Published online Mar 27, 2017. doi: 10.5313/wja.v6.i1.14
Peer-review started: September 1, 2016
First decision: September 29, 2016
Revised: December 8, 2016
Accepted: December 16, 2016
Article in press: December 19, 2016
Published online: March 27, 2017
Exsanguination from trauma, gastrointestinal bleeding, and obstetric hemorrhage remains a major source of mortality across the planet. Continued research into resuscitation strategies and evolving technology and blood product storage has allowed for patient to undergo very large volume transfusions, even to the point of replacing a patient’s blood volume several times over. As massive transfusions have become more common, more studies have been performed delineating the exact patient population that would benefit, start- and stop-points of transfusions, complications and avoidance of the same. We discuss these points and provide information and strategies for massive transfusion.
Core tip: Recognizing the patient who requires massive transfusion early is key to the most optimal outcome. Once recognized, massive transfusion protocols (MTP) should be initiated and continued until normal physiologic parameters are reached and definitive control of bleeding is achieved. Hospitals should develop their own MTP, guided by the literature, and according to their specific needs and patient populations.
- Citation: Fredericks C, Kubasiak JC, Mentzer CJ, Yon JR. Massive transfusion: An update for the anesthesiologist. World J Anesthesiol 2017; 6(1): 14-21
- URL: https://www.wjgnet.com/2218-6182/full/v6/i1/14.htm
- DOI: https://dx.doi.org/10.5313/wja.v6.i1.14
Trauma continues to be a major source of mortality, and much of this early mortality comes from exsanguination leading to death. To combat this, strategies to rapidly and effectively administer blood products and associated adjuncts to control blood loss as quickly as possible. Most deaths due to blood loss happen in the first 6 h[1]. While definitive control occurs in the operating room or interventional radiology suite, continued resuscitation is necessary to keep the patient alive during the critical first hour of the bleeding patient’s arrival to the hospital. The goal of massive transfusion is not just the replacement of intravascular volume, but the correction of trauma induced coagulopathy, in an attempt to curb further blood loss. Massive transfusion, defined as > 10 units of blood in the first 24 h can be a life-saving maneuver for a bleeding patient, but is not without complications[2]. Massive transfusion remains an area of great study throughout the critical care and trauma literature, and many hospitals now have developed their own massive transfusion protocols (MTP). This review will discuss the recent advances in massive transfusion, initiation of MTP, special populations, and complications of MTP.
Initiation of MTP was formerly firmly under clinician gestalt. However, this intuition appears to have only a 50% predictive value in identification of patients who will need MTP[3]. Recently, clinicians developed massive resuscitation scoring systems to accurately identify patients who will ultimately require large volume blood product resuscitation upon arrival to the emergency department.
Early identification of this cohort is important before the tipping point of hemorrhage spirals into the lethal triad of coagulopathy. Cotton et al[4] found that both short-term and long-term survival is increased when the initiation of MTP occurs immediately in the emergency room rather than later in the operating room. Implementation of MTP guidelines both decrease mortality and the overall amount of blood use in 24 h, due to better proximal resuscitation[5].
Therefore, scoring systems for initiation of MTP must have appropriate sensitivity and also specificity to safely rule out those who will not require large volumes of blood products thus limiting unnecessary infectious exposure and saving valuable resources. These models vary in their variables of laboratory values, physical exam findings, and physiological triggers, but all have comparable predictive abilities. Application of these scoring systems is dependent on the ability for point of care testing in individual hospitals.
The German originated trauma associated severe hemorrhage score (TASH) was the first massive transfusion scoring system. It has 7 variables involving history (gender), physical findings (FAST, long-bone or pelvic fractures), vital signs (heart rate, blood pressure), and laboratory values (base deficit, hemoglobin)[6]. The probability for MTP = 1[1 + exp(4.9 - 0.3 × TASH)].
The Assessment of Blood Consumption score was designed to be applicable on immediate arrival to the emergency room without additional laboratory testing or need for additional calculations. It assigns one point to: Penetrating mechanism, heart rate > 90 bpm, positive FAST, or SBP < 90 mmHg. An Assessment of Blood Consumption score of greater than 2 was determined to be the appropriate trigger for MTP, with a sensitivity and specificity of 75% and 86%, respectively[7].
Cessation of MTP may more nebulous and reliant on clinician gestalt given the potentially rapidly changing current of the patients’ clinical condition in the short hours after admission. Following surgical control of bleeding, the restoration of hemodynamics, correction of acidosis and coagulopathy, and signs of sufficient end-organ perfusion (mental status, urine output) all may be used as surrogates for adequate resuscitation. Callcut et al[8] identified those patients at risk of ongoing hemorrhage at 6 h after admission and those patients unlikely to need additional blood products. The authors MTP score is composed of 5 variables (INR > 1.5, hemoglobin < 11 g/dL, base deficit > 6) and vital sign (SBP < 90 mmHg, temperature < 35.5 °C). Failure of normalization of the MTP score within 3 and 6 h associated with mortality at one day and 28 d.
Compliance to MTP is vitally important, as it has been shown to negatively affect survival. In their institutional review, Bawazeer et al[9] found delays in 50% of activation and a 47% incidence of non-compliance with type of product given. The authors found significant differences in mortality between groups of high and medium compliance.
Despite the aforementioned algorithmic calculations for initiation and cessation of MTP, providers will often initiate MTP on the early evidence or concern for significant hemorrhage, i.e., initial thoracic or pelvic radiography showing hemothorax or open book pelvic fracture, respectively. It is our suggestion to place institutional protocols to limit the number of people with the power to activate MTP to prevent overutilization by providers inexperienced with the set activation points.
Many early studies in blood product ratios of fresh frozen plasma (FFP) to blood and platelet ratios were affected by survival or selection biases and mixed populations[10-12]. Most studies have focused on the risks and benefits associated with a 1:2 or 1:1 ratios of FFP to blood, although other less common ratios exist[13]. With the findings of the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) study group published in 2015, many hospitals have decided on a 1:1:1 ratio of products[14]. The PROPPR group prospectively randomized patients at 12 centers to receive 1:1:1 or 1:2:1. While 30-d mortality was similar, there was an increase in early hemostasis and decrease in deaths due to bleeding, at the expense of increased use of blood products. No increase in transfusion-related complications was noted in the 1:1:1 group[14]. The practical matter of having to thaw plasma results in many hospitals having to “catch up”, using blood first, then using plasma once it is thawed. This is disadvantageous as maintaining clotting factors and fibrinogen is important to achieving early hemostasis. A fibrinogen level lower than < 100 mg/dL substantially increases in-hospital mortality[15]. Alternatives to “catching up” include keeping a small number of units of thawed plasma and rotating it out as necessary, or using lyophilized plasma.
In addition to a balanced hemostatic resuscitation (BHR) protocol multiple other pharmacologic adjuncts have been studied. Initially utilized for procedural related hemorrhage in hemophilic patients[16], tranexamic acid (TXA) was found to have application in the management of surgical bleeding. Multiple studies demonstrated a role for reduced use of blood products in elective surgery as well as the treatment of hyperfibrinolysis associated with cardiopulmonary bypass[16-19]. TXA is a lysine analog which, similar to aminocaproic acid, inhibits fibrinolysis. The antifibrinolytic effect is attributed to its competitive inhibition of the lysine binding site on plasminogen, resulting in the molecules stability and prevents further degradation of the existing clot[20,21]. In 2010 the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH)-2 study reported the results of a 20000 patient RTC in which the effects of TXA on mortality and transfusion requirements in adult patients who were traumatically injured and in hemorrhagic shock[22]. The CRASH-2 group was able to demonstrate an improvement in all-cause mortality and mortality attributed to hemorrhage with the use of TXA within 3 h from time of injury. Subsequent studies, such as the MATTERs (Military Application of Tranexamic Acid in Trauma Emergency Resuscitation) and PED-TRAX (Pediatric Trauma and Tranexamic Acid), demonstrated a survival advantage when TXA was administered[23,24]. In the MATTERs study there was an observed lower unadjusted mortality rate, increased odds of survival and lower rate of coagulopathy[23]. With PED-TRAX, the first study to look at children independently, there was an observed decreased mortality among all patients (OR = 0.3; P = 0.03) who received TXA[24]. Interestingly both populations of the MATTERs and PED-TRAX studies who received TXA were more severely injured than those who did not yet there remained an observed survival benefit.
Regarding thromboembolic events, there was an observed increase in pulmonary embolus and deep venous thrombosis in the MATTERs study but these results were not replicated in the PED-TRAX study[23,24]. While there appears a clear benefit to use of TXA in the bleeding trauma population, several critiques have arisen specifically regarding the CRASH-2 study. These include the applicability and predictability, lack of measurement of severity of injury and the application of TXA use in traumatic brain injury[25]. Several upcoming studies like CRASH-3 and the Prehospital Antifibrinolytics for Traumatic Coagulopathy and Hemorrhage Trial will hopefully elucidate a more specified role for TXA, but what we do know is that TXA provides some benefit to the bleeding trauma patient, and is included in many transfusion protocols[26,27].
The use of supplemental fibrinogen, Recombinant Factor VII (rFVIIa), and prothrombin complex (PCC) as adjuncts to BHR have also been explored. Several nonrandomized trials have evaluated the use fibrinogen in the trauma patient[28-30]. In each of these studies there was a reduced requirement for blood product transfusion and associated reduced potential for multi-organ failure. While there has been shown a benefit, no prospect randomized trials have been reported to date. rFVIIa has also been examined for use in massive transfusion. Multiple retrospective studies have elevated the added effect of rFVIIa on volume of transfusion, mortality and organ failure with mixed results[31-34]. To date, only two randomized controlled trials have been completed[35,36]. Unfortunately, neither study revealed any mortality benefit with the addition of Factor VII though there was observed reduction in amount of blood products transfused, furthermore a Cochrane review was unable to support the use of Factor VII for use in traumatic hemorrhage[37]. PCC has also been proposed for use in BHR. Initially utilized in the treatment of hemophilia, it is the standard reversal agent for vitamin K antagonists and has been utilized in BHR[38]. Several well-constructed reviews and retrospective studies have shown efficaciousness of PCC in addressing vitamin K antagonistic bleeding in trauma, but no clear role in it use in BHR has been defined[39-41]. Hannon et al[42] in an animal based model of traumatic coagulopathy failed to show any benefit on blood volume lost when PCC was utilized and there is currently a lack of prospective randomized controlled trials to guide evidence supported use. Although promising, additional prospective study is needed of concentrated fibrinogen or prothrombin complex concentrate before they can be recommended[43].
While a balanced hemostatic resuscitation is the cornerstone of addressing the bleeding trauma patient, there remains much work to be done in the investigation of the multiple potential and available adjuncts before the optimal strategy is determined.
Speed of transfusion is important during MTP especially when exsanguination occurs at a rate greater than transfusion. Speed is augmented by optimal vascular access and utilizing Pouiselle’s law, which states flow is directly related to width and indirectly related to length of the catheter. Pressurized tubing or specialized pressurized rapid transfusers can augment flow. Rapid transfuser units can often also warm blood at the same time, providing an additional benefit against hypothermia.
Blood and fluid warming is important as to not to exacerbate hypothermia. Barthel et al[44] suggest warmed fluids cannot induce hyperthermia, but can limit additional heat loss. However, adjunctive techniques of warming including forced air devices, blankets, and high operating room temperatures are important. Hypothermia is exceedingly dangerous; in patients undergoing MTP, a cutoff of less than 35 °Cas the lowest recorded temperature in the first 24 h portended increases in mortality[45].
The elderly patient represents a unique challenge amongst the trauma population at base line. Underlying cardiovascular disease and decreased functional status can complicate resuscitation. Data from the PROMMTT and PROPPR had median ages for 37 and 34 years respectively, which questions the applicability of such practices to an elderly population[14,46]. The Trauma Outcomes Group demonstrated that age is an independent predictor of mortality in the massively transfused patient[2]. In theory the decreased need for crystalloid volume would lead to a decreased incidence of circulatory over load in the elderly population. Although no subgroup analysis of the PROMMTT or PROPPER studies exists and no prospective institutional studies exist to examine age in a massive transfusion protocol. In a retrospective analysis of 14 elderly (> 60 years) compared to 52 non-elderly patients, Murry et al[47] demonstrated similar, mortality rates (50% vs 53%). This is a limited study with high mortality in both arms, but otherwise similar patient characteristics on arrival including GCS, ISS and starting hematocrits. Mitra et al[48] retrospectively compared patients > 65 years vs a younger cohort and demonstrated that while mortality was higher (39% elderly vs 21% young) a significant number of elderly patients survived to discharge. Patient characteristics that were associated with mortality among the elderly patients included an increased systolic blood pressure (OR = 1.02), a pre hospital GCS < 8 (0.73), and acute traumatic coagulopathy (11.75). Limited data exists for the use of massive transfusions in the elderly trauma patient. Small series would suggest similar mortality to cohorts from the same institutions, further work to explore coagulopathic complications is needed. Additionally, considerations for age, frailty and cardiovascular function should be investigate and possibly included in future targeted massive transfusion protocols.
The pediatric patient population also represents unique challenges to hemorrhagic shock and acute traumatic coagulopathy. The physiologic reserve in children is robust and includes the ability to maintain normal blood pressures until 20% of blood volume loss[49]. Which make application standard triggers used in adults difficult to apply. Resuscitation in the pediatric population is defined on volume (mL) per body weight (kg) and is no different in acute hemorrhage. Children younger than 3 mo are estimated to 90 mL/kg of blood volume and those older than 3 mo are estimated at 70 mL/kg[50]. Some measures do apply: High ISS scores, shock, high base deficit and increased INR > 1.5 are associated with increased mortality in the pediatric population[51]. In an effort to better define the volume of blood loss needed to qualify for a pediatric MTP, Neff et al[52] used the DOD trauma registry and identified all pediatric patients, those to greater than 40 mL/kg of blood loss in the initial 24 h were more often in shock, hypothermic, coagulopathic and thrombocytopenic at time of presentation[52,53]. This gives a reasonable initial starting point for a volume trigger for pediatric MTP. The same principles apply including balanced transfusion strategies and a restrictive use of crystalloids. That stated there are only two small prospective trials on the use of MTP in pediatrics. Hendrickson et al[54] described the initiation of protocol involving a fixed ratio of products based on body weight. They included 102 patients, and succeeded in transfusing at nearly a 1:1 FFP to RBC ratio, although no statistic improvement was seen in mortality (38% pre vs 23% post P = 0.035) after taking into account severity of injury. In this study only 50% of patients required a massive volume of blood (> 70 mL/kg) to be transfused this may reflect our inability to identify pediatric patient in need and not the benefit of MTP. Chidester et al[55] also applied the MTP principles to the pediatric population; they included 55 patients with transfusion ratios at 1:3 and similarly didn’t see an improvement in mortality. They did note fewer thromboembolic complications with the MTP group (4 events vs 0 events).
Post-partum hemorrhage (PPH) is a major cause of up to 25% of pregnancy related deaths[56]. Physiologic changes in pregnancy including an increase in red blood cell mass (25%) and a greater increase in plasma volume (50%) allow of hemodynamic stability during the birthing process. Although changes in coagulation factors are not balanced and the relative increase in fibrinogen and factors VII, VIII and IX lead to a relative hypercoagulable state[57]. These physiologic changes make the resuscitation of such patients uniquely challenging. Primary treatment of postpartum hemorrhage includes surgery and uterotonic agents. Some investigators are starting to apply balanced and goal directed resuscitation to PPH[58]. Adjuncts such as thromboelastography allow for a real-time assessment of coagulation and fibrinolysis and have allowed for targeted treatment[59,60]. Although familiarity with the normal baseline changes in the tests are key for interpretation[59]. Despite these initial advancements in PPH care, the lack of prospective data has led to limited improvement in the national management guidelines. Dahlke et al[61] compared management guidelines from the 4 large nations obstetrics committees and only one guideline includes mention of blood bank notification and none describe a balanced or targeted transfusion practice. Further prospective analysis is required for the use of massive transfusion in the obstetric patient.
Administration of liters of inflammatory, immunomodulatory, and potentially infectious fluids into a patient already in hemorrhagic shock can assist in explanation of the common morbidities of MTP. They include: Acute respiratory distress syndrome (ARDS), transmission of viral and bacterial infection, abdominal compartment syndrome (ACS) and electrolyte abnormalities.
Massive resuscitation makes the lungs susceptible to the spectrum of lung injury via volume or immune-mediated mechanisms. For those undergoing MTP, Moss et al[62] found that 21% of patients will develop ARDS. The risks appear to increase with the amount of blood transfused and the mechanism of injury. Silverboard et al[63] demonstrated in a prospective cohort of 102 patients, development of ARDS was found at 5 or 10 units of PRBCs transfused for blunt or penetrating trauma respectively.
MTP places patients at risk for both viral and bacterial infections. In the United States, the estimated risk for HIV is 1 in 2135000. The greatest risk is for hepatitis B at 1 in 277000[64]. Patients are more susceptible to bacterial infections from platelets because of their relatively warm storage requirements (20 °C), with an estimated risk of 1 in 5000 compared to 1 in 38500 in RBC[64]. The most common bacteria transmitted are gram-positive aerobic organisms[65].
The incidence of intraabdominal hypertension or abdominal compartment syndrome appears to be declining with limitation and replacement of crystalloid with balanced blood products strategies. Joseph et al[57] showed a decrease in ACS from 7.4% to 0% with corresponding reduction in crystalloid from 12.8 to 6.6 L.
Two common electrolyte abnormalities that occur in MTP are hypocalcemia, caused by the preservative citrate and hyperkalemia. Aboudara et al[66] show that after transfusion of 7 units of PRBCs, the patient is at risk for hyperkalemia. Furthermore, in the pediatric population, there is an association between hyperkalemia during rapid blood transfusion and cardiac arrest.
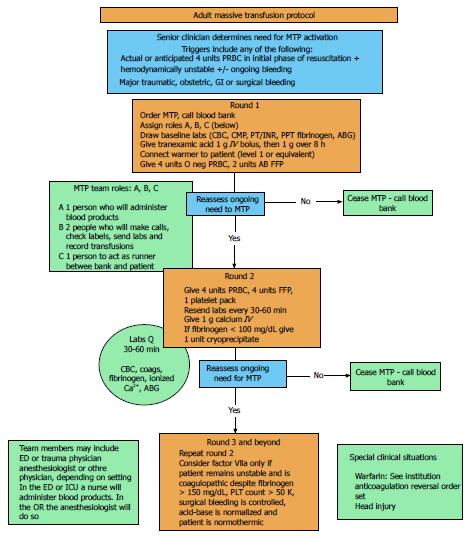
The finer points of massive transfusion, such as ratios of blood products, adjuncts, and transfusion triggers and endpoints will continue to be studied and debated in the literature for years to come. However, it cannot be argued that a massive transfusion protocol saves lives. A defined, hospital-specific MTP allows trained providers to recognize patients at risk of high-volume blood loss early, initiation of massive transfusion quickly, and has specific stop points to limit over transfusion[67]. We recommend that all centers that take care of critically ill patients of all varieties should evaluate the literature and develop their own protocol. We have included our protocol for guidance (Figure 1).
Manuscript source: Invited manuscript
Specialty type: Anesthesiology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bestas A, Hilmi I, Kvolik S S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Callcut RA, Cotton BA, Muskat P, Fox EE, Wade CE, Holcomb JB, Schreiber MA, Rahbar MH, Cohen MJ, Knudson MM. Defining when to initiate massive transfusion: a validation study of individual massive transfusion triggers in PROMMTT patients. J Trauma Acute Care Surg. 2013;74:59-65, 67-68; discussion 66-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Barbosa RR, Rowell SE, Sambasivan CN, Diggs BS, Spinella PC, Schreiber MA, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G. A predictive model for mortality in massively transfused trauma patients. J Trauma. 2011;71:S370-S374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Pommerening MJ, Goodman MD, Holcomb JB, Wade CE, Fox EE, Del Junco DJ, Brasel KJ, Bulger EM, Cohen MJ, Alarcon LH. Clinical gestalt and the prediction of massive transfusion after trauma. Injury. 2015;46:807-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Cotton BA, Dossett LA, Au BK, Nunez TC, Robertson AM, Young PP. Room for (performance) improvement: provider-related factors associated with poor outcomes in massive transfusion. J Trauma. 2009;67:1004-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, St Jacques P, Young PP. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177-1182; discussion 1182-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Yücel N, Lefering R, Maegele M, Vorweg M, Tjardes T, Ruchholtz S, Neugebauer EA, Wappler F, Bouillon B, Rixen D. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60:1228-1236; discussion 1236-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 8. | Callcut RA, Cripps MW, Nelson MF, Conroy AS, Robinson BB, Cohen MJ. The Massive Transfusion Score as a decision aid for resuscitation: Learning when to turn the massive transfusion protocol on and off. J Trauma Acute Care Surg. 2016;80:450-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Bawazeer M, Ahmed N, Izadi H, McFarlan A, Nathens A, Pavenski K. Compliance with a massive transfusion protocol (MTP) impacts patient outcome. Injury. 2015;46:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Bhangu A, Nepogodiev D, Doughty H, Bowley DM. Meta-analysis of plasma to red blood cell ratios and mortality in massive blood transfusions for trauma. Injury. 2013;44:1693-1699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Godier A, Samama CM, Susen S. Plasma/platelets/red blood cell ratio in the management of the bleeding traumatized patient: does it matter? Curr Opin Anaesthesiol. 2012;25:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Hallet J, Lauzier F, Mailloux O, Trottier V, Archambault P, Zarychanski R, Turgeon AF. The use of higher platelet: RBC transfusion ratio in the acute phase of trauma resuscitation: a systematic review. Crit Care Med. 2013;41:2800-2811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Lal DS, Shaz BH. Massive transfusion: blood component ratios. Curr Opin Hematol. 2013;20:521-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA. Transfusion of plasma, platelets, and red blood cells in a 1: 1: 1 vs a 1: 1: 2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1527] [Cited by in F6Publishing: 1462] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 15. | Inaba K, Karamanos E, Lustenberger T, Schöchl H, Shulman I, Nelson J, Rhee P, Talving P, Lam L, Demetriades D. Impact of fibrinogen levels on outcomes after acute injury in patients requiring a massive transfusion. J Am Coll Surg. 2013;216:290-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71:S9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Ker K. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;CD001886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Wei M, Jian K, Guo Z, Wang L, Jiang D, Zhang L, Tarkka M. Tranexamic acid reduces postoperative bleeding in off-pump coronary artery bypass grafting. Scand Cardiovasc J. 2006;40:105-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 570] [Article Influence: 47.5] [Reference Citation Analysis (1)] |
| 20. | Bailey AM, Baker SN, Weant KA. Tranexamic acid for trauma-related hemorrhage. Adv Emerg Nurs J. 2014;36:123-131; quiz 132-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Okamoto S, Hijikata-Okunomiya A, Wanaka K, Okada Y, Okamoto U. Enzyme-controlling medicines: introduction. Semin Thromb Hemost. 1997;23:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 23. | Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 24. | Eckert MJ, Wertin TM, Tyner SD, Nelson DW, Izenberg S, Martin MJ. Tranexamic acid administration to pediatric trauma patients in a combat setting: the pediatric trauma and tranexamic acid study (PED-TRAX). J Trauma Acute Care Surg. 2014;77:852-858; discussion 858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Stephens CT, Gumbert S, Holcomb JB. Trauma-associated bleeding: management of massive transfusion. Curr Opin Anaesthesiol. 2016;29:250-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Ker K, Kiriya J, Perel P, Edwards P, Shakur H, Roberts I. Avoidable mortality from giving tranexamic acid to bleeding trauma patients: an estimation based on WHO mortality data, a systematic literature review and data from the CRASH-2 trial. BMC Emerg Med. 2012;12:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Perel P, Al-Shahi Salman R, Kawahara T, Morris Z, Prieto-Merino D, Roberts I, Sandercock P, Shakur H, Wardlaw J. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury--a nested randomised, placebo-controlled trial. Health Technol Assess. 2012;16:iii-xii, 1-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Schöchl H, Solomon C, Traintinger S, Nienaber U, Tacacs-Tolnai A, Windhofer C, Bahrami S, Voelckel W. Thromboelastometric (ROTEM) findings in patients suffering from isolated severe traumatic brain injury. J Neurotrauma. 2011;28:2033-2041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Schöchl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 30. | Aubron C, Reade MC, Fraser JF, Cooper DJ. Efficacy and safety of fibrinogen concentrate in trauma patients--a systematic review. J Crit Care. 2014;29:471.e11-471.e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, Salinas J, Mehta S, Wade CE, Holcomb JB. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286-293; discussion 293-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Perkins JG, Schreiber MA, Wade CE, Holcomb JB. Early versus late recombinant factor VIIa in combat trauma patients requiring massive transfusion. J Trauma. 2007;62:1095-1099; discussion 1099-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Rizoli SB, Nascimento B, Osman F, Netto FS, Kiss A, Callum J, Brenneman FD, Tremblay L, Tien HC. Recombinant activated coagulation factor VII and bleeding trauma patients. J Trauma. 2006;61:1419-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Morse BC, Dente CJ, Hodgman EI, Shaz BH, Nicholas JM, Wyrzykowski AD, Salomone JP, Vercruysse GA, Rozycki GS, Feliciano DV. The effects of protocolized use of recombinant factor VIIa within a massive transfusion protocol in a civilian level I trauma center. Am Surg. 2011;77:1043-1049. [PubMed] [Cited in This Article: ] |
| 35. | Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8-15; discussion 15-18. [PubMed] [Cited in This Article: ] |
| 36. | Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 37. | Stanworth SJ, Birchall J, Doree CJ, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2007;CD005011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:160S-198S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1524] [Cited by in F6Publishing: 1441] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 39. | Matsushima K, Benjamin E, Demetriades D. Prothrombin complex concentrate in trauma patients. Am J Surg. 2015;209:413-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Nienaber U, Innerhofer P, Westermann I, Schöchl H, Attal R, Breitkopf R, Maegele M. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma-associated haemorrhage and massive transfusion. Injury. 2011;42:697-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Berndtson AE, Huang WT, Box K, Kobayashi L, Godat LN, Smith AM, Weingarten D, Coimbra R. A new kid on the block: Outcomes with Kcentra 1 year after approval. J Trauma Acute Care Surg. 2015;79:1004-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Hannon M, Quail J, Johnson M, Pugliese C, Chen K, Shorter H, Riffenburgh R, Jackson R. Fibrinogen and prothrombin complex concentrate in trauma coagulopathy. J Surg Res. 2015;196:368-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Shah A, McKechnie S, Stanworth S. Use of Plasma for Acquired Coagulation Factor Deficiencies in Critical Care. Semin Thromb Hemost. 2016;42:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Barthel ER, Pierce JR. Steady-state and time-dependent thermodynamic modeling of the effect of intravenous infusion of warm and cold fluids. J Trauma Acute Care Surg. 2012;72:1590-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Reynolds BR, Forsythe RM, Harbrecht BG, Cuschieri J, Minei JP, Maier RV, Moore EE, Billiar EE, Peitzman AB, Sperry JL. Hypothermia in massive transfusion: have we been paying enough attention to it? J Trauma Acute Care Surg. 2012;73:486-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 679] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 47. | Murry JS, Zaw AA, Hoang DM, Mehrzadi D, Tran D, Nuno M, Bloom M, Melo N, Margulies DR, Ley EJ. Activation of Massive Transfusion for Elderly Trauma Patients. Am Surg. 2015;81:945-949. [PubMed] [Cited in This Article: ] |
| 48. | Mitra B, Olaussen A, Cameron PA, O’Donohoe T, Fitzgerald M. Massive blood transfusions post trauma in the elderly compared to younger patients. Injury. 2014;45:1296-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Nystrup KB, Stensballe J, Bøttger M, Johansson PI, Ostrowski SR. Transfusion therapy in paediatric trauma patients: a review of the literature. Scand J Trauma Resusc Emerg Med. 2015;23:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Barcelona SL, Thompson AA, Coté CJ. Intraoperative pediatric blood transfusion therapy: a review of common issues. Part II: transfusion therapy, special considerations, and reduction of allogenic blood transfusions. Paediatr Anaesth. 2005;15:814-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Patregnani JT, Borgman MA, Maegele M, Wade CE, Blackbourne LH, Spinella PC. Coagulopathy and shock on admission is associated with mortality for children with traumatic injuries at combat support hospitals. Pediatr Crit Care Med. 2012;13:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Neff LP, Cannon JW, Morrison JJ, Edwards MJ, Spinella PC, Borgman MA. Clearly defining pediatric massive transfusion: cutting through the fog and friction with combat data. J Trauma Acute Care Surg. 2015;78:22-28; discussion 28-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Livingston MH, Singh S, Merritt NH. Massive transfusion in paediatric and adolescent trauma patients: incidence, patient profile, and outcomes prior to a massive transfusion protocol. Injury. 2014;45:1301-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Hendrickson JE, Shaz BH, Pereira G, Parker PM, Jessup P, Atwell F, Polstra B, Atkins E, Johnson KK, Bao G. Implementation of a pediatric trauma massive transfusion protocol: one institution’s experience. Transfusion. 2012;52:1228-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Chidester SJ, Williams N, Wang W, Groner JI. A pediatric massive transfusion protocol. J Trauma Acute Care Surg. 2012;73:1273-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Obaid TA. No woman should die giving life. Lancet. 2007;370:1287-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Joseph B, Zangbar B, Pandit V, Vercruysse G, Aziz H, Kulvatunyou N, Wynne J, O’Keeffe T, Tang A, Friese RS. The conjoint effect of reduced crystalloid administration and decreased damage-control laparotomy use in the development of abdominal compartment syndrome. J Trauma Acute Care Surg. 2014;76:457-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Jadon A, Bagai R. Blood transfusion practices in obstetric anaesthesia. Indian J Anaesth. 2014;58:629-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Ekelund K, Hanke G, Stensballe J, Wikkelsøe A, Albrechtsen CK, Afshari A. Hemostatic resuscitation in postpartum hemorrhage - a supplement to surgery. Acta Obstet Gynecol Scand. 2015;94:680-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Long N, Ng S, Donnelly G, Owens M, McNicholas M, McCarthy K, McCaul C. Anatomical characterisation of the cricothyroid membrane in females of childbearing age using computed tomography. Int J Obstet Anesth. 2014;23:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Dahlke JD, Mendez-Figueroa H, Maggio L, Hauspurg AK, Sperling JD, Chauhan SP, Rouse DJ. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213:76.e1-76.10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 62. | Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59:717-723. [PubMed] [Cited in This Article: ] |
| 64. | Bihl F, Castelli D, Marincola F, Dodd RY, Brander C. Transfusion-transmitted infections. J Transl Med. 2007;5:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Wagner SJ. Transfusion-transmitted bacterial infection: risks, sources and interventions. Vox Sang. 2005;88:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64:S86-91; discussion S91. [PubMed] [Cited in This Article: ] |
| 67. | Brown KA, Bissonnette B, McIntyre B. Hyperkalaemia during rapid blood transfusion and hypovolaemic cardiac arrest in children. Can J Anaesth. 1990;37:747-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |