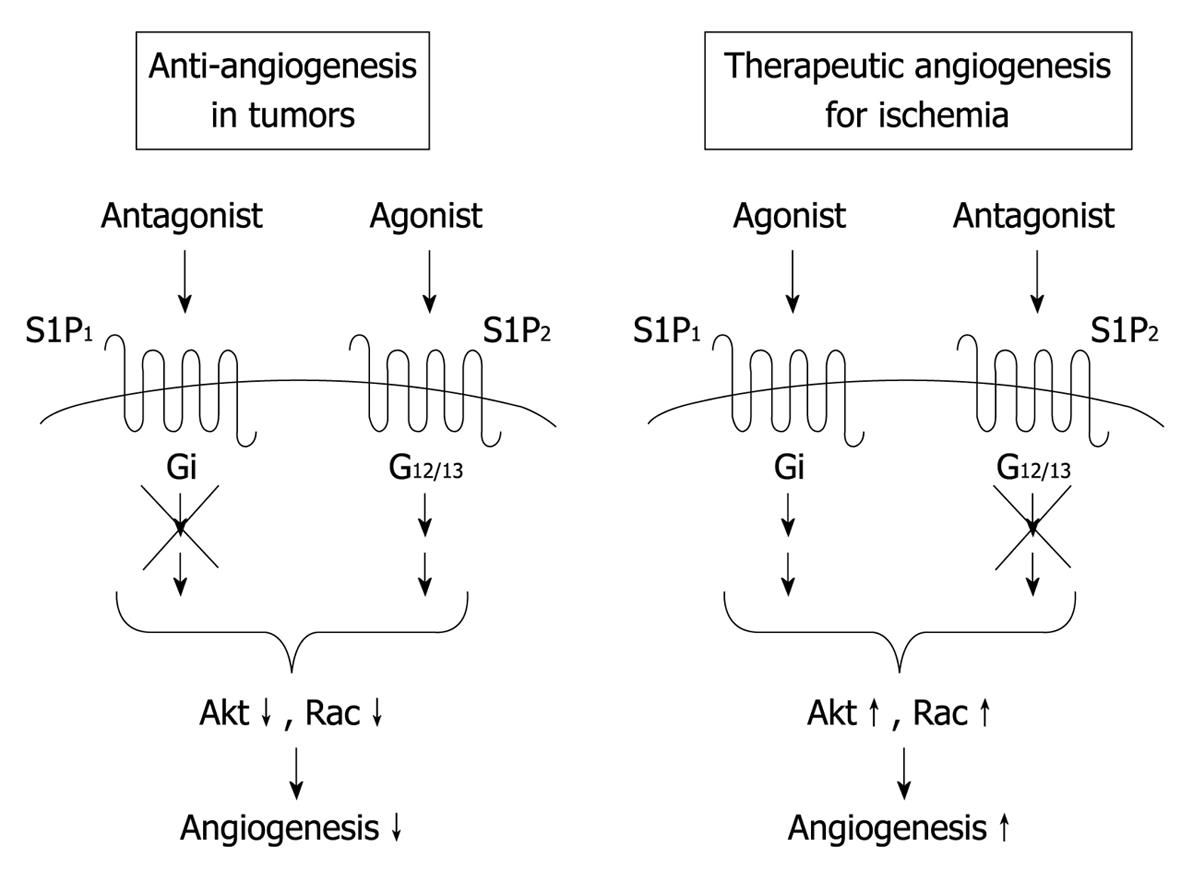
Figure 3 Possible therapeutic strategies for anti-angiogenesis in tumors and angiogenesis in ischemic diseases.
S1P1 and S1P2 mediate angiogenic and anti-angiogenic effects of S1P in vivo, respectively. Therefore, it is possible that simultaneous activation of the angiogenic receptor S1P1 and blockade of the anti-angiogenic receptor S1P2 are more effective for therapeutic angiogenesis in ischemic diseases compared with S1P1 activation or S1P2 inhibition alone. In contrast, for anti-angiogenic therapy in cancer, the combination of S1P1 inhibition and S1P2 stimulation might be favorable.