Abstract
Context:
The world health organization (WHO) recommends that all blood donations should be screened for evidence of infections, such as hepatitis B. The present study aimed to determine the prevalence of hepatitis B surface antigen (HBsAg) in blood donors at the eastern Mediterranean region office (EMRO) of the WHO and middle eastern countries.Evidence Acquisition:
A meta-analysis was carried out based on the results of an electronic literature search of PubMed, Ovid, Scopus, and Google Scholar for articles published from January 1, 2000, to August 31, 2015. In accordance with a significant homogeneity test and a large value of I2, the random effects model was used to aggregate data from the studies and produce the pooled estimates using the “Metan” command.Results:
We included 66 eligible studies. The pooled prevalence of HBsAg in blood donors of both EMRO and middle eastern (E and M) countries was 2.03% (95% confidence interval [CI]: 1.79 – 2.26). In addition, the prevalence rates in the EMRO countries was 1.99% (95% CI: 1.84 – 2.14) and 1.62% in the Middle Eastern countries (95% CI: 1.36 – 1.88). The prevalence among blood donors with more than one study was 1.58% in Egypt, 0.58% in Iran, 0.67% in Iraq, 2.84% in Pakistan, 3.02% in Saudi Arabia, 1.68% in Turkey, and 5.05% in Yemen.Conclusions:
Based on the WHO classification of hepatitis B virus (HBV) prevalence, the prevalence of HBsAg in blood donors from E and M countries reached an intermediate level. However, there were low prevalence levels in some E and M countries.Keywords
Prevalence Hepatitis B Eastern Mediterranean Middle East Meta-Analysis
1. Context
Hepatitis B virus (HBV) is a public health problem and a major cause of mortality and morbidity (1). Almost 30% of the world population is infected with HBV, and more than 600,000 people die each year from acute disease or chronic sequelae secondary to HBV infection. Hepatitis B surface antigen (HBsAg) is the earliest marker of acute infection and is helpful for the diagnosis of HBV infection. The carrier rate varies from 0.1 to 20% for this marker in different communities (2, 3).
Blood donation is an important, lifesaving intervention. The world health organization (WHO) recommends that all blood donations should be screened for evidence of infections, such as human immunodeficiency virus (HIV), hepatitis B and C, and syphilis. Information provided by 164 countries to the WHO global database on blood safety showed that worldwide, more than 92 million blood samples are donated annually. Of these, an estimated 1.6 million units are excluded due to the presence of infectious markers, including HBsAg (4, 5). In the multiple countries where pre-transfusion screening of blood donations for HBsAg is conducted systematically, and the high-risk groups are rejected from blood donation, the prevalence of HBV in blood donors is less than that in the general population; this results in underestimation of the extent of this issue. However, the risk of transmission still exists in several developing countries (3, 6).
The eastern Mediterranean regional office (EMRO) is a one of the six regional offices of the WHO around the world, consisting of 22 member states with a total population of 605 million individuals (7). It is estimated that about 4.3 million people are infected with HBV in this region (8). Unsafe blood transfusion and poor public health awareness are the major risk factors for HBV infection in this part of the world (1). The WHO has defined prevalences of < 2%, 2 – 8%, and > 8% as low, intermediate, and high prevalences of HBV, respectively (2). In the EMRO and some Middle Eastern countries, low–intermediate and high prevalence of HBV have been reported across all age groups (1, 9).
Several studies conducted in the EMRO and middle eastern (E and M) countries have investigated the prevalence of HBsAg positivity in blood donors and have reported different values. A study conducted on approximately 15 million Iranian blood donors over 10 years showed that about 1% of blood donors were HBsAg positive, and the prevalence declined over the study period (10). Another large-scale study conducted by Mohammadali et al. (2014) (11) over 6 years showed that only 0.5% of blood donors were HBsAg positive in Iran. A study performed on more than six million Turkish blood donors reported that more than 4% of this population was HBsAg positive, while the study by Tigen et al. (2015) (12) showed an HBsAg prevalence of 1.5% in Turkish blood donors (13). The prevalence increases in the blood donors of Djibouti (14) and Pakistan (15), with rates up to 10% and 6%, respectively.
There have been several single-center studies on the prevalence of HBsAg positivity in blood donors from E and M countries, but they have reported different estimates. In addition, there are no comprehensive and reliable data with a large sample size on the prevalence of HBsAg positivity in these regions. Therefore, the present study aimed to determine the prevalence of HBsAg positivity in blood donors of E and M countries using a systematic review and meta-analysis of published cross-sectional studies during 2000 – 2015.
2. Evidence Acquisition
2.1. Search Strategy
An electronic literature search through two MEDLINE and EMbase databases (PubMed and Ovid), Scopus, and Google Scholar was carried out for articles published from January 1, 2000, to August 31, 2015, using different combinations of the following keywords in titles and/or abstracts: “Prevalence,” “Epidemiology,” “Hepatitis B virus,” “HBV,” “HBsAg,” and “Blood donors.” These keywords were combined with the names of the following E and M countries: Afghanistan, Bahrain, Cyprus, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, Turkey, United Arab Emirates, and Yemen. Iranian databases, such as IranMedex, Magiran, Medlib, and the scientific information database, were also searched using relevant English and Persian keywords. PakMediNet was searched for related epidemiological studies. The eastern Mediterranean journals from the WHO–EMRO indexing site were also searched. Search sensitivity was assessed by considering overlapping articles. If the full text of articles was not available, emails were sent to authors. If the authors did not respond after 1 month, informative abstracts were used for data extraction. Articles with no informative abstracts were excluded.
2.2. Study Selection
Published studies in English, Persian, French, and Arabic were considered if they met the following criteria: 1) the studies were cross-sectional and 2) had clearly declared information on the number of HBsAg-positive cases among healthy blood donors from E and M countries. The exclusion criteria were as follows: 1) studies on blood donors who had positive HBsAg, 2) studies reporting confusing data or probable errors, and 3) studies with fewer than 1,000 samples. Studies without any information on the country were retained for reviewing. Author name or journal title had no effect on the choice to exclude or include the articles.
2.3. Data Extraction and Quality Assessment
Data extraction was conducted separately by one researcher (M.B.), and critical evaluation for quality assessment of the articles was performed by another researcher who was not involved in the literature search (S.M.A.). A meeting was scheduled before the critical evaluation, and researchers were justified about the questions. After the critical evaluation, the selected articles were checked by both the authors. The selected and included citations were reviewed, and data were extracted to excel spreadsheets, which included the first author’s name, year of publication, name of country, sample size, male percentage, and HBsAg prevalence and its standard error (SE). If there were other parameters reported besides SE, such as standard deviation, confidence interval, and/or P value, a suitable modification was performed to calculate SE. SE was estimated using the following formula: SE = √(P × (1 − P)/N) (P = prevalence, N = sample size).
2.4. Statistical Analysis
The SE of HBsAg prevalence in each study was estimated based on the binomial distribution formula. Cochran’s Q-test of heterogeneity was performed to detect homogeneity among the studies. Higgins and Thompson’s I2 was also applied due to the inherent limitations of Cochran’s Q in detecting true heterogeneity. Depending on whether homogeneity or a large value of I2 was found, a fixed or random effects model, respectively, was used to aggregate data from studies and produce the pooled estimates, with the “Metan” command. Results were provided as forest plots with description of the findings in the plots, and the point estimations and their 95% confidence intervals (CIs) were computed accordingly. The analyses were performed using Stata software, version 11.
3. Results
Based on the data gathered from titles and abstracts, out of 4,125 citations, 86 of them were potentially related to HBsAg prevalence in blood donors from E and M countries. All of the papers were carefully examined to avoid including duplicate papers; two studies with duplicate data from the same group were excluded (16, 17). Four studies were excluded because they were not original (18-21). We found three publications that were not available online, and despite contacting their authors or publishers, we could not obtain their abstracts (22-24). In addition, two papers reporting confusing or insufficient data were removed (25, 26). There were nine studies with sample sizes of less than 1,000 people from Egypt, Iran, Pakistan, Somalia, Sudan, and Yemen that we excluded from the meta-analysis to avoid the effect of lower sample size (27-35). However, because we had no information in our meta-analysis on the HBsAg prevalence in Somalia or Sudan, we reviewed three studies that reported the HBsAg prevalence in these two countries (33-35) (Figure 1). Therefore, the remaining 66 studies, involving 28,947,262 blood donors and assessing HBsAg prevalence in E and M countries, were considered for the meta-analysis. Out of these, 18 studies related to Iran (10, 11, 36-51), 14 to Pakistan (15, 52-64), 10 to Turkey (12, 13, 65-72), 7 to Egypt (73-79), 7 to Saudi Arabia (80-86), 2 to Iraq (87, 88), and 2 to Yemen (89, 90). There was one study each from other countries, which included Cyprus (91), Djibouti (14), Jordan (92), Kuwait (93), Lebanon (94), and Morocco (95). There were no data available for the following countries: Afghanistan, Bahrain, Libya, Oman, Palestine, Qatar, Saudi Arabia, Syria, Tunisia, and the United Arab Emirates.
Diagram of the Systematic Review and Meta-Analysis of HbsAg Prevalence in the EMRO and Middle Eastern Regions

3.1. Total HBsAg Prevalence in E and M Countries
Information on HBsAg prevalence in blood donors was available from 13 countries. Pooled prevalence of HBsAg in blood donors of all E and M countries was 2.03% (95% CI: 1.79 – 2.26). When these countries were divided into groups, the prevalence in the EMRO countries was 1.99% (95% CI: 1.84 – 2.14), and that in Middle Eastern countries was 1.62% (95% CI: 1.36 – 1.88; Tables 1 - 3).
Description of Studies in Eastern Mediterranean Region Office (EMRO) and Middle Eastern Countries That Met Our Eligibility Criteria
| Country | Pub. year | Male, % | Sample Size | HBsAg Prevalence (95% CI) |
|---|---|---|---|---|
| Cyprus (M) | ||||
| Altindis, M. | 2006 | NR | 5,057 | 3 (2.54 – 3.45) |
| Djibouti (E) | ||||
| Dray, X. | 2005 | NR | 9,006 | 10.4 (10.2 – 10.59) |
| Egypt (E and M) | ||||
| Eissa, S. A. | 2007 | NR | 99,757 | 1.3 (1.23 – 1.35) |
| El-Gilany, A. H. | 2006 | 62.6 | 2,157 | 4.31 (3.46 – 5.15) |
| Habil, F. E. | 2013 | NR | 12,000 | 1.98 (1.74 – 2.21) |
| Hussein, E. | 2014 | NR | 308,762 | 1.22 (1.18 – 1.25) |
| Ismail, A. M. | 2009 | 93 | 55,922 | 1.3 (1.22 – 1.37) |
| Khattab, M. A. | 2009 | 85 | 211,772 | 1.65 (1.59 – 1.7) |
| Wasfi, O. A. S. | 2011 | NR | 3,420 | 1.4 (1.00 – 1.79) |
| Iran (E and M) | ||||
| Afzali, H. | 2002 | 90 | 43,731 | 0.62 (0.54 – 0.69) |
| Aghamohamad, A. | 2014 | NR | 124,704 | 0.24 (0.21 – 0.26) |
| Kafi-Abad, S. A. | 2009 | 91 | 14,599,783 | 0.96 (0.95 – 0.96) |
| Bozorgi, S. H. | 2012 | 92 | 20,591 | 0.23 (0.17 – 0.28) |
| Mansour Ghanaei, F. | 2008 | 97.8 | 221,508 | 0.45 (0.42 – 0.47) |
| Ghavanini, A. A. | 2000 | NR | 7,879 | 1.07 (0.85 – 1.28) |
| Emamghorashi | 2006 | 93 | 3,000 | 0.4 (0.18 – 0.61) |
| Jabbari, H. | 2008 | 95.7 | 5,409 | 2.4 (2.00 – 2.79) |
| Karimi, A. | 2008 | NR | 35,124 | 0.1 (0.07 – 0.14) |
| Kasraian, L. | 2012 | NR | 96,646 | 0.27 (0.213 – 0.3) |
| Khedmat, H. | 2007 | NR | 318,029 | 0.52 (0.49 – 0.54) |
| Khedmat, H. | 2009 | NR | 1,004,889 | 0.6 (0.58 – 0.61) |
| Maneshi, H. O. | 2010 | NR | 51,884 | 0.47 (0.41 – 0.52) |
| Masaeli, Z. | 2006 | 90 | 29,458 | 0.5 (0.42 – 0.58) |
| Mohammadali, F. | 2014 | NR | 2,026,628 | 0.38 (0.38 – 0.39) |
| Samadi, M. | 2014 | 96 | 2,108 | 0.19 (0.17 – 0.21) |
| Vahid, T. | 2005 | NR | 39,598 | 1.08 (0.98 – 1.18) |
| Sorouri Zanjani, R. | 2013 | 89 | 29,716 | 0.35 (0.28 – 0.41) |
Description of Studies in Eastern Mediterranean Region Office (EMRO) and Middle Eastern Countries That Met Our Eligibility Criteria
| Country | Pub. year | Male, % | Sample Size | HBsAg Prevalence (95% CI) |
|---|---|---|---|---|
| Iraq (E and M) | ||||
| Al-Juboury, A. | 2010 | 99 | 23,336 | 0.73 (0.62 – 0.83) |
| Ataallah, T. M. | 2011 | 97 | 495,648 | 0.66 (0.63 – 0.68) |
| Jordan (E and M) | ||||
| Al-Rashed, M. | 2003 | 93 | 18,000 | 1.72 (1.53 – 1.9) |
| Kuwait (E and M) | ||||
| Ameen, R. | 2005 | NR | 12,798 | 1.92 (1.68 – 2.15) |
| Lebanon (E and M) | ||||
| Irani-Hakime, N. | 2006 | 93 | 16,084 | 0.92 (0.77 – 1.07) |
| Morocco (E) | ||||
| Baha, W. | 2013 | 79 | 169,605 | 0.95 (0.9 – 0.99) |
| Pakistan (E) | ||||
| Akhtar, S. | 2005 | 100 | 351,309 | 2 (1.95 – 2.04) |
| Asif, N. | 2004 | NR | 3,430 | 2.4 (1.89 – 2.91) |
| Attaullah, S. | 2012 | NR | 127,828 | 2.68 (2.59 – 2.76) |
| Bangash, M. H. | 2009 | NR | 1,300 | 5.07 (3.89 – 6.24) |
| Bhatti, F. A. | 2007 | NR | 94,177 | 2.16 (2.06 – 2.25) |
| Chaudhry, M. A. | 2013 | 95 | 2,155 | 1.34 (0.87 – 1.81) |
| Farooqi, J. I. | 2007 | NR | 166,189 | 2.54 (2.46 – 2.61) |
| Ijaz, R. | 2012 | NR | 3,652 | 4 (3.37 – 4.62) |
| Khan, N. U. | 2010 | NR | 7,148 | 2.05 (1.71 – 2.38) |
| Khattak, M. F. | 2002 | NR | 103,858 | 3.31 (3.2 – 3.41) |
| Abdul Mujeeb, S. | 2008 | 99 | 5,345 | 6.2 (5.55 – 6.84) |
| Abdul Mujeeb, S. | 2006 | 98 | 7,325 | 4.7 (4.23 – 5.17) |
| Zaheer, H. A. | 2014 | 99.6 | 160,376 | 2.35 (2.27 – 2.42) |
| Rahman, M. U. | 2002 | NR | 1,021,505 | 2.25 (2.22 – 2.27) |
Description of Studies in Eastern Mediterranean Region Office (EMRO) and Middle Eastern Countries That Met Our Eligibility Criteria
| Country | Pub. year | Male, % | Sample Size | HBsAg Prevalence (95% CI) |
|---|---|---|---|---|
| Saudi Arabia (E and M) | ||||
| Bashawri, L. A. | 2004 | NR | 13,443 | 2.28 (2.04 – 2.51) |
| El Beltagy, K. E. | 2008 | 100 | 3,192 | 3 (2.41 – 3.58) |
| El-Hazmi, M. M. | 2004 | 99 | 24,173 | 1.53 (1.37 – 1.68) |
| Mohammed Abdullah, S. | 2013 | NR | 29,949 | 3.8 (3.58 – 4.01) |
| Al-Bahrani, A. | 2001 | NR | 95,539 | 3.27 (3.15 – 3.38) |
| Ayoola, A. E. | 2003 | NR | 14,883 | 5.4 (5.04 – 5.75) |
| Panhotra, B. R. | 2005 | NR | 26,606 | 1.9 (1.73 – 2.06) |
| Turkey (M) | ||||
| Acar, A. | 2010 | NR | 72,695 | 1.78 (1.68 – 1.87) |
| Akalin, S. | 2011 | NR | 50,521 | 0.97 (0.88 – 1.05) |
| Gurol, E. | 2006 | NR | 6,240,130 | 4.19 (4.17 – 4.2) |
| Kader, C. | 2010 | 95 | 16,362 | 0.5 (0.4 – 0.59) |
| Oner, S. | 2011 | 96.3 | 30,716 | 2.2 (2.04 – 2.35) |
| Sakarya, S. | 2004 | 96.5 | 37,866 | 1.5 (1.38 – 1.61) |
| Tigen, E. T. | 2015 | 93.9 | 68,393 | 1.54 (1.44 – 1.63) |
| Turan, H. | 2011 | 93.5 | 17,071 | 1.53 (1.34 – 1.71) |
| Mese, S. | 2013 | 97 | 6,200 | 0.3 (0.16 – 0.43) |
| Mutlu, B. | 2004 | NR | 29,049 | 2.3 (2.12 – 2.47) |
| Yemen (E and M) | ||||
| Alodini, A. Q. | 2014 | NR | 3,000 | 2.1 (1.59 – 2.61) |
| Haidar, N. A. | 2002 | NR | 7,868 | 9.8 (9.15 – 10.44) |
| Pooled prevalence (EMRO and middle eastern countries)a | - | - | 28,947,262 | 2.03 (1.79–2.26)b |
3.2. Total HBsAg Prevalence in the E and M Region by Country
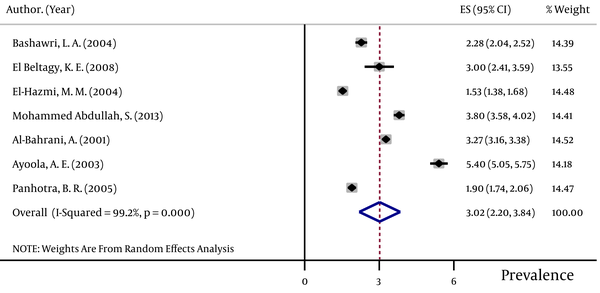
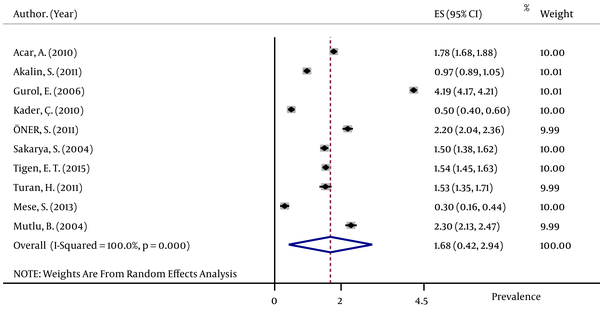
The pooled HBsAg prevalences in blood donors considered in more than one study were 1.58% (95% CI: 1.39 – 1.78) in Egypt (Figure 2), 0.58% (95% CI: 0.4 – 0.76) in Iran (Figure 3), 0.67% (95% CI: 0.61 – 0.73) in Iraq, 2.84% (95% CI: 2.62 – 3.06) in Pakistan (Figure 4), 3.02% (95% CI: 2.2 – 3.84) in Saudi Arabia (Figure 5), 1.68% (95% CI: 0.42 – 2.94) in Turkey (Figure 6), and 5.05% (95% CI: 4.64 – 5.44) in Yemen. The prevalences in countries considered in only one published article were 3% (95% CI: 2.54 – 3.45) in Cyprus, 10.4% (95% CI: 10.2 – 10.59) in Djibouti, 1.72% (95% CI: 1.53 – 1.9) in Jordan, 1.92% (95% CI: 1.68 – 2.15) in Kuwait, 0.92% (95% CI: 0.77 – 1.07) in Lebanon, and 0.95% (95% CI: 0.9 – 0.99) in Morocco.
Forest Plot of HBsAg Prevalence in Egypt
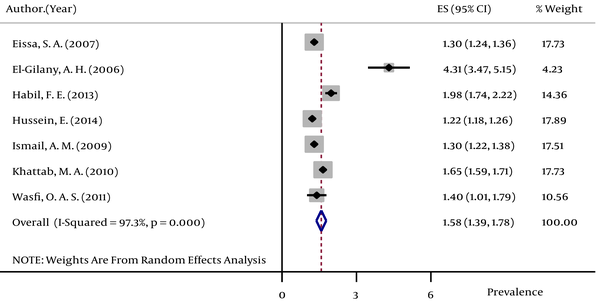
Forest Plot of HBsAg Prevalence in Iran
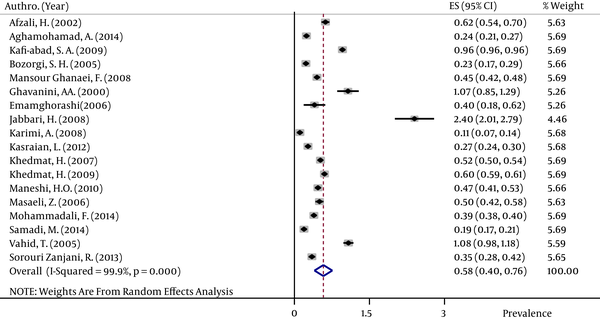
Forest Plot of HBsAg Prevalence in Pakistan
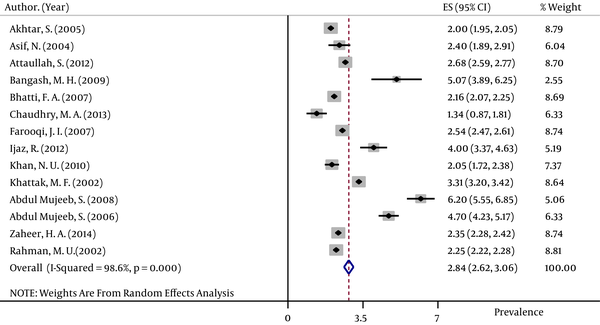
Forest Plot of HBsAg Prevalence in Saudi Arabia
Forest Plot of HBsAg Prevalence in Turkey
3.3. Review of Three Single Studies on HBsAg Prevalences in Somalia and Sudan With Fewer Than 1,000 Blood Donors
One study from Somalia showed that among 115 healthy blood donors, 22 (19.1%) were HBsAg positive (35). Two other studies from Sudan reported that among 400 and 260 male blood donors, 25 (6.25%) and 26 (10%) donors were positive for HBsAg, respectively (33, 34).
4. Conclusions
The present study showed that the pooled prevalence of HBsAg in blood donors of E and M countries was 2.03% (95% CI: 1.79 – 2.26), which is considered an intermediate prevalence as defined by the WHO. The prevalence in Iran was 0.58%, which was lower than that in other E and M countries.
Based on the world bank list of middle-income countries, among the 13 E and M countries from which data were used in our meta-analysis, 10 countries are members of middle-income countries (96). The WHO has estimated the prevalence of HBV in blood donors of middle-income countries to be in the range of 0.19 to 2.33%, which is comparable with our results (6). The results of two individual studies on 7,277 and 3,292 blood donors in the Democratic Republic of the Congo showed that 9.2% (97) and 3.7% (98) of blood donors were found to be positive for HBsAg, respectively. In addition, the prevalences of HBsAg in blood donors were 18.6% in Nigeria (99), 12% in Ghana (100), 10.1% in Equatorial Guinea (101), 4.7% in Ethiopia (102), and 14.96% in Burkina Faso (103), which shows that the prevalence of HBsAg in most of the African countries was higher than that observed in our meta-analysis of E and M countries. The prevalence of HBsAg among blood donors of the WHO south-east Asian regions showed a different pattern. The values were 1.09% (104), 1.27% (105), and 1.7% (106) in India; 1.4% in Bangladesh (107); 0.53% in Nepal (108); and 2.6% in Thailand (109), which indicate that the range of available data in these regions is somewhat comparable with the prevalence in E and M countries. A systematic review in 24 European countries (110) showed that the HBsAg prevalence in first-time blood donors was 0.74%. In addition, another study conducted on more than 1 million blood donors in the United States (111) showed that the prevalence was only 0.04%, indicating that the infection rates in Europe and US were lower than that in our region. Overall, it seems that the discussed differences in HBsAg prevalence in blood donors reflect a disparity in prevalence among the population who are suitable to donate blood, the types of donors (such as voluntary unpaid blood donors from lower risk groups), and the quality of blood processing (6).
According to our results, the pooled estimate of HBsAg infection was higher in other E and M countries than Iran, which exhibited a prevalence of 0.58% in its blood donors, while the prevalence increased for Yemen and Djibouti, by up to 5.05% and 10.4%, respectively. This lower prevalence in Iran can be attributed to the effectiveness of the screening system, the selection of eligible donors, and the high vaccination coverage for HBV in the first years of life (11, 112). In addition, low public awareness, particularly for the general population (113), unsafe services for blood donation (114), and a higher rate of HBsAg infection in the general population (115) may contribute to the higher prevalence of HBsAg in other E and M countries.
The strength of the current study was that the search strategy was performed with high sensitivity with all related keywords to include most of the available published studies. In addition, the large sample size of this study was another strength; it included 28,947,262 blood donors from E and M countries.
The first limitation of this study was that the quantity and quality of data varied by country. To decrease the effect of studies with low sample sizes on the estimated prevalence, we excluded studies with fewer than 1,000 samples. Another limitation of this study was that some databases, such as web of science, were not searched due to sanction limitations in Iran. In addition, university research projects and students’ theses were not included in our search.
Based on the WHO classification of HBV prevalence, the prevalence of HBsAg in blood donors of E and M countries represented an intermediate level (2.03%), while the individual prevalence rates in each of the EMRO (1.99%) and middle eastern (1.62%) countries was low. The available data showed a wide disparity in the prevalence of HBsAg among the E and M countries. The lowest prevalence of HBsAg was seen for Iranian blood donors, while the highest rate was observed in Djiboutian blood donors.
Acknowledgements
References
-
1.
Alavian SM, Fallahian F, Lankarani KB. The changing epidemiology of viral hepatitis B in Iran. J Gastrointestin Liver Dis. 2007;16(4):403-6. [PubMed ID: 18193122].
-
2.
2015. Hepatitis B. Available from:http://www.who.int/mediacentre/factsheets/fs204/en/.
-
3.
Emergencies preparedness, response. Hepatitis B. Disease. 2015, [cited 2/11/2015]. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en.
-
4.
Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415-26. [PubMed ID: 17679019]. https://doi.org/10.1016/S0140-6736(07)61197-0.
-
5.
2012.World Health Organization. Global Database on Blood Safety: Summary Report 2011.
-
6.
World Health Organization. Blood safety and availability. 2015, [cited 2/12/2015]. Available from: http://www.who.int/mediacentre/factsheets/fs279/en/.
-
7.
New York;
-
8.
2009. The growing threats of hepatitis B and C in the Eastern Mediterranean region: a call for action. Available from: http://applications.emro.who.int/docs/EM_RC56_3_en.pdf.
-
9.
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212-9. [PubMed ID: 22273662]. https://doi.org/10.1016/j.vaccine.2011.12.116.
-
10.
Kafi-abad SA, Rezvan H, Abolghasemi H. Trends in prevalence of hepatitis B virus infection among Iranian blood donors, 1998-2007. Transfus Med. 2009;19(4):189-94. [PubMed ID: 19708860]. https://doi.org/10.1111/j.1365-3148.2009.00935.x.
-
11.
Mohammadali F, Pourfathollah AA. Changes in frequency of HBV, HCV, HIV and syphilis infections among blood donors in Tehran province 2005 - 2011. Arch Iran Med. 2014;17(9):613-20. [PubMed ID: 25204477].
-
12.
Tigen ET, Dogru A, Karadag FY. Hepatitis B, Hepatitis C and human immunodeficiency virus prevalences among first time blood donors in Istanbul, Turkey, 2004-2011. Transfus Apher Sci. 2015;53(2):176-9. [PubMed ID: 25881737]. https://doi.org/10.1016/j.transci.2015.03.013.
-
13.
Gurol E, Saban C, Oral O, Cigdem A, Armagan A. Trends in hepatitis B and hepatitis C virus among blood donors over 16 years in Turkey. Eur J Epidemiol. 2006;21(4):299-305. [PubMed ID: 16685581]. https://doi.org/10.1007/s10654-006-0001-2.
-
14.
Dray X, Dray-Spira R, Bronstein JA, Mattera D. Prevalences of HIV, hepatitis B and hepatitis C in blood donors in the Republic of Djibouti [in Frecch]. Med Trop (Mars). 2005;65(1):39-42. [PubMed ID: 15903075].
-
15.
Mujeeb SA, Pearce MS. Temporal trends in hepatitis B and C infection in family blood donors from interior Sindh, Pakistan. BMC Infect Dis. 2008;8:43. [PubMed ID: 18402660]. https://doi.org/10.1186/1471-2334-8-43.
-
16.
Vahid T, Kafaei J, Kabir A, Yektaparast B, Alavian SM. Hepatitis B prevalence and risk factors in blood donors in Ghazvin, Iran. Hakim Res J. 2005;1(8):8-15.
-
17.
Kafi-abad SA, Rezvan H, Abolghasemi H, Talebian A. Prevalence and trends of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus among blood donors in Iran, 2004 through 2007. Transfusion. 2009;49(10):2214-20. [PubMed ID: 19527477]. https://doi.org/10.1111/j.1537-2995.2009.02245.x.
-
18.
Adouani B, Alami R, Laouina A, Benahadi A, Boulahdid S, Mokhtari A, et al. Hepatitis B in Moroccan blood donors: a decade trend of the HBsAg prevalence in a resources limited country. Transfus Med. 2013;23(6):432-7. [PubMed ID: 23841654]. https://doi.org/10.1111/tme.12054.
-
19.
Khmmaj A, Habas E, Azabi M, Rayani A. Frequency of hepatitis B, C, and HIV viruses among blood donors in Libya. Libyan J Med. 2010;5. [PubMed ID: 21483567]. https://doi.org/10.3402/ljm.v5i0.5333.
-
20.
Kasraian L, Jahromi ST. Prevalence of major transfusion-transmissible viral infections in blood donors attending fars blood transfusion center, shiraz, southern Iran: 2002–05. Iran J Med Sci. 2015;32(2):114-7.
-
21.
Ramezani H, Bozorgi SH, Nooranipour M, Sadri M, Molaverdikhani S, Alavian SM. Successful exclusion of blood-borne viral disease in blood donors. Eur J Intern Med. 2011;22(6):e71-4. [PubMed ID: 22075316]. https://doi.org/10.1016/j.ejim.2011.07.002.
-
22.
Fawzi Z, Al Hilali A, Al Malki A, Al Matawa H, Yousef B, Ali Bin Ali A, et al. Survey of Hepatitis Markers Among Donors in the State of Qatar. Qatar Med J. 2007;2007(2):16. https://doi.org/10.5339/qmj.2007.2.16.
-
23.
Krichen CH, Rekik H, Feki H, Dammak J, Gargouri J. Prevalence of viral markers among blood donors in Tunisia. Clin Lab. 2001;47(9-10):509-16. [PubMed ID: 11596914].
-
24.
Nagi AM, Altyeb H, Ahmed A. Seroprevalence of Hepatitis B and C Viral Infections among blood donors in Shendi, River Nile State, Sudan. Res J Med Med Sci. 2007;2(2):122-6.
-
25.
Bani Aghil SS, Abbasi S, Arab M, Seyedein MS. The Prevalence of HCV, HBV, HIV in Blood Donors of Golestan Province, (2006-2008). Med Lab J. 2009;3(2).
-
26.
Khoufi MT, Mrabet A, Nsiri B, Ebdelli MN, Yedeas M. Epidemiology of hepatitis B in Tunisia: retrospective study in 198157 blood donors in military environment. BMC Infect Dis. 2014;14(Suppl 2):16. https://doi.org/10.1186/1471-2334-14-s2-p16.
-
27.
Al-Waleedi AA, Khader YS. Prevalence of hepatitis B and C infections and associated factors among blood donors in Aden City, Yemen. East Mediterr Health J. 2012;18(6):624-9. [PubMed ID: 22888620].
-
28.
El-Ghitany EM, Farghaly AG. Serological pattern of hepatitis B virus among Hbsag negative blood donors in Alexandria, Egypt. East Mediterr Health J. 2013;19(7):600-7. [PubMed ID: 24975302].
-
29.
El-Zayadi AR, Ibrahim EH, Badran HM, Saeid A, Moneib NA, Shemis MA, et al. Anti-HBc screening in Egyptian blood donors reduces the risk of hepatitis B virus transmission. Transfus Med. 2008;18(1):55-61. [PubMed ID: 18279193]. https://doi.org/10.1111/j.1365-3148.2007.00806.x.
-
30.
Moniri R, Mosayebii Z, Mossavi GA. Seroprevalence of cytomegalovirus, hepatitis B, hepatitis C and human immunodeficiency virus antibodies among volunteer blood donors. Iranian J Publ Health. 2004;33(4):38-42.
-
31.
Sallam TA, Tong CY, Cuevas LE, Raja'a YA, Othman AM, Al-Kharsa KR. Prevalence of blood-borne viral hepatitis in different communities in Yemen. Epidemiol Infect. 2003;131(1):771-5. [PubMed ID: 12948378].
-
32.
Abdul Mujeeb S, Aamir K, Mehmood K. Seroprevalence of HBV, HCV and HIV infections among college going first time voluntary blood donors. J Pak Med Assoc. 2000;50(8):269-70. [PubMed ID: 10992712].
-
33.
Abou MA, Eltahir YM, Ali AS. Seroprevalence of hepatitis B virus and hepatitis C virus among blood donors in Nyala, South Dar Fur, Sudan. Virol J. 2009;6:146. [PubMed ID: 19775459]. https://doi.org/10.1186/1743-422X-6-146.
-
34.
Elfaki A, Eldour A, Elsheikh N. Sero-prevalence of immunodeficiency virus, hepatitis B and C and syphilis among blood donors at ElObeid Teaching Hospital, West Sudan. Sudan J Med Sci. 2008;3(4):333-8.
-
35.
Nur YA, Groen J, Elmi AM, Ott A, Osterhaus AD. Prevalence of serum antibodies against bloodborne and sexually transmitted agents in selected groups in Somalia. Epidemiol Infect. 2000;124(1):137-41. [PubMed ID: 10722141].
-
36.
Afzali H, Ardakani AT, Vali GR. Seroepidemiology of hepatitis B and C in blood donors in Kashan, 1996-2001. Feyz J Kashan Unive Med Sci. 2002;6(3):43-50.
-
37.
Aghamohamad A, Montazeri M, Akbari M. Prevalence of hepatitis B and hepatitis C in blood donors at Semnan province from 2008 to 2011. Koomesh. 2014;15(2):Pe162-7. En23.
-
38.
Bozorgi SH, Ramezani H, Nooranipour M, Ahmadi M, Baghernejad A, Mostajeri A, et al. Risk factors of viral hepatitis: yet to explore. Transfus Apher Sci. 2012;47(2):145-9. [PubMed ID: 22858443]. https://doi.org/10.1016/j.transci.2012.06.023.
-
39.
Emam Ghoreyshi F, Fathi GE, Mohtashami A. Evaluation of demographic characteristics and hepatitis B, C and HIV prevalence among blood donors in Jahrom. SJIBTO. 2006;2:373-8.
-
40.
Ghavanini AA, Sabri MR. Hepatitis B surface antigen and anti-hepatitis C antibodies among blood donors in the Islamic Republic of Iran. East Mediterr Health J. 2000;6(5-6):1114-6. [PubMed ID: 12197336].
-
41.
Jabbari H, Karami S, Fattahi F, Jam S, Mohraz M. Seroprevalence of Human Immunodeficiency Virus, Hepatitis B and C Viruses Among Blood Donors in Chabahar Iran. Int J Infec Dis. 2008;12. eee424. https://doi.org/10.1016/j.ijid.2008.05.1242.
-
42.
Karimi A, Hoseini S. Seroprevalence of hepatitis B and C virus and HIV markers among blood donors from Shahre-Kord, Iran (2004-2006). Kuwait Med J. 2008;40(4):279-81.
-
43.
Kasraian L. The prevalence and risk factor of hepatitis B and D in Shiraz blood donors. Africa J Microbiol Res. 2012;6(18):3976-9. https://doi.org/10.5897/ajmr11.822.
-
44.
Khedmat H, Miri SM, Amini M, Abolghasemi H, Hajibeigi B, Alaeddini F, et al. Trends in seroprevalence of hepatitis B, hepatitis C, HIV, and syphilis infections in Iranian blood donors from 2003 to 2005. Hepat Mont. 2009;2009(1, Winter):24-8.
-
45.
Khedmat H, Fallahian F, Abolghasemi H, Alavian SM, Hajibeigi B, Miri SM, et al. Seroepidemiologic study of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and syphilis infections in Iranian blood donors. Pak J Biol Sci. 2007;10(24):4461-6. [PubMed ID: 19093512].
-
46.
Maneshi HO, Zare S, Karimi M, Hajiani G. HBV and HCV viral markers seroperevalence in first-time healthy blood donors refered to transfusion centers of bushehr province, South of Iran (April 2004 to March 2008). Retrovirology. 2010;7(Suppl 1):P151. https://doi.org/10.1186/1742-4690-7-s1-p151.
-
47.
Ghanaei MF, Falah MS, Jafarshad R, Joukar F, Salari A, Tavaf Zadeh R. Prevalence of hepatitis B and hepatitis C, and their risk factors among Guilan blood donors. Sci J Iran Blood Transfus Organ. 2008;4(5):331-6.
-
48.
Masaeli Z, Jaberi MR, Magsudlu M. A comparison of seroprevalence of blood-borne infections among regular, sporadic, and first-time blood donors in Isfahan. Sci J Iran Blood Trans Org. 2006;2(7):301-7.
-
49.
Samadi M, Ghasemzade AH, Sarizade G, Ebrahimi S, Saati S, Abassinejad-Pour A, et al. The comparison of the prevalence rates of HBV, HCV, and HIV in blood donors having deferred for high risk behaviors. Sci J Iran Blood Trans Org. 2014;10(4).
-
50.
Sorouri Zanjani R, Mazloomzadeh S, Koocheki A, Noori M. Prevalence of Hepatitis B, C and HIV Infection in Blood Donors in Zanjan, 2005-2006. Prevent Care Nurs Midwif J. 2013;3(1):56-63.
-
51.
Vahid T, Alavian SM, Kabir A, Kafaee J, Yektaparast B. Hepatitis B prevalence and risk factors in blood donors in Ghazvin, IR. Iran. Hepat Mon. 2005;5(4):117.
-
52.
Abdul Mujeeb S, Nanan D, Sabir S, Altaf A, Kadir M. Hepatitis B and C infection in first-time blood donors in Karachi--a possible subgroup for sentinel surveillance. East Mediterr Health J. 2006;12(6):735-41. [PubMed ID: 17333817].
-
53.
Akhtar S, Younus M, Adil S, Hassan F, Jafri SH. Epidemiologic study of chronic hepatitis B virus infection in male volunteer blood donors in Karachi, Pakistan. BMC Gastroenterol. 2005;5:26. [PubMed ID: 16086833]. https://doi.org/10.1186/1471-230X-5-26.
-
54.
Asif N, Khokhar N, Ilahi F. Sero-prevalence of HBV, HCV, and HIV infection among voluntary non remunerated & replacement donors in Northern Pakistan. Pakistan J Med Sci. 2004;20(1):24-8.
-
55.
Attaullah S, Khan S, Khan J. Trend of transfusion transmitted infections frequency in blood donors: provide a road map for its prevention and control. J Transl Med. 2012;10:20. [PubMed ID: 22293125]. https://doi.org/10.1186/1479-5876-10-20.
-
56.
Bangash MH, Bangash TH, Alam S. Prevalance of hepatitis B and hepatatis C among healthy blood donors at Kurram agency. J Postgrad Med Inst (Peshawar-Pakistan). 2011;23(2).
-
57.
Bhatti FA, Ullah Z, Salamat N, Ayub M, Ghani E. Anti-hepatits B core antigen testing, viral markers, and occult hepatitis B virus infection in Pakistani blood donors: implications for transfusion practice. Transfusion. 2007;47(1):74-9. [PubMed ID: 17207233]. https://doi.org/10.1111/j.1537-2995.2007.01066.x.
-
58.
Chaudhry MA, Malik JR, Ashraf MZ. Seropositivity of Hepatitis B and C in Blood Donors at CMH Lahore, Pakistan. APMC.
-
59.
Farooqi JI, Farooqi RJ, Khan N. Frequency of hepatitis B and C in selected groups of population in NWFP, Pakistan. J Postgrad Med Inst (Peshawar-Pakistan). 2011;21(3).
-
60.
Ijaz R, Bhatti S, Ullah S. Prevalence of hepatitis B and C in healthy blood donors in a peripheral hospital - Ghurki trust hospital, Lahore. Pakistan J Med Health Sci. 2012;6(3):568-9.
-
61.
Khan NU, Siddique L, Ali I, Iqbal A, Munir I, Rashid F, et al. Prevalence of hepatitis B in the blood donors of NW. FP and FATA regions and the current scenario of HBV infection in Pakistan. Africa J Biotech. 2013;9(37):6162-6.
-
62.
Khattak MF, Salamat N, Bhatti FA, Qureshi TZ. Seroprevalence of hepatitis B, C and HIV in blood donors in northern Pakistan. J Pak Med Assoc. 2002;52(9):398-402. [PubMed ID: 12532573].
-
63.
Rahman M, Akhtar GN, Lodhi Y. Transfusion transmitted HIV and HBV infections in Punjab Pakistan. Pakistan J Med Sci. 2002;18(1):18-25.
-
64.
Zaheer H, Saeed U, Waheed Y, Karimi S, Waheed U. Prevalence and Trends of Hepatitis B, Hepatitis C and Human Immunodeficiency Viruses among Blood Donors in Islamabad, Pakistan 2005-2013. J Blood Disorders Transf. 2014;5(217):2.
-
65.
Acar A, Kemahli S, Altunay H, Kosan E, Oncul O, Gorenek L, et al. HBV, HCV and HIV seroprevalence among blood donors in Istanbul, Turkey: how effective are the changes in the national blood transfusion policies? Brazil J Infect Dis. 2010;14(1):41-6.
-
66.
Akalin S, Baskan B, Sacar S, Sayin Kutlu S, Turgut H. Seroprevalence of HBsAg, Anti-HCV and RPR in Blood Donors in Denizli, Turkey. Klimik Dergisi/Klimik J. 2011;24(2):101-4. https://doi.org/10.5152/kd.2011.24.
-
67.
Kader C, Erbay A, Birengel S, Gurbuz M. Seroprevalence of Hepatitis B Virus, Hepatitis C Virus, Human Immunodeficiency Virus Infections and Syphilis in Blood Donors. Klimik Dergisi/Klimik J. 2010;23(3):95-9. https://doi.org/10.5152/kd.2010.27.
-
68.
Mese S, Nergiz S, Tekes S, Gul K. Seroprevalence of serum HBsAg positivity and hepatitis delta virus infection among blood donors in Southeastern Turkey. Clin Ter. 2014;165(2):95-8. [PubMed ID: 24770811]. https://doi.org/10.7471/CT.2014.1683.
-
69.
Mutlu B, Meric M, Willke A. [Seroprevalence of hepatitis B and C virus, human immunodeficiency virus and syphilis in the blood donors]. Mikrobiyol Bul. 2004;38(4):445-8. [PubMed ID: 15700672].
-
70.
Oner S, Yapici G, Sasmaz CT, Kurt AO, Bugdayci R. Hepatitis B, hepatitis C, HIV, and VDRL seroprevalence of blood donors in Mersin, Turkey. Turk J Med Sci. 2011;41(2):335-41.
-
71.
Sakarya S, Oncu S, Ozturk B, Oncu S. Effect of preventive applications on prevalence of hepatitis B virus and hepatitis C virus infections in West Turkey. Saudi Med J. 2004;25(8):1070-2. [PubMed ID: 15322600].
-
72.
Turan H, Serefhanoglu K, Kanat-Unler G, Arslan H. Seroprevalance of HBsAg and Anti-HCV and Their Correlation to Age and Gender in Blood Donors in the Province of Konya. Klimik Dergisi/Klimik J. 2011;24(1):36-9. https://doi.org/10.5152/kd.2011.07.
-
73.
Eissa SA, Abdel Meguid LM, Ebeid SM, Abou Elfetouh RM, Abdel Moneim GM. National Cancer Institute Experience in Healthy Egyptian Blood Donors as Regards Blood Group Frequencies and Seroprevalence of Hepatitis B Virus, Hepatitis C Virus&HIV: 10 Year Evaluation. J Egypt Natl Canc Inst. 2007;19(1):71-6. [PubMed ID: 18839037].
-
74.
El-Gilany AH, El-Fedawy S. Bloodborne infections among student voluntary blood donors in Mansoura University, Egypt. East Mediterr Health J. 2006;12(6):742-8. [PubMed ID: 17333818].
-
75.
Habil FE, Mahdi WK, Abdelwahab SF, Abdel-Hamid M. Hepatitis B virus genotype D predominates HBsAg-positive egyptian blood donors and is mainly associated with a negative HBeAg serostatus. Intervirology. 2013;56(5):278-83. [PubMed ID: 23887183]. https://doi.org/10.1159/000353105.
-
76.
Hussein E. Blood donor recruitment strategies and their impact on blood safety in Egypt. Transfus Apher Sci. 2014;50(1):63-7. [PubMed ID: 24325889]. https://doi.org/10.1016/j.transci.2013.11.005.
-
77.
Ismail AM, Ziada HN, Sheashaa HA, Shehab El-Din AB. Decline of viral hepatitis prevalence among asymptomatic Egyptian blood donors: a glimmer of hope. Eur J Intern Med. 2009;20(5):490-3. [PubMed ID: 19712851]. https://doi.org/10.1016/j.ejim.2009.03.005.
-
78.
Khattab MA, Eslam M, Sharwae MA, Hamdy L. Seroprevalence of hepatitis C and B among blood donors in Egypt: Minya Governorate, 2000-2008. Am J Infect Control. 2010;38(8):640-1. [PubMed ID: 20400204]. https://doi.org/10.1016/j.ajic.2009.12.016.
-
79.
Wasfi OA, Sadek NA. Prevalence of hepatitis B surface antigen and hepatitis C virus antibodies among blood donors in Alexandria, Egypt. East Mediterr Health J. 2011;17(3):238-42. [PubMed ID: 21735965].
-
80.
Al-Bahrani A, Panhotra BR. Prevalence of HBsAG and ANTI-HCV antibodies in blood donors of the Al-Hasa Region of Saudi Arabia. Ann Saudi Med. 2001;21(3-4):234-5. [PubMed ID: 17264563].
-
81.
Ayoola AE, Tobaigy MS, Gadour MO, Ahmad BS, Hamza MK, Ageel AM. The decline of hepatitis B viral infection in South-Western Saudi Arabia. Saudi Med J. 2003;24(9):991-5. [PubMed ID: 12973485].
-
82.
Bashawri LA, Fawaz NA, Ahmad MS, Qadi AA, Almawi WY. Prevalence of seromarkers of HBV and HCV among blood donors in eastern Saudi Arabia, 1998-2001. Clin Lab Haematol. 2004;26(3):225-8. [PubMed ID: 15163322]. https://doi.org/10.1111/j.1365-2257.2004.00601.x.
-
83.
El Beltagy KE, Al Balawi IA, Almuneef M, Memish ZA. Prevalence of hepatitis B virus markers among blood donors in a tertiary hospital in Tabuk, northwestern Saudi Arabia. Int J Infect Dis. 2008;12(5):495-9. [PubMed ID: 18400539]. https://doi.org/10.1016/j.ijid.2008.01.010.
-
84.
El-Hazmi MM. Prevalence of HBV, HCV, HIV-1, 2 and HTLV-I/II infections among blood donors in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004;25(1):26-33. [PubMed ID: 14758374].
-
85.
Mohammed Abdullah S. Prevalence of hepatitis B and C in donated blood from the jazan region of saudi arabia. Malays J Med Sci. 2013;20(2):41-6. [PubMed ID: 23983576].
-
86.
Panhotra BR, Al-Bahrani A, Ul-Hassan Z. Epidemiology of antibody to hepatitis B core antigen screening among blood donors in Eastern Saudi Arabia. Need to replace the test by HBV DNA testing. Saudi Med J. 2005;26(2):270-3. [PubMed ID: 15770304].
-
87.
Al-Juboury AWF, Salih H, Al-Assadi MK, Ali AM. Seroprevalence of Hepatitis B and C among blood donors in Babylon Governorate-Iraq. Med J Babylon. 2010;7(1-2):121-9.
-
88.
Ataallah TM, Hanan KA, Maysoun KS, Sadoon AA. Prevalence of hepatitis B and C among blood donors attending the National Blood Transfusion Center in Baghdad, Iraq from 2006-2009. Saudi Med J. 2011;32(10):1046-50. [PubMed ID: 22008925].
-
89.
Alodini AQ. Prevalence of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) infections among blood donors at Al-Thawra Hospital Sana'a City-Yemen. Yemen J Med Sci. 2014;6.
-
90.
Haidar NA. Prevalence of hepatitis B and hepatitis C in blood donors and high risk groups in Hajjah, Yemen Republic. Saudi Med J. 2002;23(9):1090-4. [PubMed ID: 12370719].
-
91.
Altindis M, Yilmaz S, Dikengil T, Acemoglu H, Hosoglu S. Seroprevalence and genotyping of hepatitis B, hepatitis C and HIV among healthy population and Turkish soldiers in Northern Cyprus. World J Gastroenterol. 2006;12(42):6792-6. [PubMed ID: 17106927].
-
92.
Münzer ALR. The prevalance of hepatitis b in blood donors in the middle region of Jordan. Gulhane Tip Dergsi (Gtd) Gulhane Med J(Gmj). 2003:153.
-
93.
Ameen R, Sanad N, Al-Shemmari S, Siddique I, Chowdhury RI, Al-Hamdan S, et al. Prevalence of viral markers among first-time Arab blood donors in Kuwait. Transfusion. 2005;45(12):1973-80. [PubMed ID: 16371052]. https://doi.org/10.1111/j.1537-2995.2005.00635.x.
-
94.
Irani-Hakime N, Musharrafieh U, Samaha H, Almawi WY. Prevalence of antibodies against hepatitis B virus and hepatitis C virus among blood donors in Lebanon, 1997-2003. Am J Infect Control. 2006;34(4):241-3. [PubMed ID: 16679184]. https://doi.org/10.1016/j.ajic.2005.06.009.
-
95.
Baha W, Foullous A, Dersi N, They-they TP, El alaoui K, Nourichafi N, et al. Prevalence and risk factors of hepatitis B and C virus infections among the general population and blood donors in Morocco. BMC Public Health. 2013;13:50. [PubMed ID: 23331910]. https://doi.org/10.1186/1471-2458-13-50.
-
96.
Country and Lending Groups. [cited 2015 12/12/2015]. Available from: http://data.worldbank.org/about/country-and-lending-groups.
-
97.
Mbendi Nlombi C, Longo-Mbenza B, Mbendi Nsukini S, Muyembe Tamfum JJ, Situakibanza Nanituma H, Vangu Ngoma D. Prevalence of HIV and HBs antigen in blood donors. Residual risk of contamination in blood recipients in East Kinshasa, Democratic Republic of the Congo [in French]. Med Trop (Mars). 2001;61(2):139-42. [PubMed ID: 11582869].
-
98.
Namululi BA, Guerrieri C, Dramaix MW. [Prevalence and incidence of HIV and hepatitis B among blood donors and estimated residual risk of transmission of HIV and HBV virus by blood transfusion. A study at the Provincial General Referee Hospital Bukavu, Democratic Republic of the Congo]. Rev Epidemiol Sante Publique. 2013;61(2):139-44. [PubMed ID: 23498094]. https://doi.org/10.1016/j.respe.2012.09.005.
-
99.
Buseri FI, Muhibi MA, Jeremiah ZA. Sero-epidemiology of transfusion-transmissible infectious diseases among blood donors in Osogbo, south-west Nigeria. Blood Transfus. 2009;7(4):293-9. [PubMed ID: 20011640]. https://doi.org/10.2450/2009.0071-08.
-
100.
Dongdem JT, Kampo S, Soyiri IN, Asebga PN, Ziem JB, Sagoe K. Prevalence of hepatitis B virus infection among blood donors at the Tamale Teaching Hospital, Ghana (2009). BMC Res Notes. 2012;5:115. [PubMed ID: 22357100]. https://doi.org/10.1186/1756-0500-5-115.
-
101.
Xie DD, Li J, Chen JT, Eyi UM, Matesa RA, Obono MM, et al. Seroprevalence of Human Immunodeficiency Virus, Hepatitis B Virus, Hepatitis C Virus, and Treponema pallidum Infections among Blood Donors on Bioko Island, Equatorial Guinea. PLoS One. 2015;10(10):e0139947. [PubMed ID: 26448460]. https://doi.org/10.1371/journal.pone.0139947.
-
102.
Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, et al. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010;10(1):111. https://doi.org/10.1186/1471-2334-10-111.
-
103.
Nagalo MB, Sanou M, Bisseye C, Kabore MI, Nebie YK, Kienou K, et al. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis among blood donors in Koudougou (Burkina Faso) in 2009. Blood Transfus. 2011;9(4):419-24. [PubMed ID: 21839011]. https://doi.org/10.2450/2011.0112-10.
-
104.
Giri PA, Deshpande JD, Phalke DB, Karle LB. Seroprevalence of transfusion transmissible infections among voluntary blood donors at a tertiary care teaching hospital in rural area of India. J Family Med Prim Care. 2012;1(1):48-51. [PubMed ID: 24479001]. https://doi.org/10.4103/2249-4863.94452.
-
105.
Pallavi P, Ganesh CK, Jayashree K, Manjunath GV. Seroprevalence and trends in transfusion transmitted infections among blood donors in a university hospital blood bank: a 5 year study. Indian J Hematol Blood Transfus. 2011;27(1):1-6. [PubMed ID: 22379287]. https://doi.org/10.1007/s12288-010-0047-x.
-
106.
Arora D, Arora B, Khetarpal A. Seroprevalence of HIV, HBV, HCV and syphilis in blood donors in Southern Haryana. Indian J Pathol Microbiol. 2010;53(2):308-9. [PubMed ID: 20551540]. https://doi.org/10.4103/0377-4929.64295.
-
107.
Rudra S, Chakrabarty P, Hossain MA, Akhter H, Bhuiyan MR. Seroprevalence of Hepatitis B, Hepatitis C, HIV Infections in Blood Donors of Khulna, Bangladesh. Mymensingh Med J. 2010;19(4):515-9. [PubMed ID: 20956891].
-
108.
Karki S, Ghimire P, Tiwari B, Rajkarnikar M. HBsAg serosurveillance among Nepalese blood donors. Ann Trop Med Pub Health. 2008;1(1):15. https://doi.org/10.4103/1755-6783.43072.
-
109.
Chimparlee N, Oota S, Phikulsod S, Tangkijvanich P, Poovorawan Y. Hepatitis B and hepatitis C virus in Thai blood donors. Southeast Asian J Trop Med Public Health. 2011;42(3):609-15. [PubMed ID: 21706939].
-
110.
Hahne SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar M. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181. [PubMed ID: 23597411]. https://doi.org/10.1186/1471-2334-13-181.
-
111.
Murphy EL, Lam J, Kaidarova Z, Cyrus S, Busch MP. Prevalence and demographic determinants of hepatitis b virus in first-time us blood donors, 2004-2009. Vox Sanguinis. 2012;103.
-
112.
Mirrezaie SM, Saber HR, Hajibeigi B, Salekmoghaddam E, Abbasian A, Alavian SM. Impact of HBV Vaccination on Prevalence of Hepatitis B Virus Infection Among Volunteer Blood Donors in Tehran-Iran. Shiraz E-Medl J. 2014;15(2). https://doi.org/10.17795/semj18066.
-
113.
Manzoor I, Hashmi N, Daud S, Ajmal S, Fatima H, Rasheed Z, et al. Seroprevalence of transfusion transmissible infections (TTIS) in blood donors. Biomed. 2009;25(2):154-8.
-
114.
Ali SA, Donahue RM, Qureshi H, Vermund SH. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int J Infect Dis. 2009;13(1):9-19. [PubMed ID: 18835208]. https://doi.org/10.1016/j.ijid.2008.06.019.
-
115.
Redwan NA, Ahmed MM, Barnawi MB. Prevalence study of Hepatitis B virus (HBV) infection by serological techniques in Jeddah, Saudi Arabia. Life Sci J. 2012;9:5442-8.