Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.767
Peer-review started: September 28, 2016
First decision: October 21, 2016
Revised: October 23, 2016
Accepted: November 16, 2016
Article in press: November 18, 2016
Published online: December 24, 2016
To review negative pressure wound therapy (NPWT) as an important addition to the conventional methods of wound management.
A systematic review, performed by searching the PubMed, EMBASE and Cochrane Library databases, showed 11 case reports comprising a total of 22 kidney transplantation (KT) patients (range, 1 to 9), who were treated with NPWT. Application of NPWT was associated with successful healing of wounds, leg ulcer, lymphocele and urine leak from ileal conduit.
No complications related to NPWT were reported. However, there was paucity of robust data on the effectiveness of NPWT in KT recipients; therefore, prospective studies assessing its safety and efficacy of NPWT and randomised trials comparing the effectiveness of NPWT with alternative modalities of wound management in KT recipients is recommended.
Negative pressure incision management system, NPWT with instillation and endoscopic vacuum-assisted closure system are in investigational stage.
Core tip: Systematic review of the safety and efficacy of negative pressure wound therapy (NPWT) in kidney transplant (KT) recipients revealed 11 case reports, which have shown the effective role NPWT in the management of wound dehiscence, lymphocele, urine leak from ileal conduits and leg ulcers. Because of the lack of robust evidence on the safety and efficacy of NPWT in KT patients, prospective multicentre studies recruiting large number of patients is recommended to examine the role of NPWT in the treatment of wound-related complications in KT recipients. The efficacy of negative pressure incision management system, NPWT with instillation and endoscopic vacuum-assisted closure system remain in investigational stage.
- Citation: Shrestha BM. Systematic review of the negative pressure wound therapy in kidney transplant recipients. World J Transplant 2016; 6(4): 767-773
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/767.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.767
Kidney transplantation (KT) represents the best treatment modality for patients with end-stage renal disease, providing the best outcomes for survival, quality of life and cost-effectiveness[1]. Immunosuppressive agents administered to prevent rejection and prolong transplant survival, not only increase susceptibility to infections, but also delay wound healing. Post-operative wound infection leading to cavitation and dehiscence continue to remain serious problems resulting in extended hospital stay, readmissions, repeated surgical interventions and protracted recovery, thereby imposing extra cost to the healthcare delivery system[2]. The wound complication rate after KT ranges between 2% to 47%. The risk factors for these complications are advancing age, diabetes mellitus, body mass index, kidney failure, type of surgical incision, re-operation, operator’s experience, and immunosuppressive drugs including sirolimus and steroids[3-6]. The wound-related complications can present as superficial infection, haematomas, lymphocele, and partial or full-thickness wound dehiscence leading to incisional hernias[7].
Negative pressure wound therapy (NPWT), also referred to as, vacuum-assisted closure therapy (VACT), topical negative pressure therapy or microdeformational wound therapy has been evaluated over last two decades and is considered as an useful adjunct to the management of diverse range of lesions including open abdominal wounds, open fractures, post-traumatic wounds, split-thickness skin grafts and after clean surgery in obese patients[8-13]. Application of any new form of treatment in KT patients is associated with concerns on the part of clinicians, particularly when robust evidence supporting their safety and efficacy are lacking. A systematic review of the published literature was carried out to evaluate the effectiveness and safety of NPWT in KT recipients presenting with wound-related complications.
A systematic electronic literature search was performed in PubMed, EMBASE and Cochrane Library databases from inception to March 2016. The search terms “renal transplantation”, “kidney transplantation”, “negative pressure wound therapy”, “vacuum-assisted closure”, “wound”, and “topical negative pressure therapy” were used. EndNote software (Version X7.5, BLD 9325; Thomson Reuters, Philadelphia, PA, United States) was used to compile pertinent references.
KT is performed by using classical Gibson’s muscle-cutting incision, where the iliac vessels and urinary bladder are accessed extraperitoneally. The renal vessels are anastomosed to the iliac vessels and the ureter to the bladder by the techniques described previously[14]. Wound infection leading to muscular dehiscence exposes the kidney to the external environment, which predisposes to infection around the kidney, haemorrhage from mycotic aneurysms of the vascular anastomoses, lymph leak, urine leak, and dehiscence of muscle layers leading to incisional herniation.
The beneficial effect of negative sub-atmospheric pressure on the wound results in gradual closure of wound edges by micro- and macrodeformation of the wound surface, and by suction of infectious material and interstitial fluid, reduces tissue oedema. Decompression of tissue increases blood flow and tissue oxygenation, thereby accelerates the wound healing cascade including, angiogenesis, neurogenesis, granulation tissue formation, cellular proliferation, differentiation and migration of appropriate cellular components at the site of healing[15-20].
Glass et al[21] in a systematic review, evaluated the molecular basis for the promotion of wound healing by NPWT and observed an increase in the expression of cytokines, chemokines and growth factors, which reflected mechanoreceptor and chemoreceptor transduction in response to stress and hypoxia. There was reduction of expression of matrix metalloproteinase-1, -2, -9 and -13, with no changes on the activity of tissue inhibitor of metalloproteinase-1[21].
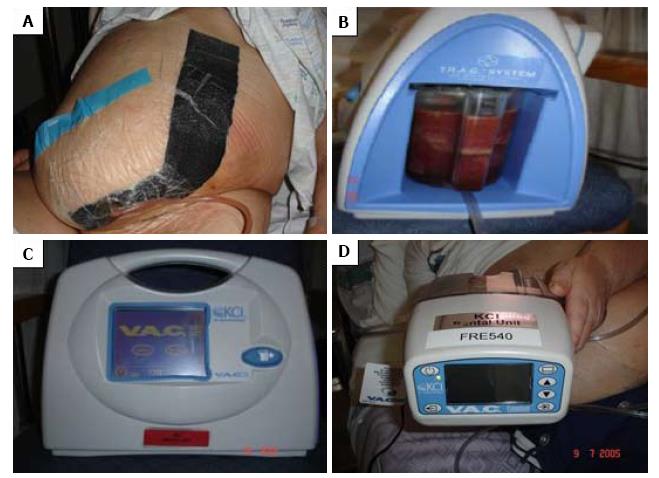
The NPWT device comprises of black polyurethane ether foam dressing or white polyvinyl alcohol foam, which is tailored to fit into the dimension of the wound. A tube with multiple perforations is placed within the foam for the evacuation of the wound discharge. The tube together with the foam is then covered with an occlusive drape, which helps to maintain uniform negative pressure. The effluent of the wound is collected in a canister, which is attached to the vacuum pump with an adjustable negative pressure, ranging from 50 and 125 mmHg (Figure 1A and B). At the interval 48-72 h, the soiled dressing is replaced with fresh dressing at the bedside, when progress of healing is assessed. The device can be used in preparation for secondary suture, a skin graft, flap or until full closure of the wound has taken place[22,23]. The oldest and most popular device in clinical practice is the vacuum-assisted closure (VAC® KCI, San Antonio, Texas) system (Figure 1C and D)[24].
The contra-indications for the applications of NPWT are excessive pain with NPWT, presence of pus or excessive bleeding and intolerance. The success of NPWT is assessed by the reduction in wound size by at least 10% per week or 50% improvement over 4 wk period, which indicates high probability of success of the therapy[25].
The literature search identified 11 case reports comprising of a total of 22 KT patients (range, 1 to 9) (Table 1), who were treated with NPWT[26-36]. Comparison between NPWT and other methods of wound treatment in KT patients has not been reported in any study.
| Ref. | Year | Country | No. of cases | No. of NPWT days | Indications |
| Iesari et al[36] | 2015 | Italy | 1 | Not described | Wound dehiscence |
| Bozkurt et al[33] | 2015 | Turkey | 1 | 5 | Primary surgery |
| Markic et al[34] | 2014 | Croatia | 2 | 14, 21 | Wound dehiscence |
| Franchin et al[35] | 2014 | Italy | 1 | 45 | Infected lymphocele |
| Zanus et al[26] | 2011 | Italy | 1 | 180 | Wound dehiscence, pancreatitis |
| Ortiz et al[27] | 2011 | United States | 1 | 15 | Wound infection |
| Heap et al[28] | 2010 | United Kingdom | 2 | Not described | Wound dehiscence, Urine leak |
| Thodis et al[29] | 2009 | Greece | 1 | Not described | Vibrio infection of leg |
| Devries et al[31] | 2009 | United States | 1 | Not described | Leg wound |
| Shrestha et al[30] | 2007 | United Kingdom | 9 | 3, 5, 5, 5, 8, 10, 10, 15, 30 | Wound dehiscence, infection |
| Hodzic et al[32] | 2003 | Germany | 2 | 15 | Wound dehiscence |
In 2003, Hodzic et al[32], for the first time, reported successful outcome of application of NPWT for 15 d in 2 KT patients prior to secondary suture of wounds. Successful treatment of an infected and dehisced laparotomy wound following liver and KT in a patient by employing NPWT was reported by Zanus et al[26], where associated complications included acute pancreatitis, abdominal compartment syndrome and wound infection by multi-drug resistant organisms. The patient required 14 successive laparotomies and NPWT for 6 mo for complete closure of the wound[26]. Similarly, Markić et al[34] have described successful treatment with application of NPWT in 2 KT patients who developed infected and dehisced wounds. NPWT was applied for 2 and 3 wk, respectively, which was followed by secondary sutures[34].
The occurrence of ureteric complications significantly delays the recovery following KT and the incidence of such complication ranges between 1.2% and 8.9%[37]. Urinary leak rate of 11% requiring re-implantation was reported by Surange et al[38] in a series of KT into ileal conduits. Two cases of urine leak and wound dehiscence following KT into ileal conduits were managed successfully by Heap et al[28] with the application of NPWT. Secondary suture of the wounds was carried out after two and three months in these patients. The renal function was restored in both patients leading to 141 µmol/L and 75 µmol/L of serum creatinine, respectively, at the end of 3 mo[28]. On the other hand, Ortiz et al[27], had negative experience of NPWT in a KT recipient with peri-renal collection and wound infection. They concluded that NPWT had encouraged and prolonged urine leak, which had healed after 5 d of discontinuation of NPWT[27]. Iesari et al[36] had applied VAC device in a KT patient who had developed spontaneous rupture of urinary bladder due to gangrenous cystitis and extensive wound dehiscence associated with multidrug resistant Acinetobacter baumanni infection. There was significant urine leak following VAC therapy, hence this was discontinued and topical homologous platelet-rich gel was used resulting in complete wound healing[36].
Infection caused by virulent organisms after skin grafts and reconstructive surgery in KT recipients not only lead to failure of treatment, but also can be life-threatening. Thodis et al[29] treated soft tissue infection caused by Vibrio vulnificus with NPWT, which involved the leg in a KT recipient. Autologous platelet concentrate spray further enhanced granulation tissue formation leading to complete epithelialization of the wound after 4 wk[29]. In a similar situation, Devries et al[31] were unsuccessful in treating soft tissue infection on the leg of a KT recipient, that culminated in amputation. As the patient was on sirolimus, wound healing could have been compromised by the same drug[5,31,39].
Lymphocele following KT can cause significant morbidity due to infection and compression of ureter and blood vessels. The reported incidence of lymphocele ranges between 0.6% to 49%[40]. Franchin et al[35] have described successful management of a large deep-seated lymphocele infected with Staphylococcus haemolyticus, Escherichia coli and Enterococcus faecal, with the application of NPWT. Following surgical drainage, the wound had completely dehisced and transplanted kidney exposed. The cavity was packed with foam dressing and device was applied. A negative pressure of 80 mmHg was maintained. The dressing was changed every 5 d. After 45 d, the lymphocele had sealed and skin closed[35].
In a prospective study reported by Shrestha et al[30], 9 KT patients had developed wound infection with cavitation and wound dehiscence. This was associated with significant amount of discharge from the wound, which failed to respond to standard method of treatment. Treatment with NPWT for a median of 9 (range 3-30) d led to cessation of discharge from the wound. Of the 9 patients, 4 patients were managed on an outpatient with portable NPWT device, where the treatment was discontinued after a median of 5.5 (range 3-7) d. The median hospital stay since the employment of NPWT was significantly shorter (5, range 2-12 d) compared to the standard method of treatment prior to application of NPWT (11 d, range, 5-20 d; P = 0.003). The wound healed completely in all 9 cases after the therapy[30].
Recently, Bozkurt et al[33], for the first time, employed Prevena incision management system (Kinetic Concept Inc. San Antonio, Texas, United States) to the clean closed surgical wound for 5 d after a KT and observed complete healing of the wound with no skin or device-related problems.
All infected wounds with associated collections require surgical drainage for early healing. Fleischmann et al[41] from Germany, in 1993, for the first time described the benefit of exposing wounds to sub-atmospheric pressure, which promoted wound debridement and healing. He applied this method in 15 patients with compound fractures and observed enhanced proliferation of the granulation tissue with no associated bone infection leading to complete healing of fractures[41]. In 1997, Louis Argenta and Michael Morykwas introduced NPWT therapy, for the first time, in the treatment of bed sores and slow healing wounds. Since then, NPWT has been extended to treat various types of wounds resulting from surgery, trauma, infection, congenital deformities and tumours[42-44]. The experience of NPWT gained over the past two decades has encouraged clinicians to treat patients globally in both hospital and domiciliary environments[44-46].
This systematic review has confirmed the available evidence on the safety and efficacy of the application of NPWT in KT recipients limited to case reports. On the other hand, the reported experiences do support NPWT in the management of complex wounds following KT, including urine leak from KT in ileal conduits and lymphoceles. The theoretical risk of haemorrhage and urine leak from transmission of suction pressure on the vascular and ureteric anastomoses cannot be ignored. Prolonged urine leak had occurred in two reported cases after KT where NPWT was applied. Discontinuation of NPWT had led to resolution of urine leak. In author’s single KT patient with a urine leak from the ureterovesical junction, treatment with NPWT led to persistence of urine leak for 1 wk. Resolution of the urine leak occurred 2 d after discontinuation of NPWT therapy. Successful outcomes of NPWT in the management of wound infections in cardiac and liver transplant recipients have been described previously[47,48].
Development of enterocutaneous fistula during the course of NPWT is always a concern, which is particularly applicable in in deep wounds after KT, where thin layer of peritoneum lies between the bowel and the foam dressing. Occurrence of enterocutaneous fistula has been observed after NPWT in open abdominal wound. However, the evidence in support of the occurrence of this complication after NPWT is weak[49-51].
Shrestha et al[30], in their largest reported series of 9 patients, observed benefit of NPWT on wound healing, reduced hospital stays and convenience of wound management. The management of 4 patients on an outpatient basis with the NPWT device in situ, was convenient to the patient and saved hospital cost significantly[30].
There is no data available comparing the safety and efficacy of NPWT over conventional methods of wound management in KT recipients. However, there are several randomised trials and meta-analyses, which have assessed the effectiveness of NPWT for skin grafts and surgical wound healing by primary and secondary intentions and in chronic wounds compared with several conventional treatment methods. With regards to healing of surgical wound by primary intention, the evidence for the effect of NPWT for reducing surgical site infections, time to complete healing and wound dehiscence remains unclear[52]. A Cochrane Database Systematic Review assessed the effect of NPWT for the treatment of chronic wounds in comparison with five different comparators, which did not show that NPWT significantly increased the healing rate. The trials did have methodical flaws, therefore need for better quality research was recommended[53]. Similarly, a recent Cochrane review did not show clinical effectiveness of NPWT over alginate dressings in the treatment of open infected groin wounds and a silicone dressing in the treatment of excised pilonidal sinus when they were allowed to heal by secondary intention[54].
NPWT with instillation (NPWTi) is a recent advancement, which is being assessed in the management of complicated surgical wounds. The wound is covered with normal saline (0.9%) and left for 10-20 min for diffusion to take place. Then, 2-4 h of negative pressure at -125 mmHg is applied. A panel of experts in the first International Consensus Guidelines for NPWTi have recommended its use in high risk patients with multiple comorbidities including diabetes, contaminated traumatic wounds, and wounds complicated by invasive infection or extensive biofilm. Available evidence suggest achievement of better outcomes with the addition of NPWTi to standard of care in properly selected cases, compared to standard care alone[55,56]. As majority of KT recipients often have associated co-morbidities, NPWTi may be an option in this group of patients.
Colli et al[57] employed the negative pressure incision management system in clean closed incisions, for the first time, in 10 patients after cardiac surgery and observed normal wound healing in patients where complications were expected after surgery. Bozkurt et al[33], have reported their experience of using Prevena incision management device in a KT recipient. A recent meta-analysis of NPWT for closed surgical incisions, (including 10 studies, 1311 incisions in 1089 patients) showed significant reduction in wound infection (RR = 0.54) and seroma formation (RR = 0.48), when NPWT was compared with standard care. The reduction in wound dehiscence was not significant. The numbers needed to treat were 3 (seroma), 17 (dehiscence) and 25 (infection). Due to heterogeneity between the included studies, no general recommendations could be made yet[58]. However, this device has a potential for its use in immunosuppressed and obese patients undergoing KT.
Endoscopic vacuum-assisted closure system (E-VAC) has developed as an important alternative in patients with upper gastrointestinal leaks not responding to standard endoscopic or surgical treatment procedures. Leak from oesophageal and gastric anastomosis sites and perforations resulting from endoscopic procedures were successfully closed using the E-VAC therapy[59,60]. Application of this device in KT recipients remains to be explored.
This systematic review has shown successful healing of wounds, leg ulcer, lymphocele and urine leak from ileal conduit following application of NPWT in KT recipients and there was no report of complications associated with NPWT. However, there is lack of robust evidence on safety and efficacy of NPWT in KT patients. Based on available evidence on the application of NPWT in KT recipients, NPWT can be considered as a valuable adjunct in the management of infected and dehisced wounds following KT. The safety and efficacy of NPWT, negative pressure incision management system, NPWT with instillation and E-VAC system, and efficacy of NPWT in comparison with standard methods of wound management, need to be examined prospectively by including large number patients in multicentre studies.
Negative pressure wound therapy (NPWT) is a useful adjunct to the conventional methods of management of infected wounds with deep cavitation in the kidney transplant (KT) recipients. A systematic review was performed to assess the safety and efficacy of NPWT in KT recipients, which showed 11 case reports including 22 KT recipients who were treated with NPWT showing beneficial outcomes.
There are no randomised trials comparing the safety and efficacy of NPWT with alternative modalities of wound management, hence multicentre prospective study by including large number of patients is recommended.
The negative pressure incision management, NPWT with instillation, and endoscopic vacuum-assisted closure system are the new developments in this field, which need to be applied and examined in the RT recipients.
NPWT has been applied in the treatment of abdominal wounds, leg ulcers, lymphoceles and urine leak from ileal conduit in RT recipients successfully.
Negative pressure wound therapy (NPWT), also referred to as, vacuum-assisted closure therapy (VACT), topical negative pressure therapy (TNPT) or microdeformational wound therapy.
The authors made a comprehensive review on NPWT on KTx recipients. It provides useful information for clinicians.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chkhotua A, Friedman EA, Wang CX S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Shrestha A, Shrestha A, Basarab-Horwath C, McKane W, Shrestha B, Raftery A. Quality of life following live donor renal transplantation: a single centre experience. Ann Transplant. 2010;15:5-10. [PubMed] [Cited in This Article: ] |
| 2. | Harris AD, Fleming B, Bromberg JS, Rock P, Nkonge G, Emerick M, Harris-Williams M, Thom KA. Surgical site infection after renal transplantation. Infect Control Hosp Epidemiol. 2015;36:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94:547-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Judson RT. Wound infection following renal transplantation. Aust N Z J Surg. 1984;54:223-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, Kremers WK, Stegall MD. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004;77:1555-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Mehrabi A, Fonouni H, Wente M, Sadeghi M, Eisenbach C, Encke J, Schmied BM, Libicher M, Zeier M, Weitz J. Wound complications following kidney and liver transplantation. Clin Transplant. 2006;20 Suppl 17:97-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Fockens MM, Alberts VP, Bemelman FJ, van der Pant KA, Idu MM. Wound morbidity after kidney transplant. Prog Transplant. 2015;25:45-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Kanakaris NK, Thanasas C, Keramaris N, Kontakis G, Granick MS, Giannoudis PV. The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury. 2007;38 Suppl 5:S9-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Stannard JP, Volgas DA, Stewart R, McGwin G, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23:552-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Stevens P. Vacuum-assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J. 2009;6:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum-assisted closure: state of clinic art. Plast Reconstr Surg. 2006;117:127S-142S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Blume PA, Key JJ, Thakor P, Thakor S, Sumpio B. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C.® therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J. 2010;7:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Dragu A, Schnürer S, Unglaub F, Wolf MB, Beier JP, Kneser U, Horch RE. Wide topical negative pressure wound dressing treatment for patients undergoing abdominal dermolipectomy following massive weight loss. Obes Surg. 2011;21:1781-1786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Mellin P, Eickenberg HU. Ureteral reimplantation: Lich-Grégoire method. Urology. 1978;11:315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg. 1998;51:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Timmers MS, Le Cessie S, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Jones SM, Banwell PE, Shakespeare PG. Advances in wound healing: topical negative pressure therapy. Postgrad Med J. 2005;81:353-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Seo SG, Yeo JH, Kim JH, Kim JB, Cho TJ, Lee DY. Negative-pressure wound therapy induces endothelial progenitor cell mobilization in diabetic patients with foot infection or skin defects. Exp Mol Med. 2013;45:e62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Borgquist O, Ingemansson R, Malmsjö M. The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg. 2011;127:551-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg. 2014;101:1627-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg. 2008;95:685-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A. Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg. 2008;36:438-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1526] [Cited by in F6Publishing: 1380] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 25. | Sheehan P. Early change in wound area as a predictor of healing in diabetic foot ulcers: knowing “when to say when”. Plast Reconstr Surg. 2006;117:245S-247S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Zanus G, Boetto R, D’Amico F, Gringeri E, Vitale A, Carraro A, Bassi D, Scopelliti M, Bonsignore P, Burra P. A novel approach to severe acute pancreatitis in sequential liver-kidney transplantation: the first report on the application of VAC therapy. Transpl Int. 2011;24:e23-e27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Ortiz J, Siddeswarappa M, Stewart S, Khanmoradi K, Campos S, Zaki R. Negative pressure therapy may delay resolution of urinary leaks. Am J Transplant. 2011;11:412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Heap S, Mehra S, Tavakoli A, Augustine T, Riad H, Pararajasingam R. Negative pressure wound therapy used to heal complex urinary fistula wounds following renal transplantation into an ileal conduit. Am J Transplant. 2010;10:2370-2373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Thodis E, Kriki P, Kakagia D, Passadakis P, Theodoridis M, Mourvati E, Vargemezis V. Rigorous Vibrio vulnificus soft tissue infection of the lower leg in a renal transplant patient managed by vacuum therapy and autologous growth factors. J Cutan Med Surg. 2009;13:209-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Shrestha BM, Nathan VC, Delbridge MC, Parker K, Throssell D, McKane WS, Karim MS, Raftery AT. Vacuum-assisted closure (VAC) therapy in the management of wound infection following renal transplantation. Kathmandu Univ Med J (KUMJ). 2007;5:4-7. [PubMed] [Cited in This Article: ] |
| 31. | Devries JG, Collier RC, Niezgoda JA, Sanicola S, Simanonok JP. Impaired lower extremity wound healing secondary to sirolimus after kidney transplantation. J Am Col Certif Wound Spec. 2009;1:86-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Hodzic J, Adams J, Staehler G, Wiesel M. [Vacuum sealing of extensive wound healing disorders after kidney transplantation]. Urologe A. 2003;42:1097-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Bozkurt B, Tokac M, Dumlu EG, Yalcin A, Kilic M. Our First Experience With Negative Pressure Incision Management System Implemented on the Clean Surgical Incision in the Renal Transplantation Recipient: A Case Report. Transplant Proc. 2015;47:1515-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Markić D, Marinović M, Sotosek S, Spanjol J, Ivancić A, Anton M, Fuckar Z. The role of negative pressure wound therapy in patients with kidney transplantation. Coll Antropol. 2014;38:1199-1201. [PubMed] [Cited in This Article: ] |
| 35. | Franchin M, Tozzi M, Soldini G, Piffaretti G. A case of continuous negative pressure wound therapy for abdominal infected lymphocele after kidney transplantation. Case Rep Transplant. 2014;2014:742161. [PubMed] [Cited in This Article: ] |
| 36. | Iesari S, Lai Q, Rughetti A, Dell’Orso L, Clemente K, Famulari A, Pisani F, Favi E. Infected Nonhealing Wound in a Kidney Transplant Recipient: Successful Treatment With Topical Homologous Platelet-Rich Gel. Exp Clin Transplant. 2015; Jun 15; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 37. | Nie ZL, Zhang KQ, Li QS, Jin FS, Zhu FQ, Huo WQ. Treatment of urinary fistula after kidney transplantation. Transplant Proc. 2009;41:1624-1626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Surange RS, Johnson RW, Tavakoli A, Parrott NR, Riad HN, Campbell BA, Augustine T. Kidney transplantation into an ileal conduit: a single center experience of 59 cases. J Urol. 2003;170:1727-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Troppmann C, Pierce JL, Gandhi MM, Gallay BJ, McVicar JP, Perez RV. Higher surgical wound complication rates with sirolimus immunosuppression after kidney transplantation: a matched-pair pilot study. Transplantation. 2003;76:426-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Lima ML, Cotrim CA, Moro JC, Miyaoka R, D’Ancona CA. Laparoscopic treatment of lymphoceles after renal transplantation. Int Braz J Urol. 2012;38:215-221; discussion 221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg. 1993;96:488-492. [PubMed] [Cited in This Article: ] |
| 42. | Aldridge B, Ladd AP, Kepple J, Wingle T, Ring C, Kokoska ER. Negative pressure wound therapy for initial management of giant omphalocele. Am J Surg. 2016;211:605-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117:121S-126S. [PubMed] [Cited in This Article: ] |
| 44. | Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563-576; discussion 577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1531] [Cited by in F6Publishing: 1325] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 45. | Clubley L, Harper L. Using negative pressure therapy for healing of a sternal wound. Nurs Times. 2005;101:44-46. [PubMed] [Cited in This Article: ] |
| 46. | O'Connor J, Kells A, Henry S, Scalea T. Vacuum-assisted closure for the treatment of complex chest wounds. Ann Thorac Surg. 2005;79:1196-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Fleck T, Moidl R, Grimm M, Wolner E, Zuckermann A. [Vacuum assisted closure therapy for the treatment of sternal wound infections after heart transplantation: preliminary results]. Zentralbl Chir. 2007;132:138-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Bettschart V, Vallet C, Majno P, Mentha G, Morel P, Gillet M, Mosimann F. Laparostomy with vacuum dressing after liver transplantation. Transplant Proc. 2002;34:777-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 49. | Terzi C, Egeli T, Canda AE, Arslan NC. Management of enteroatmospheric fistulae. Int Wound J. 2014;11 Suppl 1:17-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Richter S, Dold S, Doberauer JP, Mai P, Schuld J. Negative pressure wound therapy for the treatment of the open abdomen and incidence of enteral fistulas: a retrospective bicentre analysis. Gastroenterol Res Pract. 2013;2013:730829. [PubMed] [Cited in This Article: ] |
| 51. | Tavusbay C, Genc H, Cin N, Kar H, Kamer E, Atahan K, Haciyanli M. Use of a vacuum-assisted closure system for the management of enteroatmospheric fistulae. Surg Today. 2015;45:1102-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev. 2014;10:CD009261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Ubbink DT, Westerbos SJ, Evans D, Land L, Vermeulen H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008;CD001898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Dumville JC, Owens GL, Crosbie EJ, Peinemann F, Liu Z. Negative pressure wound therapy for treating surgical wounds healing by secondary intention. Cochrane Database Syst Rev. 2015;6:CD011278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Kim PJ, Attinger CE, Olawoye O, Crist BD, Gabriel A, Galiano RD, Gupta S, Lantis Ii JC, Lavery L, Lipsky BA. Negative Pressure Wound Therapy With Instillation: Review of Evidence and Recommendations. Wounds. 2015;27:S2-S19. [PubMed] [Cited in This Article: ] |
| 56. | Kim PJ, Attinger CE, Steinberg JS, Evans KK, Lehner B, Willy C, Lavery L, Wolvos T, Orgill D, Ennis W. Negative-pressure wound therapy with instillation: international consensus guidelines. Plast Reconstr Surg. 2013;132:1569-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Colli A, Camara ML. First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg. 2011;6:160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Hyldig N, Birke-Sorensen H, Kruse M, Vinter C, Joergensen JS, Sorensen JA, Mogensen O, Lamont RF, Bille C. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103:477-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 59. | Smallwood NR, Fleshman JW, Leeds SG, Burdick JS. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc. 2016;30:2473-2480. [PubMed] [Cited in This Article: ] |
| 60. | Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H, Schröder W. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc. 2014;28:896-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |