Abstract
Background:
One of the most important global public health concerns is chronic hepatitis C virus (HCV) infection, causing liver-related morbidity and mortality with a significant prevalence rate. Cirrhosis caused by hepatitis C is one of the most common causes of liver transplantation. Therefore, determining the prevalence of HCV infection and its geographical distribution is essential.Objectives:
The aim of this study was to estimate the point prevalence of HCV infection among Iranian general population.Methods:
Published studies reporting the prevalence of HCV infection in the Iranian general population were identified by a comprehensive literature search. Studies assessing and reporting HCV Ab positivity were evaluated in this review. Furthermore, an additional grey-literature search was performed to obtain other relevant studies.Results:
Twelve studies were eligible for inclusion in this review. The overall seroprevalence of HCV was 0.6% (95% CI: 0.4% to 0.8%). The seroprevalence of HCV infection varied considerably among different provinces ranging from 0.08% to 1.6%. Hormozgan province was reported to have the highest HCV Ab seropositivity rate while Mazandaran province had the lowest rate. The overall prevalence of actual viremia was 0.4% (range = 0.05 - 0.87), based on the results of five studies using PCR for confirmation of HCV diagnosis.Conclusions:
Our results demonstrated that the seroprevalence of HCV among Iranian general population is lower compared to other countries in the middle-east. However, the significant heterogeneity across included studies limits this conclusion. Therefore, to reduce the existing heterogeneity in the literature and strengthen the current evidence on the prevalence of HCV infection among Iranian general population, further high quality studies are required.Keywords
1. Background
Hepatitis C virus (HCV) infection is a global public health concern, which affects both developed and developing countries. In 2005, it was estimated that more than 185 million people were affected by HCV infection throughout the world (1). Based on estimation, the number of individuals affected annually by HCV infection is estimated to be three to four million. There are 170 million people chronically infected with HCV. They are prone to the development of cirrhosis and liver cancer, resulting in 350,000 deaths each year due to HCV-related complications (2). Central and East Asia, North Africa, and the Middle East are the areas with high prevalence of HCV infection, which is estimated to affect above 3.5% of the population (1).
Based on a study by Liakina et al., 60000 individuals were diagnosed with HCV in Iran in 2013, and the number of individuals diagnosed with HCV annually was estimated to be 6000. Furthermore, the number of individuals who were treated for HCV in Iran was 4500 in 2011 (3).
The prevalence of HCV infection in the general population, reported by previously published studies in Iran, varied among different geographical regions. The most important issue, however, is to provide a point estimate for prevalence of HCV infection among Iranian general population. This information paves the way to establish efficient preventive, diagnostic, and therapeutic strategies. The aim of this systematic review was to summarize the available evidence on HCV infection in Iran.
2. Methods
2.1. Information Sources and Search
This systematic review was performed according to the criteria of the PRISMA guidelines (4). We systematically searched Electronic databases of biological and health sciences including PubMed, Scopus, EMBASE, Ebsco, Science Citation Index Expanded, Ovid, Google Scholar, IranMedex, Magiran, and Scientific Information Database (SID). The Mesh terms used were as follows: “Hepatitis C”, “Prevalence”, “Epidemiology”, and “Iran”. Furthermore, reference lists of retrieved manuscripts, reviews, and meta-analyses were hand-searched to find additional eligible studies. In addition, proceedings of national and international conferences on hepatitis, infectious diseases, and blood, experts’ curriculum vitae in this relevant fields and national reports from the Iranian Ministry of health and Medical Education and the Iranian Blood Transfusion Organization (IBTO) were also reviewed to find grey literature.
2.2. Eligibility Criteria
All population-based studies assessing HCV serology in the Iranian population through diagnostic blood tests such as enzyme linked immuno-sorbent assay (ELISA), recombinant immunoblot assay (RIBA) or polymerase chain reaction (PCR) were examined in this review. The criteria of general population have been described in our recently published study (5). Briefly, general population refers to all individuals without reference to any specific characteristic and with the same male to female ratio that includes persons from different age groups. Time span, language, or study-design limit was not applied to the search.
The eligibility of publications was assessed independently by two review authors based on titles and abstracts. Any disagreement was resolved by the third review author. The quality of included studies was assessed by two review authors using a modified STROBE checklist. Any disagreement was resolved by consensus. Two review authors independently retrieved the data from each included study using a predefined data extraction form. The following study data were retrieved: title, author names, type of report, and electronic database where the article was found, as well as subjects’ demographic information such as mean age, age range, and gender, and details of the study methodology including the location and duration of the study, sampling methods, sample size, exclusion criteria, mode of hepatitis detection (serological or genetic tests), type/name of diagnostic kits, and the actual prevalence of HCV.
The meta-analysis was performed using STATA version 11.1. To combine the prevalence of HCV Ab positivity, the random effect model was used. The prevalence of HCV Ab in the whole population was the main outcome measure, as well as sex-specific prevalence. Using the Binomial Exact Method, ninety-five percent (95%) confidence intervals were calculated. Subgroup analysis and meta-regression methods were performed to explore heterogeneity. The I2 statistic was used for heterogeneity assessment. Severe heterogeneity was defined by I2 statistic greater than 90%. Begg’s test and Egger’s test were used to assess publication bias (6, 7). The map demonstrating the seroprevalence of HCV in different provinces was provided Using Photoshop software (Adobe, Mountain view, CA, USA).
3. Results
3.1. Study Characteristics
After removal of duplicates and primary screening, 237 articles remained from which, twelve studies with the sample size ranging from 919 to 49338 were eligible to be included in this systematic review (Table 1 and Figure 1). In the included studies, a total of 85611 individuals composed of 43568 males and 41124 females from the general population had been assessed. The sex distribution of participants in the sample was not reported in one study. The studies were from different geographical regions; three studies from Golestan province (25%), two studies from Sistan & Balochestan province (16.6%), and two studies from Fars province (16.6%). There was also one study (8%) from each of the following provinces: Khorasan razavi, Mazandaran, Kermanshah, and Chaharmahal and Bakhtiari. In addition, one study had been conducted in three provinces including Tehran, Hormozgan, and Golestan. Of these manuscripts, 7 (58.3%) had been conducted in both urban and rural areas while 4 (33.3%) of them had been only performed in urban areas and one study (8.3%) only in rural areas. In 11 out of the 12 studies included in the systematic review, the sample population was composed of both genders. As mentioned earlier, the gender of participants was not reported in one study. Finally, cluster sampling method had been used for sample selection by 75% of the studies.
Characteristics of Studies Included in the HCV Prevalence Meta-analysis in the Iranian General Population
| First Author | Publication Date | Province | Sample Size | Age Range | Prevalence (%) “Total” (HCVAb Positive) | Prevalence (%) “Total” (HCV Ab + HCV-RNA Positive) | Prevalence (%) “Total” (Based on Final Method) | Prevalence (%) “Male” | Prevalence (%) “Female” | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Salehi (8) | 2001 | Sistan & Baluchestan | 919 | NR | 0.1 | - | 0.1 | NR | NR |
| 2 | Ansari Moghadam (9) | 2012 | Sistan & Baluchestan | 2587 | 10-88 | 0.5 | - | 0.5 | 0.4 | 0.7 |
| 3 | Jamali (10) | 2008 | Golestan | 2049 | NR | 1 | - | 1 | 1.1 | 0.8 |
| 4 | Merat (11) | 2010 | Tehran | 2326 | NR | 0.3 | - | 0.3 | 0.8 | 0 |
| 5 | Merat (11) | 2010 | Hormozgan | 1463 | NR | 1.6 | - | 1.6 | 2.6 | 0.9 |
| 6 | Merat (11) | 2010 | Golestan | 1895 | NR | 1 | - | 1 | 1.3 | 0.8 |
| 7 | Poustchi (12) | 2011 | Golestan | 49338 | NR | 0.5 | 0.31 | 0.31 | NR | NR |
| 8 | Shakeri (13) | 2013 | Mashhad | 3870 | NR | 0.2 | 0.13 | 0.13 | 0.23 | 0.07 |
| 9 | Zamani (14) | 2013 | Mazandaran | 6145 | NR | 0.08 | 0.05 | 0.05 | 0.08 | 0 |
| 10 | Ghadir (15) | 2006 | Golestan | 2123 | 18 - 75 | 1 | - | 1 | 0.54 | 1.29 |
| 11 | Sayad (16) | 2008 | Kermanshah | 1721 | 15 - 64 | 0.87 | 0.87 | 0.87 | 1.4 | 0.34 |
| 12 | Shamsdin (17) | 2012 | Fars | 2080 | 35 - 83 | NR | 0.72 | 0.72 | NR | NR |
| 13 | Fattahi (18) | 2015 | Fars | 6095 | 7 - 95 | 0.25 | - | 0.25 | 0.31 | 0.2 |
| 14 | Moezzi (19) | 2015 | Chaharmahal & Bakhtiari | 3000 | 15 - 90 | 0.9 | - | 0.9 | 1.26 | 0.68 |
Flow Diagram of the Literature Search
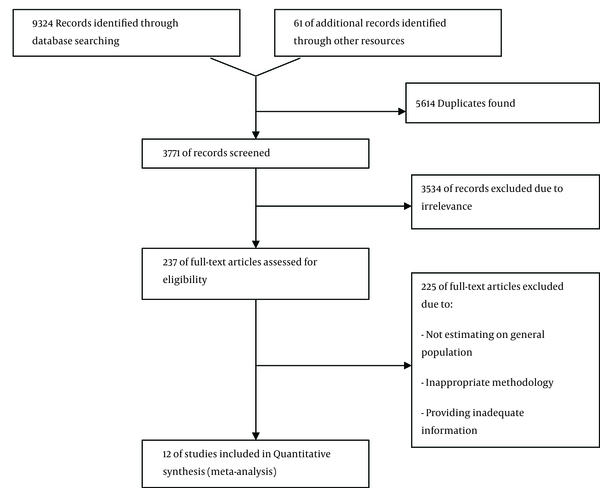
All studies used ELISA methods with Hepanostica as the most commonly used diagnostic kit. Four studies used RIBA method in addition to ELISA for confirmation of HCV diagnosis. The genetic method polymerase chain reaction (PCR) was used in addition to serological methods for confirmation of HCV diagnosis in five studies.
3.2. Meta-analysis
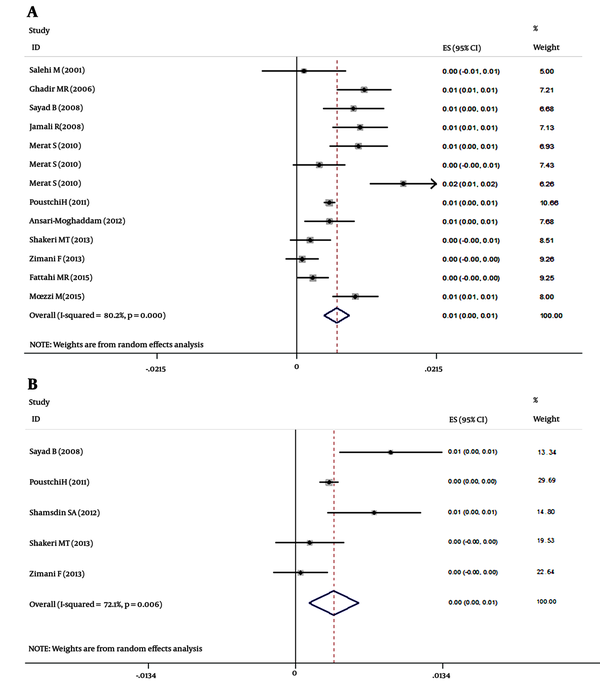
The HCV seroprevalence reported by the studies included in this review ranged from 0.08% (in Mazandaran province) to 1.6% (in Hormozgan province). As shown in Figure 2A and B, the overall seroprevalence was 0.6% (95% CI: 0.4% to 0.8%) estimated by meta-analysis (df = 12, I^2 = 80.2%, P < 0.001) while the overall prevalence of true viremia was 0.4 (95% CI: 0.1% to 0.6%, d f= 4, I^2 = 72.1, P = 0.006). The possible role of gender as a source of heterogeneity was confirmed by the analysis, since the HCV seroprevalence ranged from 0.14% to 2.6% in males and 0% to 1.29% in females (df = 9, I^2 = 80.8%, P < 0.001, Male) (df = 9, I^2 = 70.2%, P < 0.001, Female), (Figure 3A and B, respectively). The overall seroprevalence of HCV infection in males and females was 0.8% (95% CI: 0.4% to 1.2%) and 0.5% (95% CI: 0.2% to 0.8%), respectively. The overall prevalence of HCV viremia in males and females was 0.5% (df = 2, I^2 = 83.4%, P = 0.002) and 0.1% (df = 2, I^2 = 74.5%, P = 0.02), respectively.
A, Forest plot of studies on HCV seroprevalence in Iran- including both genders; B, Forest plot of studies on HCV viremia in Iran- including both genders
A, Forest plot of studies on HCV seroprevalence in Iran- males; B, Forest plot of studies on HCV seroprevalence in Iran- females
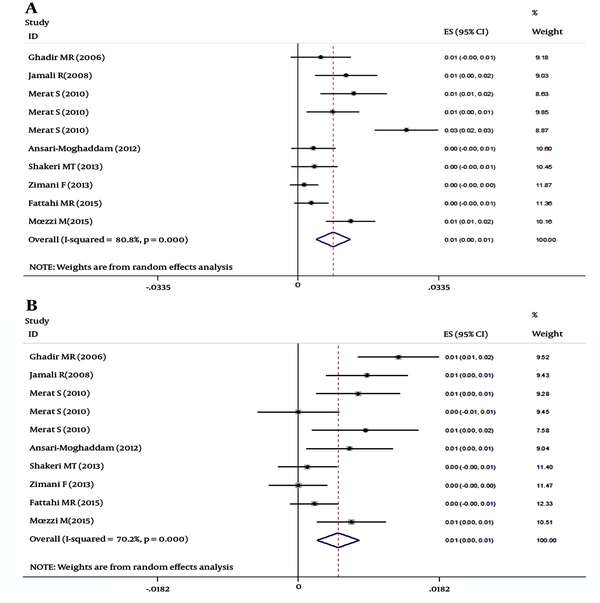
The possible role of study location as a source of heterogeneity was confirmed by meta-analysis (df = 12, I^2 = 80.2%, P < 0.001).
Begg’s test and Egger’s test did not suggest the possibility of publication bias for this systematic review. According to Begg’s test, the value for Z equaled 1.71 (P = 0.08) and based on Egger’s test, the bias score equaled 1.12 (P = 0.3).
4. Discussion
The factors affecting the prevalence of HCV including historical and current risk factors, screening programs, and treatment rates vary considerably among different countries. Therefore, establishing appropriate country-specific strategies regarding prevention, diagnosis, and treatment is crucial with the aim of reducing the disease burden represented by HCV. There are variations in the prevalence and epidemiology of HCV in different regions throughout the country.
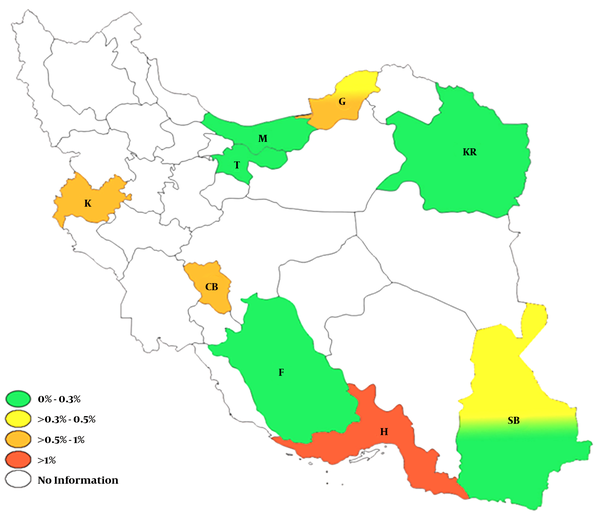
Our results demonstrated that the seroprevalence of HCV infection in Iran was 0.6% and the prevalence of viremia was 0.4%. These values indicate a spontaneous eradication rate of almost 35%, which is in agreement with other reports from Iran (12). Therefore, Iran could be considered a country with low frequency of HCV infection among general population. However, the seroprevalence of HCV infection showed a wide variation ranging from 0.08 in a study conducted in Mazandaran province to 1.6% in a study conducted in Hormozgan province. The variables that could explain this heterogeneity include lifestyle, rates of high risk behaviors, and quality of public health services.
The highest HCV infection rate in Iranian general population was reported by a study conducted in Hormozgan province (11). This high prevalence could be explained by the fact that Hormozgan province is located in the northern littoral of Persian Gulf and much of the economy of this province is dependent on frequent travelling to the Arab countries located in the south cost of Persian Gulf. Therefore, Hormozgan inhabitants might have increased exposure to HCV infection compared to people from other regions of the country. The HBs Ag seroprevalence was also relatively high in Hormozgan compared to other provinces (5). However, the HCV diagnosis in that study was made based on serological experiments rather than genetic tests. This may overestimate the prevalence of HCV infection in Hormozgan province.
Our results demonstrated higher prevalence of HCV infection among general population compared to the results of a previous systematic review by Alavian et al. (0.6% vs. 0.16%) (20). This difference could be explained by the fact that the reported prevalence of HCV infection in two of the articles included in the Alavian et al. study was 0%. These studies were conducted among street residing children and pregnant women. These subpopulations could not be actually representative of the general population and may have led to underestimate of HCV infection prevalence in that study. Our definition of general population has been previously presented in our recently published study (5). We included studies in which, specific characteristics regarding gender, socioeconomic indicators, or setting of the study (such as inpatient or outpatient) could not be attributed to participants.
The current evidence regarding prevalence of HCV infection among general population in Middle East countries is scarce. Based on the published literature, the seroprevalence of HCV infection in Iranian general population is lower compared to other countries in the Middle East region. The general population-based studies that have assessed the prevalence of HCV infection in the middle-east countries include two studies from Turkey with the reported seroprevalence of 1% and 2.1% (21, 22), a study from the Gaza strip with the reported seroprevalence of 2.2% (23), and a systematic review study from Yemen with the reported seroprevalence of 1.7% (24). Based on expert consensus, the seroprevalence of HCV infection is estimated to be 1.5% among United Arab Emirates general population (3).
The most important causative factor for the lower prevalence of HCV infection rate in our country in comparison with other developing countries is the strict program of HCV infection screening prior to transfusion, which started in Iran in 1996 (25). High risk groups including thalassemia, hemophilic and hemodialysis patients as well as intravenous drug users (IDU) are primary sources of HCV infection that can potentially transmit infection to healthy individuals. Therefore, screening programs for blood transfusion besides health programs focusing on IDU play a significant role in maintaining the rate of HCV infection in the lower range (26). Furthermore, the introduction of safe and highly effective direct-acting antiviral agents (DAAs) paves the way toward the elimination of HCV infection. Low cost DAAs are currently available in Iran. HCV elimination could be an achievable goal in Iran provided that required financial investments for increasing awareness about HCV, early diagnosis, and treatment of infected patients are met (27, 28).
The strength of this study is that the full-text of inaccessible manuscripts was obtained by contacting the authors, and information not available in the abstract was acquired to ensure the completeness and correctness of data.
On the other hand, the main limitation of this study was finding grey literature evidence. The other limitation of this systematic review is the heterogeneity of the included studies. Methodological and statistical issues were the most important sources of heterogeneity. Furthermore, the other parameter that should be considered when interpreting study results is that various diagnostic laboratory methods had been used in the surveyed studies to detect HCV Ab. Therefore, future studies should apply standardized guidelines for certain aspects of research methodology such as sample size calculation or diagnostic kits. Also, the possible impact of demographic factors such as age on the prevalence of HCV infection should be considered when reporting the results.
To sum up, our results demonstrated that the overall prevalence of HCV infection among Iranian general population is relatively low compared to other developing countries. However, the significant heterogeneity among included studies limits this conclusion. Therefore, further high-quality studies are recommended to provide more robust evidence on the prevalence of HCV among general population.
Geographic Distribution of HCV Infection in Iran (Seroprevalence)
Acknowledgements
References
-
1.
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-42. [PubMed ID: 23172780]. https://doi.org/10.1002/hep.26141.
-
2.
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529-38. [PubMed ID: 16879891]. https://doi.org/10.1016/j.jhep.2006.05.013.
-
3.
Liakina V, Hamid S, Tanaka J, Olafsson S, Sharara AI, Alavian SM, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 3. J Viral Hepat. 2015;22 Suppl 4:4-20. [PubMed ID: 26513445]. https://doi.org/10.1111/jvh.12475.
-
4.
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9. W64. [PubMed ID: 19622511].
-
5.
Mohammadi Z, Keshtkar A, Eghtesad S, Jeddian A, Pourfatholah AA, Maghsudlu M, et al. Epidemiological Profile of Hepatitis B Virus Infection in Iran in the Past 25 years; A Systematic Review and Meta-analysis of General Population Studies. Middle East J Dig Dis. 2016;8(1):5-18. [PubMed ID: 26933476]. https://doi.org/10.15171/mejdd.2016.01.
-
6.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-101. [PubMed ID: 7786990].
-
7.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-34. [PubMed ID: 9310563].
-
8.
Salehi M, Sanei Moghaddam E, Bozorg Zadeh SR, Haghighi MR. Seroepidemiology of Hepatitis C in Sistan & Baloochestan province. Zahedan J Res Medi Sci.
-
9.
Ansari-Moghaddam A, Ostovaneh MR, Sharifi Mood B, Sanei-Moghaddam E, Modabbernia A, Poustchi H. Seroprevalence of hepatitis B surface antigen and anti hepatitis C antibody in zahedan city, iran: a population-based study. Hepat Mon. 2012;12(9). eee6618. [PubMed ID: 23087764]. https://doi.org/10.5812/hepatmon.6618.
-
10.
Jamali R, Khonsari M, Merat S, Khoshnia M, Jafari E, Bahram Kalhori A, et al. Persistent alanine aminotransferase elevation among the general Iranian population: prevalence and causes. World J Gastroenterol. 2008;14(18):2867-71. [PubMed ID: 18473412].
-
11.
Merat S, Rezvan H, Nouraie M, Jafari E, Abolghasemi H, Radmard AR, et al. Seroprevalence of hepatitis C virus: the first population-based study from Iran. Int J Infect Dis. 2010;14 Suppl 3:e113-6. [PubMed ID: 20362479]. https://doi.org/10.1016/j.ijid.2009.11.032.
-
12.
Poustchi H, Esmaili S, Mohamadkhani A, Nikmahzar A, Pourshams A, Sepanlou SG, et al. The impact of illicit drug use on spontaneous hepatitis C clearance: experience from a large cohort population study. PLoS One. 2011;6(8). eee23830. [PubMed ID: 21887326]. https://doi.org/10.1371/journal.pone.0023830.
-
13.
Shakeri MT, Nomani H, Ghayour Mobarhan M, Sima HR, Gerayli S, Shahbazi S, et al. The prevalence of hepatitis C virus in mashhad, iran: a population-based study. Hepat Mon. 2013;13(3). eee7723. [PubMed ID: 23745128]. https://doi.org/10.5812/hepatmon.7723.
-
14.
Zamani F, Sohrabi M, Poustchi H, Keyvani H, Saeedian FS, Ajdarkosh H, et al. Prevalence and risk factors of hepatitis C virus infection in amol city, north of iran: a population-based study (2008-2011). Hepat Mon. 2013;13(12). eee13313. [PubMed ID: 24358039]. https://doi.org/10.5812/hepatmon.13313.
-
15.
Ghadir MR, Jafari E, Amiriani MT, Rezvan H, Amini S, Pourshams A. Hepatitis C in Golestan Province-Iran. Govaresh. 2006;11(3):158-62.
-
16.
Janbakhash A, Mansouri F, Vaziri S, Sayad B, Afsharian M, Abedanpor A. Seroepidemiology of herpes simplex virus type 2 (HSV2) in HIV infected patients in Kermanshah-Iran. Caspian J Intern Med. 2012;3(4):546-9. [PubMed ID: 24009932].
-
17.
Shamsdin SA, Fattahi MR, editors. The Prevalence of Hepatitis C Infection In General Population In Shiraz, Southern Iran. The 13th Iranian congress of Microbiology. 2012; Ardabil University of Medical Sciences.
-
18.
Fattahi MR, Safarpour A, Sepehrimanesh M, Hosseini Asl SM, Mohamaddoust F. The prevalence of hepatitis C virus infection and its related risk factors among the rural population of fars province, southern iran. Hepat Mon. 2015;15(2). eee24734. [PubMed ID: 25788957]. https://doi.org/10.5812/hepatmon.24734.
-
19.
Moezzi M, Imani R, Karimi A, Pourheidar B. Hepatitis C Seroprevalence and Risk Factors in Adult Population of Chaharmahal and Bakhtiari Province of Iran in 2013. J Clin Diagn Res. 2015;9(10):LC13-7. [PubMed ID: 26557546]. https://doi.org/10.7860/JCDR/2015/14986.6694.
-
20.
Alavian SM, Ahmadzad-Asl M, Bagheri Lankarani K, Shahbabaei MA, Bahrami Ahmadi A, Kabir A. Hepatitis C Infection in the General Population of Iran: A Systematic Review. Hepat mon. 2009;9(3):211-23.
-
21.
Akcam FZ, Uskun E, Avsar K, Songur Y. Hepatitis B virus and hepatitis C virus seroprevalence in rural areas of the southwestern region of Turkey. Int J Infect Dis. 2009;13(2):274-84. [PubMed ID: 18945630]. https://doi.org/10.1016/j.ijid.2008.07.005.
-
22.
Yildirim B, Barut S, Bulut Y, Yenisehirli G, Ozdemir M, Cetin I, et al. Seroprevalence of hepatitis B and C viruses in the province of Tokat in the Black Sea region of Turkey: A population-based study. Turk J Gastroenterol. 2009;20(1):27-30. [PubMed ID: 19330732].
-
23.
Shemer-Avni Y, el Astal Z, Kemper O, el Najjar KJ, Yaari A, Hanuka N, et al. Hepatitis C virus infection and genotypes in Southern Israel and the Gaza Strip. J Med Virol. 1998;56(3):230-3. [PubMed ID: 9783690].
-
24.
Bajubair MA, Elrub AA, Bather G. Hepatic viral infections in Yemen between 2000--2005. Saudi Med J. 2008;29(6):871-4. [PubMed ID: 18521468].
-
25.
Rezvan H, Abolghassemi H, Kafiabad SA. Transfusion-transmitted infections among multitransfused patients in Iran: a review. Transfus Med. 2007;17(6):425-33. [PubMed ID: 18067646]. https://doi.org/10.1111/j.1365-3148.2007.00794.x.
-
26.
Alavian SM. We Need a New National Approach to Control Hepatitis C: It is Becoming too Late. Hepat mon. 2008;8(3):1-3.
-
27.
Alavian SM, Hajarizadeh B, Bagheri Lankarani K, Sharafi H, Ebrahimi Daryani N, Merat S, et al. Recommendations for the Clinical Management of Hepatitis C in Iran: A Consensus-Based National Guideline. Hepat Mon. 2016;16(8). eee40959. [PubMed ID: 27799966]. https://doi.org/10.5812/hepatmon.guideline.
-
28.
Hesamizadeh K, Sharafi H, Rezaee-Zavareh MS, Behnava B, Alavian SM. Next Steps Toward Eradication of Hepatitis C in the Era of Direct Acting Antivirals. Hepat Mon. 2016;16(4). eee37089. [PubMed ID: 27275164]. https://doi.org/10.5812/hepatmon.37089.