Detection of PCV2e strains in Southeast China
- Published
- Accepted
- Received
- Academic Editor
- Fernando Spilki
- Subject Areas
- Veterinary Medicine, Epidemiology, Infectious Diseases
- Keywords
- Phylogenetic analysis, Recombination, PCV2, Genotype
- Copyright
- © 2018 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. Detection of PCV2e strains in Southeast China. PeerJ 6:e4476 https://doi.org/10.7717/peerj.4476
Abstract
Porcine circovirus 2 (PCV2) has been prevalent in swine herds in China since 2002, causing severe economic loss to the pig industry. The number of live pigs in southeast China is > 20 million. Since information on the genetic variation of PCV2 in the Fujian province is limited, the objective of the present work was to investigate the epidemiological and evolutionary characteristics of PCV2 in southeast China from 2013 to 2017. Of the 685 samples collected from 90 different swine herds from 2013 to 2017, 356 samples from 84 different swine herds were positive for PCV2. PCV2a, PCV2b, PCV2d, and PCV2e co-existed in the Fujian province, with PCV2d being the predominant circulating strain in swineherds and PCV2e being reported for the first time in China. Strikingly, PCV2-FJ-water DNA comes from contaminated river water and not infected animals. Sequence comparison among all isolates indicated that 95 isolates shared approximately 78.7%–100% nucleotide identity and 74.5%–100% amino acid identity for open reading frame 2 (ORF2). Amino acid alignment showed that the Cap protein of PCV2e differed markedly from those of PCV2a, PCV2b, PCV2c, and PCV2d. These results indicated that various PCV2 genotypes exist in China, and that PCV2 is continuously evolving, leading to rapid emergence of new variant stains.
Introduction
The porcine circovirus (PCV) is a small non-enveloped single-stranded circular DNA virus of swine. Currently, three major PCV2 genotypes have been recognized: PCV type 1 (PCV1), PCV2 type 2 (PCV2), and PCV type 3 (PCV3) (Opriessnig, Meng & Halbur, 2007; Palinski et al., 2016). PCV1 is non-pathogenic to pigs, whereas PCV2 is the primary causative agent of porcine circovirus-associated disease (PCVAD) in growing pigs, which includes systemic, respiratory and enteric manifestations, and PCV3 is associated with porcine dermatitis and nephropathy syndrome (PDNS) (Opriessnig, Meng & Halbur, 2007).
Currently, PCV2 is the primary causative agent of swine viral disease worldwide, which has resulted in considerable economic loss since it was first identified in Canada in 1991 (Harding & Clark, 1997; Krakowka et al., 2000; Bolin et al., 2001). The viral genomes range from 1,766 to 1,768 nucleotides (nt) in length and contain four major identified open reading frames (ORFs) (ORF1–ORF4). ORF2 encodes the capsid protein (Cap), which is the only structural protein of PCV2 and is related to viral antigenicity; hence, the gene encoding the Cap protein is suitable as a phylogenetic and epidemiological marker (Gomes et al., 2007; Choi & Chae, 2008; Jiang et al., 2017). Currently, at least five distinct genotypes have been described in swine based on the ORF2 sequence, which have been classified as PCV2a, PCV2b, PCV2c, PCV2d, and PCV2e (Segalés et al., 2008; Harmon et al., 2015; Xiao, Halbur & Opriessnig, 2015; Davies et al., 2016; Karuppannan & Opriessnig, 2017). PCV2e is a new genetic group which was recently proposed by Davies et al. (2016). Currently, PCV2a, PCV2b, and PCV2d are found globally, whereas PCV2c has only been identified in Denmark and Brazil (Dupont et al., 2008; Franzo et al., 2015). PCV2e is a new genotype that has been circulating in the USA since 2015, the ORF2 sequences of which have 12 or 15 additional nts compared to those of PCV2a–PCV2d (Harmon et al., 2015; Davies et al., 2016).
PCV2 was first reported in China in 1999, and has become the causative agent of one of the country’s most serious swine diseases (Lang et al., 2000; Shuai et al., 2007; Wang et al., 2009; Guo et al., 2012; Jiang et al., 2017). Previous studies showed that various genotypes, including PCV2a, PCV2b, and PCV2d are present in China. Among these genotypes, PCV2b and PCV2d have been predominant in China since 2004 (Lang et al., 2000; Shuai et al., 2007; Wang et al., 2009; Guo et al., 2012; Jiang et al., 2017). Recently, several groups showed that PCV2 isolates in pig farms had recombined owing to the co-existence of different PCV2 genotypes (Hesse, Kerrigan & Rowland, 2008; Cai et al., 2011; Huang et al., 2013; Ramos et al., 2013; Anoopraj et al., 2015). Despite continuous reports of newly emerging strains worldwide, information on the genetic variation of PCV2 in the Fujian province is limited. Therefore, to understand the current molecular epidemiology of PCV2 in Southeast China, the objective of the present work was to investigate the epidemiological and evolutionary characteristics of PCV2 in the Fujian province from 2013 to 2017.
Materials and Methods
Animal ethics
Sampling procedures were approved by the Animal Ethics Committee of the South China Agricultural University, with a reference number of SCAU-AEC-2014-10.
Clinical samples
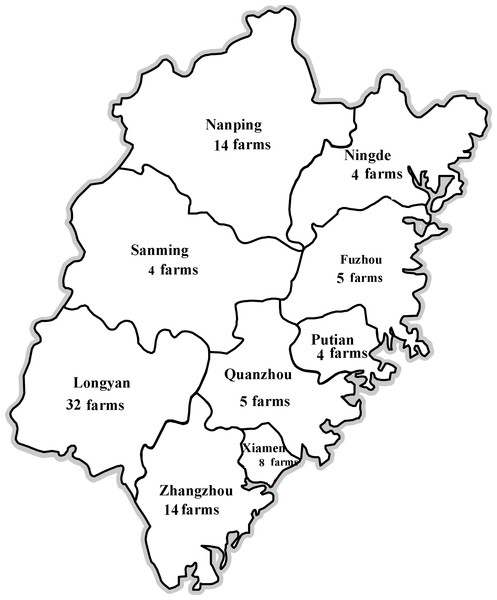
Six hundred and eighty-five clinical samples (blood, lungs, kidneys, livers, and lymph nodes) were collected from unthrifty pigs (4–10 weeks of age) in different farms of Fujian province, China, between 2013 and 2017. In particular, samples were collected from 32 farms in Longyan (196 samples), 14 farms in Zhangzhou (105 samples), eight farms in Xiamen (75 samples), five farms in Fuzhou (54 samples), four farms in Ningde (40 samples), 14 farms in Nanping (92 samples), four farms in Sanming (40 samples), five farms in Quanzhou (45 samples), and four farms in Putian (38 samples) from 2013 to 2017 (Fig. 1). These pigs were suspected to have clinical signs of Postweaning Multisystemic Wasting Syndrome (PMWS) and/or PDNS. In addition, 200 water samples were collected from the river or pool covering a geographic area of about 5 km2 near every pig farm.
Figure 1: Locations of PCV2-positive swine farms where samples were collected in Fujian Province.
Polymerase chain reaction (PCR) amplification and nucleotide sequencing
Total viral DNA was extracted from tissue samples using DNA extraction kit ver.3.0 (TaKaRa, Dalian, China) following the manufacturer’s protocol. ORF2 was amplified from the extracted DNA using specific primers according to previous studies (Fort et al., 2007). PCR products were purified using the gel extraction kit (Tiangen Biotech Co. Ltd., Beijing, China) and cloned into the pGEM-T Easy vector (Promega). Recombinant clones were sequenced by Sangon Biotech (Shanghai) Co. Ltd. Each fragment was independently sequenced at least thrice.
Phylogenetic analysis
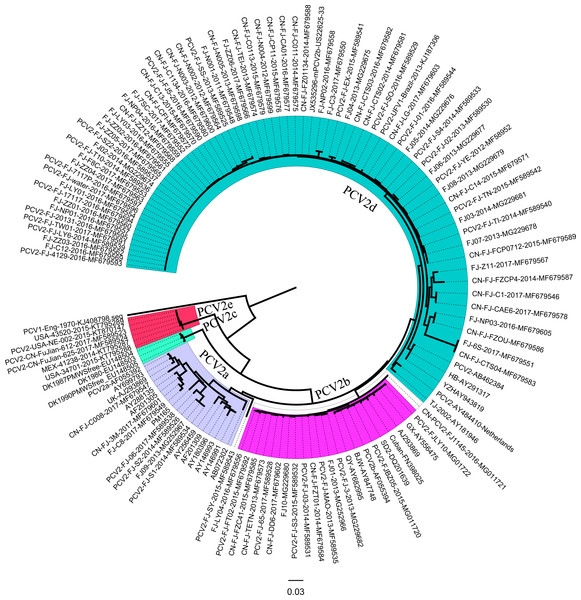
Ninety-five ORF2 genes of PCV2 isolates were identified in the Fujian province during 2013–2017. Representative PCV2 sequences, including PCV2a, PCV2b, PCV2c, PCV2d, and PCV2e isolates in the GenBank, were used for sequence alignments and phylogenetic analyses.
Multiplex sequencing alignments were performed using CLUSTAL X (version 1.83). The phylogenetic tree was constructed using the neighbor joining (NJ) method with MEGA 6.0, the maximum composite likelihood model, and a bootstrap confidence value of 1,000 replicates.
Recombination analysis
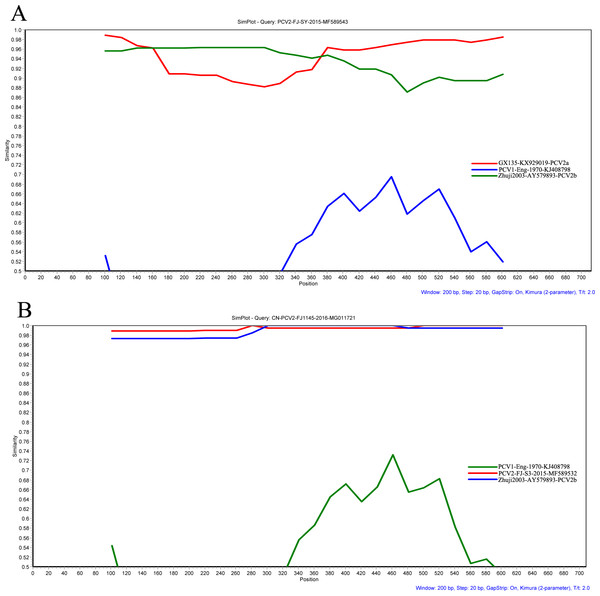
To detect putative recombination events in PCV2, recombinant strains were investigated on the complete genome database using a recombination detection program (RDP v.4.24) (Martin et al., 2010) as described by Ramos et al. (2013). Briefly, seven methods (RDP, GeneConv, BootScan, MaxChi, Chimera, SiScan, and 3Seq) were used for preliminary scan and only events detected by more than two methods with a highest acceptable p-value of 0.01 were considered. The recombination event was further confirmed using SimPlot program (v.3.5.1) and boot scanning analysis was performed with a 200-bp window sliding along a step size of 20 bp (Lole et al., 1999).
Results
PCV2 detection
Of the 685 samples collected from 90 different swine herds located in Fujian province from 2013 to 2017, 356 samples from 84 different swine herds were positive for PCV2. Ninety-five positive samples were selected for further analysis of PCV2 characteristics (Table 1).
Phylogenetic analysis of ORF2
Phylogenetic analysis based on ORF2 showed that Fujian PCV2 strains were classified into five subtypes (PCV2a, PCV2b, PCV2d, and PCV2e) (Fig. 2). Among Fujian PCV2 strains, 7.37% (7/95) of the sequences corresponded to PCV2a, 15.8% (15/95) to PCV2b, 72.6% (69/95) to PCV2d, and 2.1% (2/95) to PCV2e. PCV2-CN-FuJian-612-2017 and PCV2-CN-FuJian-625-2017 were classified as PCV2e, which has been reported for the first time in China. Interestingly, PCV2-FJ-water DNA comes from contaminated water instead of from infected animals.
| Name | Geographic origin | Source | Time | Genetype | Accession No. |
|---|---|---|---|---|---|
| CN-FJ-C1-2017 | Putian | lymph node | 2017 | PCV2d | MF679546 |
| CN-FJ-C008 | Putian | serum | 2017 | PCV2a | MF679547 |
| CN-FJ-N001-2011 | Longayan | lymph node | 2011 | PCV2d | MF679548 |
| CN-FJ-C8-2017 | Longayan | Spleen/Lung | 2017 | PCV2a | MF679549 |
| CN-FJ-C3-2017 | Longayan | lymph node | 2017 | PCV2d | MF679550 |
| CN-FJ-6S-2017 | Zhangzhou | lymph node | 2017 | PCV2d | MF679551 |
| CN-FJ-7SC-2017 | Putian | serum | 2017 | PCV2d | MF679552 |
| CN-FJ-F8C-2017 | Xiamen | lymph node | 2017 | PCV2d | MF679553 |
| CN-FJ-LY01-2016 | Quanzhou | Lung | 2016 | PCV2d | MF679554 |
| CN-FJ-LY03-2016 | Ningde | lymph node | 2016 | PCV2d | MF679555 |
| PCV2-FJ-3 | Ningde | serum | 2013 | PCV2b | MG229682 |
| FJ01 | Longyan | lymph node | 2013 | PCV2a | MG252966 |
| FJ02 | Zhangzhou | Spleen/Lung | 2014 | PCV2d | MG229674 |
| FJ03 | Xiamen | lymph node | 2014 | PCV2d | MG229681 |
| FJ04 | Nanping | lymph node | 2013 | PCV2d | MG229675 |
| FJ05 | Fuzhou | lymph node | 2014 | PCV2d | MG229676 |
| FJ06 | Longyan | serum | 2013 | PCV2d | MG229677 |
| FJ07 | Quanzhou | lymph node | 2013 | PCV2d | MG229678 |
| FJ08 | Zhangzhou | Lung | 2013 | PCV2d | MG229679 |
| FJ09 | Longyan | lymph node | 2013 | PCV2b | MG252967 |
| FJ10 | Nanping | lymph node | 2014 | PCV2b | MG229680 |
| CN-FJ-LY04-2016 | Quanzhou | serum | 2016 | PCV2b | MF679556 |
| CN-FJ-NP01-2016 | Nanping | lymph node | 2016 | PCV2d | MF679557 |
| CN-FJ-NP02-2016 | Nanping | Spleen/Lung | 2016 | PCV2d | MF679558 |
| CN-FJ-NP03-2016 | Nanping | lymph node | 2016 | PCV2d | MF679605 |
| FJ-NP04-2017 | Nanping | serum | 2016 | PCV2d | MF679559 |
| CN-FJ-ZZ01-2016 | Zhangzhou | lymph node | 2016 | PCV2d | MF679560 |
| CN-FJ-ZZ02-2016 | Zhangzhou | Spleen/Lung | 2016 | PCV2d | MF679561 |
| CN-FJ-ZZ03-2016 | Zhangzhou | lymph node | 2016 | PCV2d | MF679562 |
| CN-FJ-ZZ04-2017 | Zhangzhou | Lung | 2017 | PCV2d | MF679563 |
| CN-FJ-N002-2012 | Quanzhou | lymph node | 2012 | PCV2d | MF679564 |
| CN-FJ-ZZ05-2017 | Zhangzhou | spleen | 2017 | PCV2d | MF679565 |
| CN-FJ-ZZ06-2017 | Zhangzhou | lymph node | 2017 | PCV2d | MF679566 |
| CN-FJ-C011 | Xiamen | serum | 2015 | PCV2d | MF679575 |
| CN-FJ-Z11 | Xiamen | lymph node | 2017 | PCV2d | MF679567 |
| CN-FJ-ZZ12 | Xiamen | serum | 2015 | PCV2d | MF679568 |
| CN-FJ-C12 | Xiamen | lymph node | 2016 | PCV2d | MF679569 |
| CN-FJ-C13 | Quanzhou | Lung | 2015 | PCV2d | MF679570 |
| CN-FJ-C14 | Longyan | Spleen/Lung | 2015 | PCV2d | MF679571 |
| CN-FJ-CFI | Longyan | lymph node | 2013 | PCV2d | MF679572 |
| CN-FJ-TETN | Longyan | serum | 2013 | PCV2b | MF679573 |
| CN-FJ-TEI | Quanzhou | serum | 2013 | PCV2d | MF679574 |
| CN-FJ-CP11 | Quanzhou | lymph node | 2015 | PCV2d | MF679576 |
| CN-FJ-CA01 | Quanzhou | lymph node | 2016 | PCV2d | MF679577 |
| CN-FJ-CAE6 | Quanzhou | spleen | 2017 | PCV2d | MF679578 |
| CN-FJ-C0113 | Zhangzhou | lymph node | 2015 | PCV2d | MF679579 |
| CN-FJ-C1134 | Xiamen | serum | 2016 | PCV2d | MF679580 |
| CN-FJ-CTS02 | Longyan | lymph node | 2014 | PCV2d | MF679581 |
| CN-FJ-CTS03 | Longyan | lymph node | 2016 | PCV2d | MF679582 |
| CN-FJ-CTS04 | Longyan | Spleen/Lung | 2015 | PCV2d | MF679583 |
| CN-FJ-FZT01 | Xiamen | lymph node | 2014 | PCV2b | MF679584 |
| CN-FJ-FZC41 | Quanzhou | serum | 2015 | PCV2b | MF679585 |
| CN-FJ-FZOU | Ningde | lymph node | 2013 | PCV2d | MF679586 |
| CN-FJ-FZCP4 | Ningde | lymph node | 2014 | PCV2d | MF679587 |
| CN-FJ-FZ01134 | Ningde | Spleen/Lung | 2014 | PCV2d | MF679588 |
| CN-FJ-FCP0712 | Ningde | lymph node | 2015 | PCV2d | MF679589 |
| PCV2-FJ-FJ5 | Quanzhou | spleen | 2016 | PCV2d | MF679590 |
| PCV2-FJ-TW01 | Quanzhou | lymph node | 2017 | PCV2d | MF679591 |
| PCV2-FJ-FT02 | Putian | lymph node | 2015 | PCV2b | MF679592 |
| PCV2-FJ-4129 | Putian | Spleen/Lung | 2016 | PCV2d | MF679593 |
| PCV2-FJ-17117 | Putian | lymph node | 2016 | PCV2d | MF679594 |
| PCV2-FJ-20131 | Putian | lymph node | 2016 | PCV2d | MF679595 |
| PCV2-FJ-water | Longyan | Water | 2017 | PCV2d | MF679596 |
| PCV2-FJ-7117P | Putian | lymph node | 2016 | PCV2d | MF679597 |
| CN-FJ-N005-2013 | Zhangzhou | serum | 2013 | PCV2d | MF679598 |
| CN-FJ-N004-2012 | Fuzhou | lymph node | 2012 | PCV2d | MF679599 |
| CN-FJ-N003-2012 | Nanping | Lung | 2012 | PCV2d | MF679600 |
| CN-FJ-3M-2017 | Nanping | lymph node | 2017 | PCV2a | MF679601 |
| CN-FJ-DD6-2017 | Fuzhou | lymph node | 2017 | PCV2b | MF679602 |
| CN-FJ-LG-2017 | Longyan | Lung | 2017 | PCV2d | MF679603 |
| PCV2-CN/FuJian-612-2017 | Longyan | lymph node/spleen | 2017 | PCV2e | MF589523 |
| PCV2-CN/FuJian-625-2017 | Longyan | lymph node/spleen | 2017 | PCV2e | MF589524 |
| PCV2-FJ-SS | Fuzhou | lymph node | 2013 | PCV2d | MF589525 |
| PCV2-FJ-S2 | Nanping | Spleen/Lung | 2014 | PCV2a | MF589526 |
| PCV2-FJ-YE | Longyan | lymph node | 2012 | PCV2d | MF589527 |
| PCV2-FJ-65 | Longyan | Lung | 2017 | PCV2b | MF589528 |
| PCV2-FJ-SO | Longyan | lymph node | 2016 | PCV2d | MF589529 |
| PCV2-FJ-02 | Longyan | Spleen/Lung | 2013 | PCV2d | MF589530 |
| PCV2-FJ-03 | Longyan | lymph node | 2014 | PCV2b | MF589531 |
| PCV2-FJ-S3 | Longyan | serum | 2015 | PCV2b | MF589532 |
| PCV2-FJ-S4 | Longyan | lymph node | 2014 | PCV2d | MF589533 |
| PCV2-FJ-S1 | Fuzhou | lymph node | 2014 | PCV2a | MF589534 |
| PCV2-FJ-MAO | Fuzhou | Lung | 2013 | PCV2b | MF589535 |
| PCV2-FJ-T10 | Fuzhou | lymph node | 2014 | PCV2d | MF589536 |
| PCV2-FJ-S22 | Fuzhou | Spleen/Lung | 2016 | PCV2d | MF589537 |
| PCV2-FJ-06 | Fuzhou | Lung | 2017 | PCV2a | MF589538 |
| PCV2-FJ-LY6 | Zhangzhou | lymph node | 2014 | PCV2d | MF589539 |
| PCV2-FJ-TI | Zhangzhou | Spleen | 2014 | PCV2d | MF589540 |
| PCV2-FJ-EX | Zhangzhou | lymph node | 2015 | PCV2d | MF589541 |
| PCV2-FJ-TN | Zhangzhou | lymph node | 2015 | PCV2d | MF589542 |
| PCV2-FJ-SY | Longyan | lymph node | 2015 | mPCV2d | MF589543 |
| PCV2-FJ-01 | Quanzhou | serum | 2016 | PCV2d | MF589544 |
| PCV2-FJBZ05 | Longyan | lymph node | 2015 | PCV2b | MG011720 |
| CN-PCV2-FJ1145 | Longyan | Spleen/Lung | 2016 | mPCV2b | MG011721 |
| PCV2-FJLY10 | Longyan | lymph node | 2016 | PCV2b | MG011722 |
Figure 2: Neighbor-joining (NJ) phylogenetic analysis of Fujian PCV2 ORF2 genes.
Reliability of the tree was assessed by bootstrap analysis of 1,000 replications. PCV2b (GenBank accession no. AF055394).Amino acid analysis of ORF2
The lengths of the Fujian PCV2 ORF2 sequences were 702 nt (PCV2a, PCV2b, or PCV2d), 705 nt (PCV2c and PCV2d), and 717 nt (PCV2e), which encoded Cap proteins of 233, 234, and 238 amino acids, respectively. These 95 isolates shared approximately 78.7%–100% nucleotide identity and 74.5%–100% amino acid identity for ORF2. Amino acid alignments showed that the Cap protein of PCV2e and two strains (CN-FJ-CTS04 and FJ-6S-2017) differed markedly from those of PCV2a, PCV2b, PCV2c, and PCV2d. Unlike the sequence of other known PCV2 ORF2, the PCV2e ORF2 possesses 12 or 15 additional nts, which generate a terminal seven amino acid sequence (PPPLSYM) that is different from the PK and NPK termini of the 233 and 234 amino acid capsids, respectively (Davies et al., 2016). The PCV2 strains in genotype PCV2d harbored four unique amino acid mutations (Y8 → F/T8, A68 → N68, F53 → I53, and V215 → I215), whereas PCV2 in genotype PCV2e harbored ten unique amino acid mutations (K58 → V8, D115 → E115, A133 → T133, A135 → N135, T137 → S137, T170 → I170, V196 → T196, N204 → H204, Y207 → T207 and L226 → M226).
Recombination analysis
Seven methods (RDP, GeneConv, BootScan, MaxChi, Chimera, SiScan, and 3Seq) were implemented in RDP 4.24 to detect potential recombinants. The results showed that inter-genotypic (PCV2-FJ-SY) and intra-genotypic (CN-PCV2-FJ1145) recombination events of PCV2 have been detected (Fig. 3). Additionally, the results of SIMPLOT software (v3.5.1) confirmed the putative recombinant events obtained with the RDP 4.24 software.
Figure 3: Recombination analysis of the PCV2 ORF2 gene of PCV2-FJ-SY (A) and CN-PCV2-FJ1145 (B) using a sliding window of 200 nt moving in 20 nt steps.
Recombination events were analyzed by Simplot software (v. 3.5.1). A PCV1 isolate (KJ408798) was used as outgroup.Discussion
PCV2 has been prevalent in Chinese swine herds since it was first identified in China in 1999. Previous studies demonstrated that various PCV2 genotypes exist in China, including PCV2a, PCV2b, PCV2c, PCV2d, and a minor recombinant group. Surprisingly, different PCV2 genotypes or strains have co-existed in Chinese swine herds. Currently, the amount of live pigs in the Fujian province (Southeast China) is >20 million heads, although the epidemic status and genetic diversity of PCV2 is still unknown. Thus, we sought to investigate the genetic diversity and epidemiology of PCV2 in southeast China in 2013–2017.
On the basis of ORF2 nucleotide sequences, the genotypes of Fujian PCV2 have been classified as PCV2a, PCV2b, PCV2d, and PCV2e (Opriessnig, Meng & Halbur, 2007; Guo et al., 2010; Guo et al., 2012; Harmon et al., 2015; Davies et al., 2016), of which, PCV2d was predominant in Fujian from 2014 to 2017. The present data indicate that the prevalence of PCV2d increased from 57.1% in 2013 to 87.5% in 2016, suggesting that PCV2d has replaced PCV2b and has now become the predominant PCV2 genotype in Fujian since 2013. Additionally, CN-FJ-CTS04 and FJ-6S-2017 strains emerged in cases of in cases of apparent vaccine failure. Overuse of vaccine has probably contributed to the variation of PCV2. PCV2e, a new PCV2 genotype, was identified in the USA in 2015, the ORF2 sequence of which contains 12 or 15 additional nt compared to other known PCV2 ORF2 sequences and shows approximately 85% identity with other known PCV2 ORF2 sequences (Harmon et al., 2015; Davies et al., 2016). In the present study, only two PCV2e sequences were detected in Fujian, suggesting that PCV2e replication is low in the swine herds. The group members encoded a capsid protein of 238 amino acids, which is markedly different from all known capsid proteins of 233 or 234 amino acids (Xiao et al., 2016). The pathogenicity of PCV2e warrants further investigation.
Recently, several groups detected PCV2 DNA in water samples, human stool, beef, calf bone marrow, farm air, house flies, and mosquito (Verreault et al., 2010; Blunt et al., 2011; Garcia et al., 2012; Yang et al., 2012). In the present study, PCV2 DNA was also detected in river water, which is indicative of a potential human health hazard.
PCV2 nucleotide substitution rate has been calculated to vary between 1.2 × 10−3 and 6.6 × 10−3 substitutions/site/year, which is close to that of RNA viruses (Firth et al., 2009; Pérez et al., 2011). Recombination is critical for viral evolution, and recombination events between PCV2 strains could generate PCV2 of varying pathogenicity (Olvera, Cortey & Segales, 2007; Hesse, Kerrigan & Rowland, 2008; Wang et al., 2009). Both inter-genotypic and intra-genotypic recombination events of PCV2 have been recorded; inter-genotypic recombination occurs within ORF1 and ORF2, whereas intra-genotypic recombination mainly occurs within ORF2. Recently, a PCV2c genotype strain (GenBank accession no. KC823058) was reported in China, and three strains were generated from PCV2b and PCV2c recombination (Liu et al., 2016). In the present study, we detected that one natural strain (PCV2-FJ-SY) was generated by recombination between different lineages of PCV2, while CN-PCV2-FJ1145 was generated by intra-genotypic recombination. These results indicated that PCV2 is continuously evolving through genome recombination, which leads to rapid emergence of new variant stains.
In summary, PCV2a, PCV2b, PCV2d, and PCV2e co-exist in Fujian, and PCV2d-2 is the predominant strain that has been circulating in Fujian since 2013. Our results enhance the understanding of the PCV2 epidemic in Fujian, and may assist veterinary workers in establishing suitable prevention and control policies for the novel strains emerging from viral evolution.
Conclusions
The findings of this study demonstrated that PCV2 is ubiquitous in China, with PCV2d being the predominant strain circulating from 2013 to 2017. PCV2 is continuously evolving through genome recombination or new viral introductions from distant geographic regions, which leads to rapid emergence of new variant stains.