Published online Jul 20, 2022. doi: 10.5493/wjem.v12.i4.53
Peer-review started: February 9, 2022
First decision: April 13, 2022
Revised: April 27, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: July 20, 2022
Coronavirus disease 2019 (COVID-19) causes acute microvascular thrombosis in both venous and arterial structures which is highly associated with increased mortality. The mechanisms leading to thromboembolism are still under investigation. Current evidence suggests that excessive complement activation with severe amplification of the inflammatory response (cytokine storm) hastens disease progression and initiates complement-dependent cytotoxic tissue damage with resultant prothrombotic complications. The concept of thromboinflammation, involving overt inflammation and activation of the coagulation cascade causing thrombotic microangiopathy and end-organ damage, has emerged as one of the core components of COVID-19 pathogenesis. The complement system is a major mediator of the innate immune response and inflammation and thus an appealing treatment target. In this review, we discuss the role of complement in the development of thrombotic microangiopathy and summarize the current data on complement inhibitors as COVID-19 therapeutics.
Core Tip: Current evidence supports the role of excessive complement activation with subsequent illness progression and development of a complement-dependent cytotoxic tissue damage with detrimental effects in coronavirus disease 2019 (COVID-19) patients, including thromboembolic complications. Based on its role in the development of the cytokine storm and thrombogenesis in COVID-19, the complement system is an appealing treatment target with promising results from preliminary reports. Whether inhibition of upstream (C3, C1) or terminal (C5, C5a, or C5aR) components is of equal importance remains to be elucidated, however, preliminary results from several ongoing clinical trials show benefit in terms of 28-d mortality and pulmonary embolism.
- Citation: Gianni P, Goldin M, Ngu S, Zafeiropoulos S, Geropoulos G, Giannis D. Complement-mediated microvascular injury and thrombosis in the pathogenesis of severe COVID-19: A review. World J Exp Med 2022; 12(4): 53-67
- URL: https://www.wjgnet.com/2220-315x/full/v12/i4/53.htm
- DOI: https://dx.doi.org/10.5493/wjem.v12.i4.53
Coronaviruses are a large family of enveloped viruses that can cause serious respiratory infections, including severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19) [from SARS coronavirus 2 (SARS-CοV-2)]. Although COVID-19 symptoms are often mild, up to 20%-25% of hospitalized patients require intensive care[1,2]. A substantial proportion of patients develop respiratory complications such as pneumonia and acute respiratory distress syndrome (ARDS) as part of a dysregulated systemic inflammatory response, in addition to acute renal, cardiac, and hepatic injury and disseminated intravascular coagulation[3]. Disease severity and mortality appear to be associated with patient age and comorbidities, suggesting a dynamic relationship between viral replication and host immune response. Patients with high levels of pro-inflammatory cytokines and chemokines show a greater degree of pulmonary inflammation, a phenomenon that has also been observed with SARS and MERS[4]. Although the molecular mechanisms of viral pathogenicity are not fully understood, immune-mediated damage is a major contributor to SARS-CοV-2-associated morbidity and mortality[5]. Rapid cardiorespiratory failure and multiorgan injury, common features of severe SARS-CoV-2 infection, can be partly explained by an aberrant immune response[6].
The complement system is an important part of the innate immune system and participates in the perivascular and intravascular clearance of pathogens, as well as in coagulation and fibrinolysis. In severe cases, SARS-CoV-2 induces a dysregulated immune response that becomes detrimental to the host, described as ‘cytokine storm’ or ‘cytokine release syndrome’[7]. During cytokine storm, serum levels of complement components 3 and 4 (C3 and C4) and other components of the classical complement pathway as measured by the CH50 assay are decreased due to increased complement factor consumption[8]. Post-mortem cadaveric analysis of patients with severe SARS-CoV-2 infection has demonstrated thrombotic microangiopathy (TMA) implicating the activation of the complement cascade[9]. These observations, coupled with results of proteomic studies, highlight the role of complement activation in the pathogenesis of SARS-CoV-2[10]. In this review, we summarize the current evidence of complement involvement in microvascular injury and thrombosis in SARS-CoV-2 infection, as well as current data on complement inhibitors in the treatment of severe COVID-19.
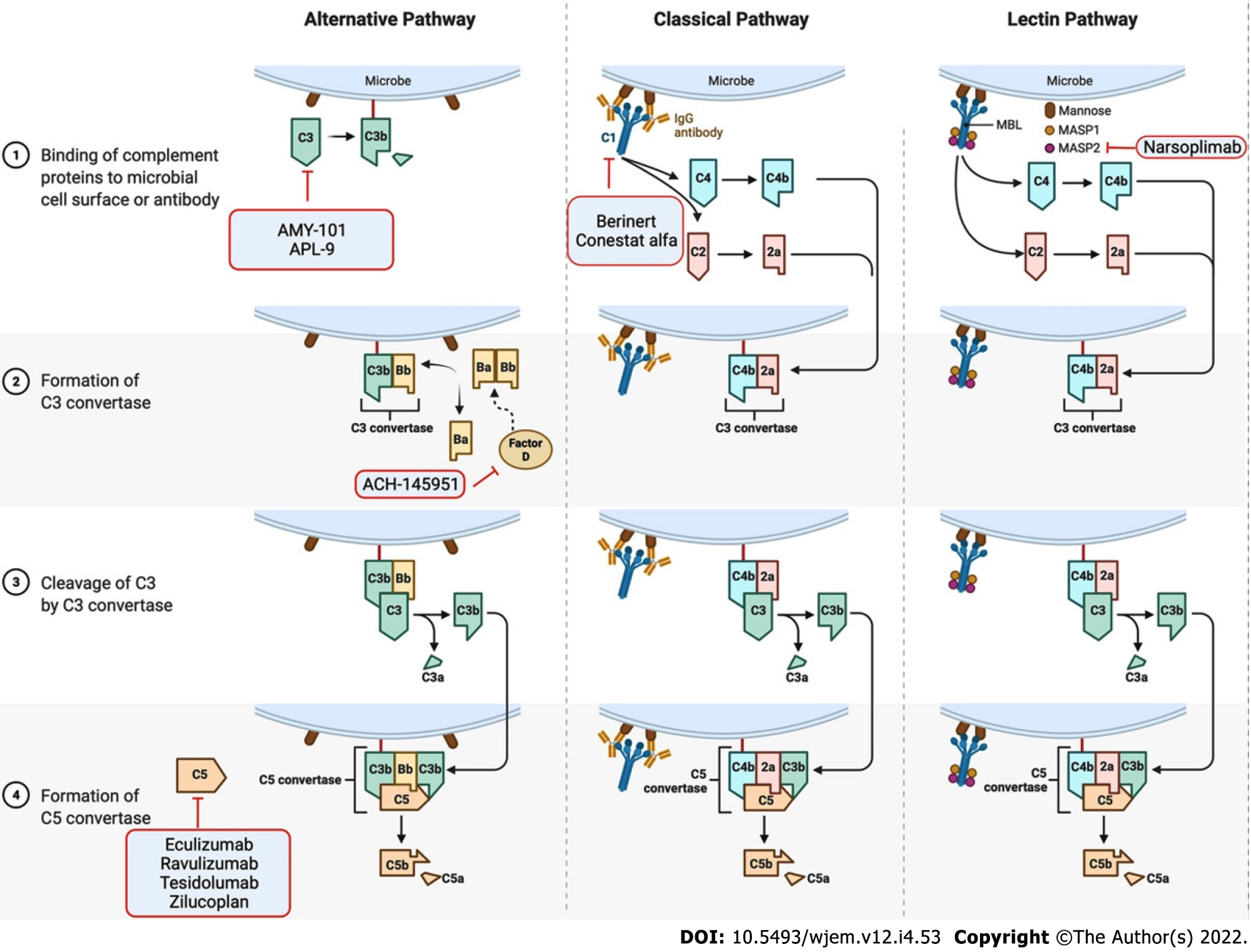
The complement system is an integral part of the innate immune system consisting of over 30 proteins. There are 3 distinct pathways of complement activation: the classical complement pathway, the alternative complement pathway, and the lectin pathway (Figure 1). The complement cascade mediates several immunoprotective and anti-inflammatory functions, enables clearance of viral pathogens and infected cells via opsonization, results in the formation of the C5b-9 membrane attack complex (MAC) on infected cells, targets intracellular viral components for proteasomal degradation, promotes chemotaxis, and enhances the adaptive immune response[11]. In addition, the complement system promotes the survival of germinal center B cells and enhances the production of antigen-specific antibodies[12,13]. Further, activation of the complement system leads to the production of anaphylatoxins, such as C3a and C5a, which triggers endothelial and mast cell degranulation, enhances phagocytic activity of neutrophils and monocytes, and elicits a local inflammatory response.
Complement pathway activation plays an important role in the development of acute lung injury induced by highly pathogenic viruses, and accordingly, inhibition of complement components has been associated with protective effects[14]. High levels of C5a have been found in the upper respiratory tract and in serum samples in patients infected with the H1N1 virus and have been moderately associated with disease severity[15,16].
In preclinical studies with rodents, inhibition of C5a and upstream factors, such as C3 and C3a, reduced lung injury caused by coronavirus (SARS-CoV) and non-coronavirus (avian influenza H5N1 virus) infections[17,18]. Interestingly, there was no change in viral titers, suggesting that complement inhibition may prevent lung damage independent of viral load[17]. Further, Jiang et al[19] showed that MERS-CoV infection in transgenic human dipeptidyl peptidase 4 (hDPP4) mice was associated with elevated cytokine release and excessive complement activation, resulting in increased concentrations of C5 cleavage products in sera and lungs, while competitive antibody-mediated inhibition of the C5a receptor (C5aR) decreased viral replication and mitigated alveolar damage by limiting alveolar macrophage infiltration and interferon (IFN)-gamma receptor expression. Gralinski et al[17] showed significantly milder airway inflammation, decreased inflammatory cell infiltration, and lower cytokine levels in both lungs and serum of transgenic C3-deficient mice infected with SARS-CoV compared to wild-type control mice. Similar findings were observed in a primate model of influenza H7N9 virus, where inhibition of C5aR significantly decreased cytokine levels and neutrophil infiltration of the lungs[20].
Clinical studies of patients with COVID-19 have supported the theory that excessive complement activation and complement-dependent cytotoxic tissue damage drive disease progression[21]. Peffault de Latour et al[22] showed that the level of circulating MAC (sC5b-9) was increased in 64% of patients and plasma levels of sC5b9 were significantly higher in infected patients compared to healthy donors. Serum C5 may be associated with COVID-19 severity; those with critical disease have significantly elevated sC5b9 compared to those with mild or moderate symptoms[21,22]. Yu et al[23] demonstrated that serum from patients with severe COVID-19 promotes complement-mediated cell death by increasing MAC deposition on the cell surface. A positive modified Ham test (complement-mediated cell-death assay) was detected in 41.2% of intubated patients compared to 6.3% of patients requiring minimal respiratory support. Similarly, Carvelli et al[24] reported increased plasma C5a levels associated with disease severity and ARDS. Lastly, Holter et al[25] showed that sC5b9 and C4d were significantly higher in patients with respiratory failure and systemic inflammation.
The activation of the complement system results in consumption of C3 and C4 and relevant changes have been investigated as markers of disease severity, intensive care unit (ICU) admission, thromboembolism, and mortality[26]. Both C3 and C4 Levels were significantly lower in severe COVID-19 or deceased patients in a meta-analysis of 19 studies including 3764 patients[27]. Serum levels of C3 were reduced in the majority of a small cohort of healthcare workers with COVID-19, suggesting activation of the complement cascade and C3 consumption[28], while case series demonstrated that lower serum C3 on hospital admission or its progressive decline during hospitalization were associated with up to a 4-fold higher risk of disease progression[29,30]. Confirming these findings, Sinkovits et al[31] revealed an association between an increased C3a/C3 ratio and need for intubation/mechanical ventilation and in-hospital mortality, while Zhao et al[32] identified decreased C3 and C4 Levels in a cohort of 125 non-survivors hospitalized during the early stages of the pandemic in Wuhan. In contrast to the aforementioned findings, adjusted analysis in a cohort of 100 ICU patients including 81 patients with acute kidney injury demonstrated no association between kidney injury and the level of C3[33].
Dynamic changes of complement levels have been reported in patients with COVID-19. Alosaimi et al[34] reported higher C3a, C5a, and factor P (properdin) levels in severe COVID-19 that were also higher in critical COVID-19 non-survivors. Further, the levels were increased during the early stage and gradually decreased during hospital course. Continuous sampling in hemodialysis patients with severe COVID-19 identified that C5a levels were elevated prior to clinical deterioration. C3a levels remained elevated during the severe phase, whereas C5a levels started decreasing on day 7[35]. Interestingly, erythrocytes have been proposed as a diagnostic marker of disease progression based on the expression of complement receptors and complement binding. COVID-19 patients admitted to the ICU had an increased percentage of RBCs coated with C3b/iC3b/C3dg and C4d during the first 72 h of admission and the percentage increased further by day 7 in the study by Lam et al[36].
Complement component profiles were investigated by Defendi et al[37], who performed an extensive analysis of the functional activities and antigenic levels of individual complement components [C1q, C4, C3, C5, Factor B, and mannose-binding lectin (MBL)] and evaluated their association with clinical outcomes, including rate of ICU admission, corticosteroid treatment, oxygen requirement, and mortality. Two distinct profiles emerged: patients with greater disease severity and mortality exhibited activation of the lectin and alternative pathways and low levels of MBL, C4, C3, Factor B, and C5, while patients with more moderate disease showed inflammatory markers compatible with classical pathway activation.
Genetic polymorphisms of C3 have been identified and associated with COVID-19 susceptibility and mortality[38]. Gavriilaki et al used targeted next-generation sequencing and identified C3 variants as independent predictors of disease severity, ICU admission, and/or mortality, strengthening the hypothesis of genetic susceptibility in severe COVID-19[39,40]. Other genetic polymorphisms associated with severe disease include the mannose binding lectin gene 2 (rs1800450)[41,42] and the chromosome 3 rs11385942 G>GA variant that has been associated with complement overactivation (formation of C5a and MAC)[43].
Post-mortem histopathological studies of patients with severe COVID-19 revealed endothelial deposition of complement activation products in the lungs and skin, including C5b9, C3d, C4d, and the mannan-binding lectin serine protease 2 (MASP-2), an important mediator of the lectin pathway activation[9,44]. Similarly, Kim et al[45] identified immune complexes and MAC deposition in airways and vasculature of lung biopsies, enhanced viral antigen-specific responses in lung-derived myeloid cells, and significant increases in concentrations of C3a and C5a in critical COVID-19 patients. In a retrospective study of 74 patients with COVID-19, SARS-CoV-2 membrane and spike proteins and MASP-2 were also detected and co-localized in small bowel vessels of those patients with microvascular injury, supporting the role of thromboinflammation and complement activation[46]. Interestingly, binding of the SARS-CoV-2 spike protein S1 and S2 subunits to heparan sulfate on cell surfaces and binding of the S and N proteins to lectin pathway molecules cause excessive activation of the alternative and lectin pathways, respectively, resulting in end-organ damage[47,48]. In contrast to the lung and small bowel findings, Santana et al[49] reported a low rate of C4d deposition (22%) in liver histopathologic specimens of 27 deceased patients, suggesting that hepatocellular injury is a result of systemic rather than intrahepatic thrombotic events.
Coagulopathy resulting in a high frequency of thrombotic complications, including venous thromboembolism (VTE) such as deep vein thrombosis and pulmonary embolism, and arterial thromboembolism such as myocardial infarction and ischemic stroke, is common in critically ill COVID-19 patients and is among the leading causes of death[50,51]. The incidence of VTE has been estimated at 5.5% to 14.1% or more – an over two-fold higher risk compared to historical matched cohorts[52-54]. Microvascular thrombosis has been associated with progression to ARDS[55], while autopsy studies have identified VTE or in situ pulmonary arterial thrombosis in at least 60% of patients with COVID-19, suggesting thrombosis as a major cause of mortality[56,57].
The causal mechanisms of the COVID-19 coagulopathy are diverse and include dysregulated inflammation (cytokine storm) with subsequent activation of the coagulation cascade and platelets[50,58], virus induced endothelial changes[59-61], or patient comorbidities and limited mobility related to prolonged hospitalization[62]. Increased plasma levels of D-dimer, a marker of coagulation cascade activation, especially greater than 4 times the upper limit of normal, predict a more than two-fold increased risk of VTE or mortality[2,60], while thrombocytopenia and prothrombin time prolongation have also been observed[63].
In the context of thromboinflammation, the complement pathways are capable of activating the coagulation cascade through the induction of tissue factor expression[64,65]. Furthermore, serine proteases of the lectin pathway can cleave prothrombin to form activated thrombin, and MBL has been shown to be significantly increased in critically ill COVID-19 patients with symptomatic thromboembolism[66,67]. Complement system inhibitors, such as C1-esterase inhibitors, can additionally inhibit the coagulation cascade[68].
Current histopathologic data suggest TMA – manifesting as thrombocytopenia, microangiopathic hemolytic anemia, and organ damage – as a potential cause of severe COVID-19. TMA has been widely reported in postmortem studies, particularly as pulmonary capillary stasis and presence of microthrombi in the lungs, along with erythrocyte aggregation, endothelial injury, and fibrin thrombi in kidneys, despite anticoagulation[69,70].
Diffuse alveolar damage and complementmediated endothelial injury of septal microvasculature and microthrombi have been observed in critically ill patients with increased serum D-dimer levels and fibrinogendegradation products, further strengthening the concept of immunemediated pulmonary vascular injury and thrombosis in COVID-19[71]. Lung histopathologic data have also shown that severe COVID-19 is characterized by innate-immunity cell-mediated inflammatory endothelial damage manifesting as an obliterating endarteritis, associated with accumulation of C5aR1+ lung macrophages around the arteries and within thrombi[24,72]. This finding supports the notion that C5a production attracts and activates myeloid cells in the lungs, causing excessive inflammation and endothelial damage[24].
Complement-mediated renal TMA has been investigated in both adults and children with COVID-19, with evidence showing a constitutional complement dysregulation and intrarenal complement activation. These findings have been associated with genetic alterations of the alternative complement pathway and suggest SARS-CoV-2 as an emerging infectious trigger for atypical hemolytic uremic syndrome (aHUS), in accordance with previous cases precipitated by influenza strains[73]. COVID-19-associated renal TMA is characterized by increased deposition of complement components (C1q, C3, C5b9) and total immunoglobulin[74] and unrestrained formation of C5b9[75], which has also been observed in children with COVID-19 independent of disease severity and in the presence of clinical and diagnostic criteria of TMA[76]. Further confirming these findings, Cugno et al[77] identified an association between high levels of C5b9 levels and von-Willebrand factor and a positive association with disease severity, suggesting that complement activation and endothelial injury are major determinants of the clinical course of COVID-19 and potential treatment targets.
Cutaneous histopathologic data, derived from chilblain-like lesions, also known as “COVID toes” – inflammatory erythematous papules involving fingers and toes – are characterized by a significant transcriptomic activation of systemic immune response (type I IFN, IgA ANCA), complement activation (upregulation of C1q, C1s and C1 inhibitor, C2, properdin, and downregulation of MAC components C5 and C6), angiogenesis factors (VEGF-A, VEGFR-2 and c-Kit), and endothelial dysfunction (angiopoietin-1, angiopoietin-2 and VEGF-A)[78]. Skin findings may be associated with antiphospholipid antibodies as supported by an analysis of skin samples in a patient with severe COVID-19 with complement-induced vascular injury and severe thrombosis[79] and deposition of C5b9, MASP2, and C4d as shown by skin biopsies in three patients with treatment-resistant COVID-19[80].
Transcriptomic and proteomic analyses have provided important insights in the interaction between inflammation and coagulation pathways in COVID-19. Transcriptomic profiling of leukocytes from intensive care patients revealed the upregulated expression of genes involved in inflammation, coagulation, and platelet function, concordant with the activation of complement pathways, including SERPINE1 (plasminogen activator inhibitor-1; PAI-1), von Willebrand factor, and Granzyme B, factors involved in the Toll-like receptor-mediated cascades, and tumor necrosis factor/interleukin 6 (IL-6) signaling[81]. In order to further investigate the proteomic signature and identify biomarkers of disease severity in COVID-19, Barberis et al[82] conducted a proteomic profile characterization of plasma-derived exosomes from COVID-19 patients and healthy controls. They reported a specific proteomic signature of strongly regulated proteins in both critically and non-critically ill patients, compared to healthy subjects, including proteins involved in the acute phase response (C-reactive protein [CRP], serum amyloid A, and ferritin), immune-response (C1R, C4A/C4B, MBL2 and SERPING1), and coagulation (proteins of the intrinsic and extrinsic coagulation cascade, Kininogen-1), and reported that the C1r complement subcomponent is highly associated with disease severity, with an AUC of 0.93 (sensitivity: 89%; specificity: 82%). Consistent with the above findings, Freda et al[83] observed significant increases in thrombotic and inflammatory marker expression (thrombomodulin, PECAM-1) in human endothelial cells exposed to SARS-CoV-2 structural proteins. Kaiser et al[84] analyzed the proteome of neutrophils in severe COVID-19 and reported a unique proteomic signature of increased IL-8 secretion associated with increased D-dimer and neutrophil extracellular trap (NET) production, elevated complement factors (C1R, C1S, C5, C6, C7, C8 and C9), and fibrinogen binding, further uncovering a procoagulant role of inflammation and complement pathways. Lastly, NETs have been implicated in cytokine storm, and inhibition of C3aR and C5aR has been shown to attenuate thromboinflammation driven by NETs[75,85].
The complement system has garnered interest as a therapeutic target in the treatment of COVID-19. Several clinical trials investigating C1 esterase, C3, C5, C5a, or C5aR inhibition (Table 1) show reduced incidence of 28-d mortality and pulmonary embolism[86].
| NCT number | Drug | Mechanism of action | Status | Sponsor |
| NCT04395456 | AMY-101 | C3 inhibitor | Not yet recruiting | Amyndas Pharmaceuticals S.A. |
| NCT04402060 | APL-9 | C3 inhibitor | Completed | Apellis Pharmaceuticals, Inc. |
| NCT04346797 | Eculizumab | C5 inhibitor | Recruiting | Assistance Publique- Hôpitaux de Paris |
| NCT04355494 | Eculizumab | C5 inhibitor | Expanded access no longer available | Alexion Pharmaceuticals |
| NCT04288713 | Eculizumab | C5 inhibitor | Expanded access available | Hudson Medical |
| NCT04351503 | Eculizumab | C5 inhibitor | Recruiting | University Hospital, Basel, Switzerland |
| NCT04369469 | Ravulizumab | C5 inhibitor | Terminated (Met futility bar at interim analysis) | Alexion Pharmaceuticals |
| NCT04382755 | Zilucoplan (RA101495) | C5 inhibitor | Completed | University Hospital, Ghent |
| NCT04371367 | Avdoralimab | Anti-C5aR | Completed | Assistance Publique Hopitaux De; Marseille &Innate Pharma |
| NCT04414631 | Conestat alfa | C1 esterase inhibitors | Terminated | University Hospital, Basel, Switzerland & Pharming Technologies B.V. |
| NCT04530136 | Ruconest | C1 esterase inhibitors | Recruiting | Pharming Technologies B.V. |
| NCT04333420 | Vilobelimab (IFX-1) | C5a | Recruiting | InflaRx GmbH |
| NCT04570397 | Ravulizumab | C5 inhibitor | Recruiting | Brigham and Women's Hospital |
| NCT04390464 | Ravulizumab | C5 inhibitor | Recruiting | Cambridge University Hospitals NHS; Foundation Trust; Frances Hall |
Most available evidence from case reports, small case series, and ongoing studies has focused on inhibition of C5, C5a, or C5aR. Eculizumab and ravulizumab are humanized monoclonal antibodies, currently used for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and aHUS, that bind to terminal complement component C5 with high affinity, preventing the subsequent formation of C5b9. C5 inhibition can attenuate hyperinflammatory lung damage caused by SARS-CoV-2 in PNH patients with active COVID-19 infection[87]. Those without underlying PNH or aHUS may also potentially derive benefit. In a case series of four patients with severe pneumonia or ARDS, patients received up to 4 infusions of eculizumab and showed a marked improvement in respiratory status and need for non-invasive ventilation within 48 h of the first dose and recovered completely[88]. Similarly, in eight patients with severe or critical COVID-19, six patients showed improved oxygenation after receiving a first dose of eculizumab and were ultimately discharged, while two patients died from septic shock and massive pulmonary embolism, respectively[22]. These findings were further reinforced by Zelek et al[89] who reported that Tesidolumab (LFG316), a C5-blocking monoclonal antibody, can rapidly decrease the hyperinflammatory response in 4 out of 5 critical patients with high levels of MAC not responding to standard treatment. Interestingly, in accordance with in vivo findings, in vitro C5aR inhibition in human airway epithelial cells results in epithelial integrity and promotes anti-inflammatory effects[90].
In patients with established TMA or PNH and concomitant COVID-19, the disease course was milder in those receiving eculizumab or ravulizumab[91,92]. In one of the largest studies (n = 80) of complement-targeted therapy in COVID-19, 35 ICU patients treated with eculizumab showed an improved 15-d survival of 82.9% (95%CI: 70.4%-95.3%) compared to 62.2% (95%CI: 48.1%-76.4%) without eculizumab, and improved 28-d survival of 80.0% (95%CI: 66.8%-93.3%) with eculizumab vs 51.1% (95%CI: 36.5%-65.7%) without eculizumab accompanied by reduction in key biomarkers (IL-6, IL-17, IFN a2 and C5b9)[93]. However, eculizumab administered in a regular schedule in the treatment of PNH was inadequate in the prevention of ARDS, raising questions regarding the optimal dose and administration in patients with severe COVID-19[94]. A combination of eculizumab with other immunomodulatory agents, such as ruxolitinib, a Janus Associated Kinase inhibitor, may result in improved outcomes and supports the hypothesis that the ideal treatment regimen may be multifaceted[95].
C3 inhibition is also under investigation as a potential therapeutic strategy. Genetic variants of the C3 protein can independently predict risk of developing severe COVID-19, need for ICU-level care, and mortality; this may provide a theoretical foundation for the early use of complement inhibitors[39]. The compstatin-based C3 inhibitor AMY-101 was safely and successfully used in a patient with SARS–CoV-2 associated pneumonia[96]. Further data from an exploratory study by Mastellos et al[97] in severe COVID-19 patients treated with eculizumab (n = 10) or AMY-101 (n = 3), showed attenuation of the hyperinflammatory response, especially with AMY-101. Both agents resulted in a significant decrease in inflammatory markers such as CRP and IL-6 and improved lung function. AMY-101 attenuated C3a and C5b9 levels, decreased fibrinogen consumption, neutrophil counts and NET formation, and enhanced lymphocyte recovery.
The classical pathway has been targeted at the level of C1 esterase with inhibitors, such as Conestat alfa and Berinert, that have been previously used in patients with hereditary angioedema[98,99]. Berinert has similar anti-complement effects as heparin, which has demonstrated efficacy in COVID-19 treatment[100-102]. An exploratory study by Urwyler et al[103], which investigated Conestat alfa in 5 patients with severe COVID-19, showed improved clinical outcomes such as defervescence and recovery, and improved inflammatory markers levels including CRP, C4d and C5a. Common side effects for Conestat alfa and Berinert include nausea and vomiting alongside with other gastrointestinal symptoms and coinfections[104,105].
SARS-CoV-2 spike protein subunits 1 and 2 can directly activate the alternative pathway through interaction with heparan sulfate on host cell surfaces. This offers another potential therapeutic target as it could be prevented by small molecule inhibitors of factor D (ACH145951)[47]. These molecules bind factor D with high affinity and limit its proteolytic activity against proconvertase (Factor B in complex with C3b). Factor D deficiency is associated with increased risk for recurrent infections with encapsulated organisms comparable to other terminal complement deficiency syndromes[106].
The lectin pathway has been targeted with narsoplimab, an anti-MASP-2 monoclonal antibody, in the treatment of six critically ill or mechanically-ventilated patients, resulting in reduced endothelial damage and inflammation. Recipients showed an increased survival rate and improved inflammatory markers, including circulating endothelial cells, IL-6, IL-8, CRP, and LDH[107]. Common side effects include headache, upper respiratory infection, fatigue, nausea, vomiting, diarrhea, hypokalemia, neutropenia and fever[108,109]. A recently identified variant in the MBL gene 2 (rs1800450) has been associated with the need for hospitalization, severe disease, ICU admission, and development of pneumonia potentially suggesting a new therapeutic target[41,42].
Other potential targets include antibodies against SARS-CoV-2, such as nCoV396, a monoclonal antibody against the SARS-CoV-2 nucleocapsid (N) protein that has been shown to prevent the MASP-2-dependent complement activation. Binding of nCoV396 to the SARS-CoV 2 N protein leads to conformational changes that may lead to allosteric modulation of its protein function[110]. The precise interaction between the SARS-CoV-2 N protein and MASP2 remains under investigation. Overall, targeting N protein may be a feasible therapeutic strategy.
Given the fact that only a small proportion of patients will develop aggressive disease, reliable clinical indicators to identify these patients in the early phase of disease progression are of utmost importance. The time window for optimal intervention and the patient populations that could benefit from therapeutic complement inhibition have yet to be determined. Currently available biomarkers of complement activity are too unstable and short-lived to be used predictively. Nevertheless, clinical predictors of ARDS progression combined with inflammatory biomarkers (CRP, IL-6, ferritin, and D-dimer) could potentially allow the identification of patients that could benefit from early intervention[2,111].
Theoretically, upstream targets in the complement pathway would provide the most potent anti-inflammatory results[96]. Despite the fact that the use of anti-C5a antibodies has been associated with prominent clinical improvement and decreased systemic inflammation, C5 inhibition can be partial, allowing residual terminal pathway activity in cases of excessive complement activation, as seen in severe COVID-19. In these advanced stages of COVID-19, C3 inhibition has the ability to control both ARDS and the systemic inflammation that damages the microcirculation of vital organs. Proximal complement inhibitors which target C3 or its upstream activators are appealing targets, but their benefit in mortality was not confirmed in a randomized, double-blinded, multicenter study that compared APL-9 (C3 inhibitor) to standard of care in mild to moderate COVID-19[112]. Further randomized studies comparing different complement inhibitors are necessary to identify the most appropriate therapeutic agents, as well as the benefits of upstream inhibition or pathway specific targeting.
The available data should be interpreted with caution. Concurrent use of antiviral drugs, corticosteroids, heparin, and antibiotics in these studies significantly limits their generalizability. The increased risk of opportunistic infections, most notoriously with encapsulated organisms (Neisseria, Haemophilus, or Streptococcus species) in unvaccinated individuals and those with asplenia or functional asplenia, through the inhibition of terminal complement proteins has historically limited complement inhibitor use. However, growing clinical experience with C5-inhibitors and C3-inhibitors such as APL-2 and AMY-101/Cp40 along with prophylactic antibiotics or planned vaccination schedules has assuaged these concerns. Additionally, individualized treatment strategies based on specific immunologic profiles and complement-driven disease should be further investigated[113].
The complement system can also be theoretically exploited alongside the use of COVID-19 vaccines and antibody-based therapies. Complement activation is known to enhance the efficacy of pathogen-neutralizing antibodies through formation of larger antibody-C1q complexes, and thus may require fewer IgG molecules bound to virus surfaces to facilitate their neutralization[114,115]. Monoclonal antibodies or vaccines can potentially be engineered to promote enhanced C1q binding and complement activation leading to a more robust immunologic response, confronting the problem of waning antibody concentrations with traditional immunization approaches[115,116]. The risk of vaccine-induced thrombotic thrombocytopenia seen with the use of COVID-19 adenovirus-vector vaccines that is thought to be mediated by anti-platelet factor 4 antibodies and subsequent complement activation remains a concern[117]. Thus, a careful weighing of risks is essential.
Current evidence suggests excessive complement activation and subsequent complement-dependent cytotoxic tissue damage drives COVID-19 progression and thromboembolic complications. In the context of thromboinflammation, the three complement pathways can activate the coagulation cascade causing TMA and end-organ damage, mostly manifesting as lung, kidney, and cutaneous disease. Considering its role in cytokine storm and thrombogenesis, the complement system is an appealing treatment target. Preliminary reports have produced promising results. Whether inhibition of upstream (C3, C1) or terminal (C5, C5a, or C5aR) components is of greater importance remains to be elucidated. Current data indicate the need for evaluation of complement inhibitors as COVID-19 therapeutics, and many are under investigation in prospective randomized trials. Limitations such as the cost of inhibitors or their association with opportunistic infections may preclude their generalized use in the treatment of COVID-19.
Access to Biorender.com and publishing rights of Figure 1 were kindly provided by Dr. Georgios Geropoulos, who had access on a paid subscription.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Han J, China; Papadopoulos K, Thailand A-Editor: Zhu JQ, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14113] [Cited by in F6Publishing: 14170] [Article Influence: 3542.5] [Reference Citation Analysis (0)] |
| 2. | Cohen SL, Gianos E, Barish MA, Chatterjee S, Kohn N, Lesser M, Giannis D, Coppa K, Hirsch JS, McGinn TG, Goldin ME, Spyropoulos AC; Northwell Health COVID-19 Research Consortium. Prevalence and Predictors of Venous Thromboembolism or Mortality in Hospitalized COVID-19 Patients. Thromb Haemost. 2021;121:1043-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Li X, Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020;24:198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 412] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 4. | Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 955] [Cited by in F6Publishing: 865] [Article Influence: 216.3] [Reference Citation Analysis (0)] |
| 5. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2835] [Cited by in F6Publishing: 3176] [Article Influence: 794.0] [Reference Citation Analysis (0)] |
| 6. | Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970-10975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1811] [Cited by in F6Publishing: 1695] [Article Influence: 423.8] [Reference Citation Analysis (0)] |
| 7. | Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383:2255-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1324] [Cited by in F6Publishing: 1644] [Article Influence: 411.0] [Reference Citation Analysis (0)] |
| 8. | Keshavarz F, Ghalamfarsa F, Javdansirat S, Hasanzadeh S, Azizi A, Sabz G, Salehi M, Ghalamfarsa G. Patients with Covid 19 have significantly reduced CH50 activity. Virusdisease. 2021;1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1635] [Cited by in F6Publishing: 1543] [Article Influence: 385.8] [Reference Citation Analysis (1)] |
| 10. | Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, Quan S, Zhang F, Sun R, Qian L, Ge W, Liu W, Liang S, Chen H, Zhang Y, Li J, Xu J, He Z, Chen B, Wang J, Yan H, Zheng Y, Wang D, Zhu J, Kong Z, Kang Z, Liang X, Ding X, Ruan G, Xiang N, Cai X, Gao H, Li L, Li S, Xiao Q, Lu T, Zhu Y, Liu H, Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59-72.e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 927] [Article Influence: 231.8] [Reference Citation Analysis (0)] |
| 11. | Mellors J, Tipton T, Longet S, Carroll M. Viral Evasion of the Complement System and Its Importance for Vaccines and Therapeutics. Front Immunol. 2020;11:1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Pandya PH, Wilkes DS. Complement system in lung disease. Am J Respir Cell Mol Biol. 2014;51:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Barrington RA, Schneider TJ, Pitcher LA, Mempel TR, Ma M, Barteneva NS, Carroll MC. Uncoupling CD21 and CD19 of the B-cell coreceptor. Proc Natl Acad Sci U S A. 2009;106:14490-14495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4:e28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Bjornson AB, Mellencamp MA, Schiff GM. Complement is activated in the upper respiratory tract during influenza virus infection. Am Rev Respir Dis. 1991;143:1062-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Ohta R, Torii Y, Imai M, Kimura H, Okada N, Ito Y. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol Immunol. 2011;55:191-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, Whitmore A, Heise MT, Baric RS. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio. 2018;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 493] [Article Influence: 82.2] [Reference Citation Analysis (1)] |
| 18. | Sun S, Zhao G, Liu C, Wu X, Guo Y, Yu H, Song H, Du L, Jiang S, Guo R, Tomlinson S, Zhou Y. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am J Respir Cell Mol Biol. 2013;49:221-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo Y, Li J, Du L, Jiang S, Guo R, Sun S, Zhou Y. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 20. | Sun S, Zhao G, Liu C, Fan W, Zhou X, Zeng L, Guo Y, Kou Z, Yu H, Li J, Wang R, Li Y, Schneider C, Habel M, Riedemann NC, Du L, Jiang S, Guo R, Zhou Y. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin Infect Dis. 2015;60:586-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Fernández S, Moreno-Castaño AB, Palomo M, Martinez-Sanchez J, Torramadé-Moix S, Téllez A, Ventosa H, Seguí F, Escolar G, Carreras E, Nicolás JM, Richardson E, García-Bernal D, Carlo-Stella C, Moraleda JM, Richardson PG, Díaz-Ricart M, Castro P. Distinctive Biomarker Features in the Endotheliopathy of COVID-19 and Septic Syndromes. Shock. 2022;57:95-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | Peffault de Latour R, Bergeron A, Lengline E, Dupont T, Marchal A, Galicier L, de Castro N, Bondeelle L, Darmon M, Dupin C, Dumas G, Leguen P, Madelaine I, Chevret S, Molina JM, Azoulay E, Fremeaux-Bacchi V; Core Group. Complement C5 inhibition in patients with COVID-19 - a promising target? Haematologica. 2020;105:2847-2850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Yu J, Gerber GF, Chen H, Yuan X, Chaturvedi S, Braunstein EM, Brodsky RA. Complement dysregulation is associated with severe COVID-19 illness. Haematologica. 2022;107:1095-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 24. | Carvelli J, Demaria O, Vély F, Batista L, Chouaki Benmansour N, Fares J, Carpentier S, Thibult ML, Morel A, Remark R, André P, Represa A, Piperoglou C; Explore COVID-19 IPH group; Explore COVID-19 Marseille Immunopole group, Cordier PY, Le Dault E, Guervilly C, Simeone P, Gainnier M, Morel Y, Ebbo M, Schleinitz N, Vivier E. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 331] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 25. | Holter JC, Pischke SE, de Boer E, Lind A, Jenum S, Holten AR, Tonby K, Barratt-Due A, Sokolova M, Schjalm C, Chaban V, Kolderup A, Tran T, Tollefsrud Gjølberg T, Skeie LG, Hesstvedt L, Ormåsen V, Fevang B, Austad C, Müller KE, Fladeby C, Holberg-Petersen M, Halvorsen B, Müller F, Aukrust P, Dudman S, Ueland T, Andersen JT, Lund-Johansen F, Heggelund L, Dyrhol-Riise AM, Mollnes TE. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117:25018-25025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 26. | de Nooijer AH, Grondman I, Janssen NAF, Netea MG, Willems L, van de Veerdonk FL, Giamarellos-Bourboulis EJ, Toonen EJM, Joosten LAB; RCI-COVID-19 study group. Complement Activation in the Disease Course of Coronavirus Disease 2019 and Its Effects on Clinical Outcomes. J Infect Dis. 2021;223:214-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 27. | Zinellu A, Mangoni AA. Serum Complement C3 and C4 and COVID-19 Severity and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front Immunol. 2021;12:696085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Wei XS, Wang XR, Zhang JC, Yang WB, Ma WL, Yang BH, Jiang NC, Gao ZC, Shi HZ, Zhou Q. A cluster of health care workers with COVID-19 pneumonia caused by SARS-CoV-2. J Microbiol Immunol Infect. 2021;54:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 29. | Jiang H, Chen Q, Zheng S, Guo C, Luo J, Wang H, Zheng X, Weng Z. Association of Complement C3 with Clinical Deterioration Among Hospitalized Patients with COVID-19. Int J Gen Med. 2022;15:849-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Fang S, Wang H, Lu L, Jia Y, Xia Z. Decreased complement C3 levels are associated with poor prognosis in patients with COVID-19: A retrospective cohort study. Int Immunopharmacol. 2020;89:107070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Sinkovits G, Réti M, Müller V, Iványi Z, Gál J, Gopcsa L, Reményi P, Szathmáry B, Lakatos B, Szlávik J, Bobek I, Prohászka ZZ, Förhécz Z, Mező B, Csuka D, Hurler L, Kajdácsi E, Cervenak L, Kiszel P, Masszi T, Vályi-Nagy I, Prohászka Z. Associations between the von Willebrand Factor-ADAMTS13 Axis, Complement Activation, and COVID-19 Severity and Mortality. Thromb Haemost. 2022;122:240-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Zhao Y, Nie HX, Hu K, Wu XJ, Zhang YT, Wang MM, Wang T, Zheng ZS, Li XC, Zeng SL. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect Dis Poverty. 2020;9:108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | Joseph A, Zafrani L, Mabrouki A, Azoulay E, Darmon M. Acute kidney injury in patients with SARS-CoV-2 infection. Ann Intensive Care. 2020;10:117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 34. | Alosaimi B, Mubarak A, Hamed ME, Almutairi AZ, Alrashed AA, AlJuryyan A, Enani M, Alenzi FQ, Alturaiki W. Complement Anaphylatoxins and Inflammatory Cytokines as Prognostic Markers for COVID-19 Severity and In-Hospital Mortality. Front Immunol. 2021;12:668725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Prendecki M, Clarke C, Medjeral-Thomas N, McAdoo SP, Sandhu E, Peters JE, Thomas DC, Willicombe M, Botto M, Pickering MC. Temporal changes in complement activation in haemodialysis patients with COVID-19 as a predictor of disease progression. Clin Kidney J. 2020;13:889-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Lam LKM, Reilly JP, Rux AH, Murphy SJ, Kuri-Cervantes L, Weisman AR, Ittner CAG, Pampena MB, Betts MR, Wherry EJ, Song WC, Lambris JD, Meyer NJ, Cines DB, Mangalmurti NS. Erythrocytes identify complement activation in patients with COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;321:L485-L489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Defendi F, Leroy C, Epaulard O, Clavarino G, Vilotitch A, Le Marechal M, Jacob MC, Raskovalova T, Pernollet M, Le Gouellec A, Bosson JL, Poignard P, Roustit M, Thielens N, Dumestre-Pérard C, Cesbron JY. Complement Alternative and Mannose-Binding Lectin Pathway Activation Is Associated With COVID-19 Mortality. Front Immunol. 2021;12:742446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Delanghe JR, De Buyzere ML, Speeckaert MM. Genetic Polymorphisms in the Host and COVID-19 Infection. Adv Exp Med Biol. 2021;1318:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Gavriilaki E, Asteris PG, Touloumenidou T, Koravou EE, Koutra M, Papayanni PG, Karali V, Papalexandri A, Varelas C, Chatzopoulou F, Chatzidimitriou M, Chatzidimitriou D, Veleni A, Grigoriadis S, Rapti E, Chloros D, Kioumis I, Kaimakamis E, Bitzani M, Boumpas D, Tsantes A, Sotiropoulos D, Sakellari I, Kalantzis IG, Parastatidis ST, Koopialipoor M, Cavaleri L, Armaghani DJ, Papadopoulou A, Brodsky RA, Kokoris S, Anagnostopoulos A. Genetic justification of severe COVID-19 using a rigorous algorithm. Clin Immunol. 2021;226:108726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Asteris PG, Gavriilaki E, Touloumenidou T, Koravou EE, Koutra M, Papayanni PG, Pouleres A, Karali V, Lemonis ME, Mamou A, Skentou AD, Papalexandri A, Varelas C, Chatzopoulou F, Chatzidimitriou M, Chatzidimitriou D, Veleni A, Rapti E, Kioumis I, Kaimakamis E, Bitzani M, Boumpas D, Tsantes A, Sotiropoulos D, Papadopoulou A, Kalantzis IG, Vallianatou LA, Armaghani DJ, Cavaleri L, Gandomi AH, Hajihassani M, Hasanipanah M, Koopialipoor M, Lourenço PB, Samui P, Zhou J, Sakellari I, Valsami S, Politou M, Kokoris S, Anagnostopoulos A. Genetic prediction of ICU hospitalization and mortality in COVID-19 patients using artificial neural networks. J Cell Mol Med. 2022;26:1445-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Speletas M, Dadouli K, Syrakouli A, Gatselis N, Germanidis G, Mouchtouri VA, Koulas I, Samakidou A, Nikolaidou A, Stefos A, Mimtsoudis I, Hatzianastasiou S, Koureas M, Anagnostopoulos L, Tseroni M, Tsinti G, Metallidis S, Dalekos G, Hadjichristodoulou C. MBL deficiency-causing B allele (rs1800450) as a risk factor for severe COVID-19. Immunobiology. 2021;226:152136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Medetalibeyoglu A, Bahat G, Senkal N, Kose M, Avci K, Sayin GY, Isoglu-Alkac U, Tukek T, Pehlivan S. Mannose binding lectin gene 2 (rs1800450) missense variant may contribute to development and severity of COVID-19 infection. Infect Genet Evol. 2021;89:104717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Valenti L, Griffini S, Lamorte G, Grovetti E, Uceda Renteria SC, Malvestiti F, Scudeller L, Bandera A, Peyvandi F, Prati D, Meroni P, Cugno M. Chromosome 3 cluster rs11385942 variant links complement activation with severe COVID-19. J Autoimmun. 2021;117:102595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Macor P, Durigutto P, Mangogna A, Bussani R, De Maso L, D'Errico S, Zanon M, Pozzi N, Meroni PL, Tedesco F. Multiple-Organ Complement Deposition on Vascular Endothelium in COVID-19 Patients. Biomedicines. 2021;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 45. | Kim DM, Kim Y, Seo JW, Lee J, Park U, Ha NY, Koh J, Park H, Lee JW, Ro HJ, Yun NR, Kim DY, Yoon SH, Na YS, Moon DS, Lim SC, Kim CM, Jeon K, Kang JG, Jang NY, Jeong H, Kim J, Cheon S, Sohn KM, Moon JY, Kym S, Han SR, Lee MS, Kim HJ, Park WY, Choi JY, Shin HW, Kim HY, Cho CH, Jeon YK, Kim YS, Cho NH. Enhanced eosinophil-mediated inflammation associated with antibody and complement-dependent pneumonic insults in critical COVID-19. Cell Rep. 2021;37:109798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Plotz B, Castillo R, Melamed J, Magro C, Rosenthal P, Belmont HM. Focal small bowel thrombotic microvascular injury in COVID-19 mediated by the lectin complement pathway masquerading as lupus enteritis. Rheumatology (Oxford). 2021;60:e61-e63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 48. | Ali YM, Ferrari M, Lynch NJ, Yaseen S, Dudler T, Gragerov S, Demopulos G, Heeney JL, Schwaeble WJ. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front Immunol. 2021;12:714511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 49. | Santana MF, Guerra MT, Hundt MA, Ciarleglio MM, Pinto RAA, Dutra BG, Xavier MS, Lacerda MVG, Ferreira AJ, Wanderley DC, Borges do Nascimento IJ, Araújo RFA, Pinheiro SVB, Araújo SA, Leite MF, Ferreira LCL, Nathanson MH, Vieira Teixeira Vidigal P. Correlation Between Clinical and Pathological Findings of Liver Injury in 27 Patients With Lethal COVID-19 Infections in Brazil. Hepatol Commun. 2022;6:270-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 622] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 51. | Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859-1865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 513] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 52. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1017] [Cited by in F6Publishing: 1132] [Article Influence: 283.0] [Reference Citation Analysis (0)] |
| 53. | Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 300] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 54. | Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020;142:184-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 837] [Article Influence: 209.3] [Reference Citation Analysis (0)] |
| 55. | Berger JS, Connors JM. Anticoagulation in COVID-19: reaction to the ACTION trial. Lancet. 2021;397:2226-2228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 585] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 57. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1577] [Cited by in F6Publishing: 1657] [Article Influence: 414.3] [Reference Citation Analysis (0)] |
| 58. | Spyropoulos AC, Weitz JI. Hospitalized COVID-19 Patients and Venous Thromboembolism: A Perfect Storm. Circulation. 2020;142:129-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950-2973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2080] [Cited by in F6Publishing: 2065] [Article Influence: 516.3] [Reference Citation Analysis (0)] |
| 60. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3992] [Cited by in F6Publishing: 3857] [Article Influence: 964.3] [Reference Citation Analysis (0)] |
| 61. | Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med. 2020;48:1358-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 327] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 62. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1532] [Cited by in F6Publishing: 1549] [Article Influence: 387.3] [Reference Citation Analysis (0)] |
| 63. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17476] [Cited by in F6Publishing: 17282] [Article Influence: 4320.5] [Reference Citation Analysis (0)] |
| 64. | Wojta J, Huber K, Valent P. New aspects in thrombotic research: complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype. Pathophysiol Haemost Thromb. 2003;33:438-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Tiwari R, Mishra AR, Mikaeloff F, Gupta S, Mirazimi A, Byrareddy SN, Neogi U, Nayak D. In silico and in vitro studies reveal complement system drives coagulation cascade in SARS-CoV-2 pathogenesis. Comput Struct Biotechnol J. 2020;18:3734-3744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Krarup A, Wallis R, Presanis JS, Gál P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2:e623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 67. | Eriksson O, Hultström M, Persson B, Lipcsey M, Ekdahl KN, Nilsson B, Frithiof R. Mannose-Binding Lectin is Associated with Thrombosis and Coagulopathy in Critically Ill COVID-19 Patients. Thromb Haemost. 2020;120:1720-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Davis AE 3rd. Biological effects of C1 inhibitor. Drug News Perspect. 2004;17:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 892] [Cited by in F6Publishing: 865] [Article Influence: 216.3] [Reference Citation Analysis (0)] |
| 70. | Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 1193] [Article Influence: 298.3] [Reference Citation Analysis (0)] |
| 71. | Bhandari S, Solanki R, Jindal A, Rankawat G, Pathak D, Bagarhatta M, Singh A. Demystifying COVID-19 lung pathology: A clinicopathological study of postmortem core needle biopsy. Lung India. 2021;38:343-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4227] [Cited by in F6Publishing: 4268] [Article Influence: 1067.0] [Reference Citation Analysis (0)] |
| 73. | El Sissy C, Saldman A, Zanetta G, Martins PV, Poulain C, Cauchois R, Kaplanski G, Venetz JP, Bobot M, Dobosziewicz H, Daniel L, Koubi M, Sadallah S, Rotman S, Mousson C, Pascual M, Frémeaux-Bacchi V, Fakhouri F. COVID-19 as a potential trigger of complement-mediated atypical HUS. Blood. 2021;138:1777-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Jamaly S, Tsokos MG, Bhargava R, Brook OR, Hecht JL, Abdi R, Moulton VR, Satyam A, Tsokos GC. Complement activation and increased expression of Syk, mucin-1 and CaMK4 in kidneys of patients with COVID-19. Clin Immunol. 2021;229:108795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, Tektonidou M, Konstantinidis T, Papagoras C, Mitroulis I, Germanidis G, Lambris JD, Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151-6157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 499] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 76. | Diorio C, McNerney KO, Lambert M, Paessler M, Anderson EM, Henrickson SE, Chase J, Liebling EJ, Burudpakdee C, Lee JH, Balamuth FB, Blatz AM, Chiotos K, Fitzgerald JC, Giglia TM, Gollomp K, Odom John AR, Jasen C, Leng T, Petrosa W, Vella LA, Witmer C, Sullivan KE, Laskin BL, Hensley SE, Bassiri H, Behrens EM, Teachey DT. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4:6051-6063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 77. | Cugno M, Meroni PL, Gualtierotti R, Griffini S, Grovetti E, Torri A, Lonati P, Grossi C, Borghi MO, Novembrino C, Boscolo M, Uceda Renteria SC, Valenti L, Lamorte G, Manunta M, Prati D, Pesenti A, Blasi F, Costantino G, Gori A, Bandera A, Tedesco F, Peyvandi F. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116:102560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 78. | Frumholtz L, Bouaziz JD, Battistella M, Hadjadj J, Chocron R, Bengoufa D, Le Buanec H, Barnabei L, Meynier S, Schwartz O, Grzelak L, Smith N, Charbit B, Duffy D, Yatim N, Calugareanu A, Philippe A, Guerin CL, Joly B, Siguret V, Jaume L, Bachelez H, Bagot M, Rieux-Laucat F, Maylin S, Legoff J, Delaugerre C, Gendron N, Smadja DM, Cassius C; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 79. | Showers CR, Nuovo GJ, Lakhanpal A, Siegel CH, Aizer J, Elreda L, Halevi A, Lai AR, Erkan D, Magro CM. A Covid-19 Patient with Complement-Mediated Coagulopathy and Severe Thrombosis. Pathobiology. 2021;88:28-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Laurence J, Mulvey JJ, Seshadri M, Racanelli A, Harp J, Schenck EJ, Zappetti D, Horn EM, Magro CM. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020;219:108555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 81. | Gill SE, Dos Santos CC, O'Gorman DB, Carter DE, Patterson EK, Slessarev M, Martin C, Daley M, Miller MR, Cepinskas G, Fraser DD; Lawson COVID19 Study Team. Transcriptional profiling of leukocytes in critically ill COVID19 patients: implications for interferon response and coagulation. Intensive Care Med Exp. 2020;8:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Barberis E, Vanella VV, Falasca M, Caneapero V, Cappellano G, Raineri D, Ghirimoldi M, De Giorgis V, Puricelli C, Vaschetto R, Sainaghi PP, Bruno S, Sica A, Dianzani U, Rolla R, Chiocchetti A, Cantaluppi V, Baldanzi G, Marengo E, Manfredi M. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front Mol Biosci. 2021;8:632290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 83. | Freda CT, Yin W, Ghebrehiwet B, Rubenstein DA. SARS-CoV-2 Structural Proteins Exposure Alter Thrombotic and Inflammatory Responses in Human Endothelial Cells. Cell Mol Bioeng. 2021;1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Kaiser R, Leunig A, Pekayvaz K, Popp O, Joppich M, Polewka V, Escaig R, Anjum A, Hoffknecht ML, Gold C, Brambs S, Engel A, Stockhausen S, Knottenberg V, Titova A, Haji M, Scherer C, Muenchhoff M, Hellmuth JC, Saar K, Schubert B, Hilgendorff A, Schulz C, Kääb S, Zimmer R, Hübner N, Massberg S, Mertins P, Nicolai L, Stark K. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight. 2021;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 85. | Porto BN, Stein RT. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front Immunol. 2016;7:311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 86. | Vlaar APJ, de Bruin S, Busch M, Timmermans SAMEG, van Zeggeren IE, Koning R, Ter Horst L, Bulle EB, van Baarle FEHP, van de Poll MCG, Kemper EM, van der Horst ICC, Schultz MJ, Horn J, Paulus F, Bos LD, Wiersinga WJ, Witzenrath M, Rueckinger S, Pilz K, Brouwer MC, Guo RF, Heunks L, van Paassen P, Riedemann NC, van de Beek D. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020;2:e764-e773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 87. | Kulasekararaj AG, Lazana I, Large J, Posadas K, Eagleton H, Lord Villajin J, Zuckerman M, Gandhi S, Marsh JCW. Terminal complement inhibition dampens the inflammation during COVID-19. Br J Haematol. 2020;190:e141-e143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 88. | Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A, De Negri P, Di Gennaro C, Pagano A, Allegorico E, Bressy L, Bosso G, Ferrara A, Serra C, Montisci A, D'Amico M, Schiano Lo Morello S, Di Costanzo G, Tucci AG, Marchetti P, Di Vincenzo U, Sorrentino I, Casciotta A, Fusco M, Buonerba C, Berretta M, Ceccarelli M, Nunnari G, Diessa Y, Cicala S, Facchini G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040-4047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 173] [Reference Citation Analysis (0)] |
| 89. | Zelek WM, Cole J, Ponsford MJ, Harrison RA, Schroeder BE, Webb N, Jolles S, Fegan C, Morgan M, Wise MP, Morgan BP. Complement Inhibition with the C5 Blocker LFG316 in Severe COVID-19. Am J Respir Crit Care Med. 2020;202:1304-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 90. | Posch W, Vosper J, Noureen A, Zaderer V, Witting C, Bertacchi G, Gstir R, Filipek PA, Bonn GK, Huber LA, Bellmann-Weiler R, Lass-Flörl C, Wilflingseder D. C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected primary human airway epithelia. J Allergy Clin Immunol. 2021;147:2083-2097.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 91. | Araten DJ, Belmont HM, Schaefer-Cutillo J, Iyengar A, Mattoo A, Reddy R. Mild Clinical Course of COVID-19 in 3 Patients Receiving Therapeutic Monoclonal Antibodies Targeting C5 Complement for Hematologic Disorders. Am J Case Rep. 2020;21:e927418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Iluta S, Pasca S, Dima D, Mester G, Urian L, Bojan A, Zdrenghea M, Trifa A, Balacescu O, Tomuleasa C. Haematology patients infected with SARS-CoV-2, pretreated with eculizumab or siltuximab, develop oligosymptomatic disease. Eur J Hosp Pharm. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Annane D, Heming N, Grimaldi-Bensouda L, Frémeaux-Bacchi V, Vigan M, Roux AL, Marchal A, Michelon H, Rottman M, Moine P; Garches COVID 19 Collaborative Group. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. EClinicalMedicine. 2020;28:100590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 94. | Genthon A, Chiarabini T, Baylac P, Valin N, Urbina T, Pacanowski J, Mekinian A, Brissot E, M 'Hammedi-Bouzina F, Lapusan S, Mohty M, Lacombe K, Ingiliz P. Severe COVID-19 infection in a patient with paroxysmal nocturnal hemoglobinuria on eculizumab therapy. Leuk Lymphoma. 2021;62:1502-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Giudice V, Pagliano P, Vatrella A, Masullo A, Poto S, Polverino BM, Gammaldi R, Maglio A, Sellitto C, Vitale C, Serio B, Cuffa B, Borrelli A, Vecchione C, Filippelli A, Selleri C. Combination of Ruxolitinib and Eculizumab for Treatment of Severe SARS-CoV-2-Related Acute Respiratory Distress Syndrome: A Controlled Study. Front Pharmacol. 2020;11:857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 96. | Mastaglio S, Ruggeri A, Risitano AM, Angelillo P, Yancopoulou D, Mastellos DC, Huber-Lang M, Piemontese S, Assanelli A, Garlanda C, Lambris JD, Ciceri F. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. 2020;215:108450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 224] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 97. | Mastellos DC, Pires da Silva BGP, Fonseca BAL, Fonseca NP, Auxiliadora-Martins M, Mastaglio S, Ruggeri A, Sironi M, Radermacher P, Chrysanthopoulou A, Skendros P, Ritis K, Manfra I, Iacobelli S, Huber-Lang M, Nilsson B, Yancopoulou D, Connolly ES, Garlanda C, Ciceri F, Risitano AM, Calado RT, Lambris JD. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 98. | Bouillet L, Boccon-Gibod I, Gompel A, Floccard B, Martin L, Blanchard-Delaunay C, Launay D, Fain O. Hereditary angioedema with normal C1 inhibitor: clinical characteristics and treatment response with plasma-derived human C1 inhibitor concentrate (Berinert®) in a French cohort. Eur J Dermatol. 2017;27:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Valerieva A, Caccia S, Cicardi M. Recombinant human C1 esterase inhibitor (Conestat alfa) for prophylaxis to prevent attacks in adult and adolescent patients with hereditary angioedema. Expert Rev Clin Immunol. 2018;14:707-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Weiler JM, Edens RE, Linhardt RJ, Kapelanski DP. Heparin and modified heparin inhibit complement activation in vivo. J Immunol. 1992;148:3210-3215. [PubMed] [Cited in This Article: ] |
| 101. | Gozzo L, Viale P, Longo L, Vitale DC, Drago F. The Potential Role of Heparin in Patients With COVID-19: Beyond the Anticoagulant Effect. A Review. Front Pharmacol. 2020;11:1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 102. | Schoenfeld AK, Lahrsen E, Alban S. Regulation of Complement and Contact System Activation via C1 Inhibitor Potentiation and Factor XIIa Activity Modulation by Sulfated Glycans - Structure-Activity Relationships. PLoS One. 2016;11:e0165493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Urwyler P, Moser S, Charitos P, Heijnen IAFM, Rudin M, Sommer G, Giannetti BM, Bassetti S, Sendi P, Trendelenburg M, Osthoff M. Treatment of COVID-19 With Conestat Alfa, a Regulator of the Complement, Contact Activation and Kallikrein-Kinin System. Front Immunol. 2020;11:2072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 104. | Riedl MA, Bygum A, Lumry W, Magerl M, Bernstein JA, Busse P, Craig T, Frank MM, Edelman J, Williams-Herman D, Feuersenger H, Rojavin M; Berinert Registry investigators. Safety and Usage of C1-Inhibitor in Hereditary Angioedema: Berinert Registry Data. J Allergy Clin Immunol Pract. 2016;4:963-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 105. | Davis B, Bernstein JA. Conestat alfa for the treatment of angioedema attacks. Ther Clin Risk Manag. 2011;7:265-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 106. | Yuan X, Gavriilaki E, Thanassi JA, Yang G, Baines AC, Podos SD, Huang Y, Huang M, Brodsky RA. Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica. 2017;102:466-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 107. | Rambaldi A, Gritti G, Micò MC, Frigeni M, Borleri G, Salvi A, Landi F, Pavoni C, Sonzogni A, Gianatti A, Binda F, Fagiuoli S, Di Marco F, Lorini L, Remuzzi G, Whitaker S, Demopulos G. Endothelial injury and thrombotic microangiopathy in COVID-19: Treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;225:152001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 108. | Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, Tolerability and Efficacy of Narsoplimab, a Novel MASP-2 Inhibitor for the Treatment of IgA Nephropathy. Kidney Int Rep. 2020;5:2032-2041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 109. | Rambaldi A, Smith M, Whitaker S, Khaled, Samer. Narsoplimab (OMS721) for the treatment of adult hematopoietic stem cell transplant-associated thrombotic microangiopathy. Eur Hematol Assoc Congr. 2020. Available from: https://Library.ehaweb.org/eha/2020/eha25th/295082/alessandro.rambaldi.narsoplimab.28oms72129.for.the.treatment.of.adult.html. [Cited in This Article: ] |
| 110. | Kang S, Yang M, He S, Wang Y, Chen X, Chen YQ, Hong Z, Liu J, Jiang G, Chen Q, Zhou Z, Huang Z, Huang X, He H, Zheng W, Liao HX, Xiao F, Shan H, Chen S. A SARS-CoV-2 antibody curbs viral nucleocapsid protein-induced complement hyperactivation. Nat Commun. 2021;12:2697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 111. | Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, Lambris JD. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20:343-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 350] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 112. | Apellis Pharmaceuticals, Inc. A Randomized, Double-Blinded, Vehicle-Controlled, Multicenter, Parallel-Group Study of APL-9 in Mild to Moderate Acute Respiratory Distress Syndrome Due to COVID-19. clinicaltrials.gov. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04402060. [Cited in This Article: ] |
| 113. | Dupont T, Caillat-Zucman S, Fremeaux-Bacchi V, Morin F, Lengliné E, Darmon M, Peffault de Latour R, Zafrani L, Azoulay E, Dumas G. Identification of Distinct Immunophenotypes in Critically Ill Coronavirus Disease 2019 Patients. Chest. 2021;159:1884-1893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Mehlhop E, Nelson S, Jost CA, Gorlatov S, Johnson S, Fremont DH, Diamond MS, Pierson TC. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe. 2009;6:381-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 115. | Kurtovic L, Beeson JG. Complement Factors in COVID-19 Therapeutics and Vaccines. Trends Immunol. 2021;42:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, Hemmings O, O'Byrne A, Kouphou N, Galao RP, Betancor G, Wilson HD, Signell AW, Winstone H, Kerridge C, Huettner I, Jimenez-Guardeño JM, Lista MJ, Temperton N, Snell LB, Bisnauthsing K, Moore A, Green A, Martinez L, Stokes B, Honey J, Izquierdo-Barras A, Arbane G, Patel A, Tan MKI, O'Connell L, O'Hara G, MacMahon E, Douthwaite S, Nebbia G, Batra R, Martinez-Nunez R, Shankar-Hari M, Edgeworth JD, Neil SJD, Malim MH, Doores KJ. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1007] [Cited by in F6Publishing: 876] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 117. | Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384:2092-2101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1614] [Cited by in F6Publishing: 1514] [Article Influence: 504.7] [Reference Citation Analysis (0)] |