Evaluation of five regions as DNA barcodes for identification of Lepista species (Tricholomataceae, Basidiomycota) from China
- Published
- Accepted
- Received
- Academic Editor
- Nancy Keller
- Subject Areas
- Biodiversity, Mycology
- Keywords
- Agaricales, Intra-specific diversity, DNA barcoding, Inter-specific diversity, Species delineation
- Copyright
- © 2019 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Evaluation of five regions as DNA barcodes for identification of Lepista species (Tricholomataceae, Basidiomycota) from China. PeerJ 7:e7307 https://doi.org/10.7717/peerj.7307
Abstract
Background
Distinguishing among species in the genus Lepista is difficult because of their similar morphologies.
Methods
To identify a suitable DNA barcode for identification of Lepista species, we assessed the following five regions: internal transcribed spacer (ITS), the intergenic spacer (IGS), nuclear ribosomal RNA subunit, mitochondrial small subunit rDNA, and tef1. A total of 134 sequences from 34 samples belong to eight Lepista species were analyzed. The utility of each region as a DNA barcode was assessed based on the success rates of its PCR amplification and sequencing, and on its intra- and inter-specific variations.
Results
The results indicated that the ITS region could distinguish all species tested. We therefore propose that the ITS region can be used as a DNA barcode for the genus Lepista. In addition, a phylogenetic tree based on the ITS region showed that the tested eight Lepista species, including two unrecognized species, formed eight separate and well-supported clades.
Introduction
Lepista (Fr.) W.G. Sm., a genus in the family Tricholomataeae, was erected by Smith in 1870 and contains about 50 species (Kirk et al., 2008). A total of 12 Lepista species have been reported in China where they are widely distributed (Mao, 2000; Li et al., 2011). Some Lepista species are popular edible mushrooms in China, and these include Lepista nuda (Bull.) Cooke, L. sordida (Schumach.) Singer, and L. irina (Fr.) H.E. Bigelow (Dai et al., 2010).
The genus Lepista can be distinguished from other genera by the coarse surface of its spores, a white to pale-pink spore print, and clamped hyphae (Singer, 1986; Bon, 1987). Within the genus, however, the limited morphological characteristics make it difficult to distinguish among the species. As a result, misidentification is common both between and within species of Lepista. For example, L. irina and L. panaeola have a similar whitish pileus. According to Alvarado et al. (2015), the two species differ in spore size and spore wall structure but the assessment of these characters varies among observers. In addition, other morphological characteristics including the color of the pileus often vary with environmental factors. The pileus of L. nuda, for example, was described as gray brown or russet brown in some studies but as purple brown in others (Bon, 1987; Hansen & Knudsen, 1992).
Accurate identification of species is important for conserving the genetic resources of Lepista, and rapid and reliable species identification is now possible via DNA barcoding. DNA barcoding, which uses short DNA sequences of standard genomic regions, has become increasingly important in identifying species (Badotti et al., 2017; Li et al., 2017) and discovering new species (Zhao et al., 2011; Al-Hatmi et al., 2016). DNA barcoding also could provide the primary information for species delimitation in poorly known groups (Vogler & Monaghan, 2007) and help identify candidate exemplar taxa for a comprehensive phylogenetic study (Hajibabaei et al., 2007). Based on the requirements for standardized DNA barcoding, the sequences of all candidate markers should be short and should have high rates of successful amplification and high rates of successful sequencing. DNA barcoding also requires that candidate markers have substantial inter-specific variation but not intra-specific variation. The internal transcribed spacer (ITS) region of the nuclear ribosomal RNA gene has been used as a general barcode marker for some groups in the Basidiomycota (Dentinger, Didukh & Moncalvo, 2011; Cai, Tang & Yang, 2012; Buyck et al., 2014; Badotti et al., 2017). Other candidate segments that have been used as barcoding markers for mushrooms previously, including the mitochondrial cytochrome oxidase I gene (cox1) (Vialle et al., 2009), the second subunit of RNA polymerase II (RPB2) (Li et al., 2017), and the β-tubulin and elongation factor 1-α (tef1) (Guo et al., 2016).
The goal of the present study was to test the utility of DNA barcodes to the identification of the Lepista species as edible species to address the question, that is, due to the limited morphological characteristics within the genus Lepista, misidentification often happened. To address the question, we evaluated the following five markers as DNA barcodes for identification of eight Lepista species: ITS region, the intergenic spacer (IGS), the large nuclear ribosomal RNA subunit (nLSU), the mitochondrial small subunit rDNA (mtSSU), and tef1.
Materials and Methods
Ethics statement
Lepista species are neither protected nor endangered in the sampled areas, and all samples were collected by researchers following current Chinese regulations. None of the sampled locations are privately owned or protected by law.
Sampling
In a previous study (Alvarado et al., 2015), the genus Lepista was divided into three clades. The current study included two species from each of the three clades plus two unidentified Lepista species. A total of 34 samples of the eight Lepista species were collected from September 2012 to August 2017 (Table 1). Tissue blocks were removed from the inner part of the fresh basidiomata for DNA analyses. The specimens were dried with an electric air ventilation drier and deposited in the Fungal Herbarium of Shenyang Agricultural University (SYAU-FUNGI).
| Taxon | Specimen vouchera | ITSb | IGSb | nLSUb | mtSSUb | tef1b |
|---|---|---|---|---|---|---|
| Lepista densifolia | SYAU-FUNGI-022 | MK116588 | MK389519 | – | MK389570 | – |
| Lepista irina | SYAU-FUNGI-023 | MK116589 | MK389520 | MK389546 | MK389571 | MK551215 |
| Lepista irina | SYAU-FUNGI-024 | MK116590 | MK389521 | MK389547 | MK389572 | MK551216 |
| Lepista irina | SYAU-FUNGI-025 | MK116591 | – | MK389548 | MK389573 | – |
| Lepista nuda | SYAU-FUNGI-021 | MH428843 | MK389523 | MK389549 | MK389575 | MK440311 |
| Lepista nuda | SYAU-FUNGI-026 | MK116594 | – | – | – | MK440315 |
| Lepista nuda | SYAU-FUNGI-017 | MH428839 | MK389524 | MK389550 | MK389576 | MK440312 |
| Lepista nuda | SYAU-FUNGI-019 | MH428841 | – | MK389551 | MK389577 | MK440313 |
| Lepista nuda | SYAU-FUNGI-027 | MK116593 | MK389525 | MK389552 | MK389578 | MK440314 |
| Lepista nuda | SYAU-FUNGI-014 | MH428836 | MK389526 | MK389553 | MK389579 | MK440315 |
| Lepista nuda | SYAU-FUNGI-028 | MK116595 | – | MK389554 | MK389580 | MK440317 |
| Lepista nuda | SYAU-FUNGI-029 | MK116592 | MK389522 | – | MK389574 | MK440310 |
| Lepista panaeola | SYAU-FUNGI-030 | MK116597 | MK389527 | – | MK389581 | – |
| Lepista panaeola | SYAU-FUNGI-031 | MK116598 | – | – | – | – |
| Lepista panaeola | SYAU-FUNGI-032 | MK116599 | MK389529 | – | MK389583 | MK551218 |
| Lepista panaeola | SYAU-FUNGI-033 | MK116600 | MK389530 | MK389555 | MK389584 | – |
| Lepista panaeola | SYAU-FUNGI-034 | MK116601 | MK389531 | MK389556 | MK389585 | – |
| Lepista panaeola | SYAU-FUNGI-035 | MK116596 | MK389528 | MK389557 | MK389582 | MK551217 |
| Lepista saeva | SYAU-FUNGI-036 | MK116602 | MK389532 | MK389558 | MK389586 | – |
| Lepista saeva | SYAU-FUNGI-037 | MK116603 | MK389533 | MK389559 | MK389587 | – |
| Lepista saeva | SYAU-FUNGI-038 | MK116604 | MK389534 | MK389560 | MK389588 | – |
| Lepista sordida | SYAU-FUNGI-039 | MK116605 | MK389535 | MK389561 | MK389589 | MK551219 |
| Lepista sordida | SYAU-FUNGI-040 | MK116606 | MK389536 | – | MK389590 | – |
| Lepista sordida | SYAU-FUNGI-041 | MK116607 | MK389537 | MK389563 | MK389591 | – |
| Lepista sordida | SYAU-FUNGI-042 | MK116609 | MK389539 | MK389564 | MK389594 | MK551221 |
| Lepista sordida | SYAU-FUNGI-043 | MK116610 | MK389540 | MK389565 | MK389593 | MK551222 |
| Lepista sordida | SYAU-FUNGI-044 | MK116608 | MK389538 | MK389562 | MK389592 | MK551220 |
| Lepista sp 1 | SYAU-FUNGI-045 | MK116611 | – | – | – | MK440305 |
| Lepista sp 1 | SYAU-FUNGI-046 | MK116612 | – | MK389567 | – | MK440306 |
| Lepista sp 1 | SYAU-FUNGI-047 | MK116613 | MK389541 | MK389568 | MK389597 | MK440307 |
| Lepista sp 1 | SYAU-FUNGI-048 | MK116614 | MK389542 | MK389566 | MK389595 | MK440308 |
| Lepista sp 1 | SYAU-FUNGI-049 | MK116615 | MK389543 | – | MK389596 | MK440309 |
| Lepista sp 2 | SYAU-FUNGI-050 | MK116617 | MK389544 | – | – | – |
| Lepista sp 2 | SYAU-FUNGI-051 | MK116616 | MK389545 | MK389569 | – | – |
Morphological observations
Morphological identification was based on previous studies (Singer, 1986; Bon, 1987; Li et al., 2015). Microscopic characteristics of the basidiomata were assessed by examining dried specimens that had been treated with 5% KOH solution and Melzer’s reagent with a light microscope.
DNA extraction, amplification, and sequencing
Genomic DNA was extracted from fresh blocks of tissue with a plant DNA extraction kit (Sunbiotech, Beijing, China). Crude DNA extracts were used as templates for PCR, and a total of five primers were used for amplification (Table 2). Reaction mixtures were as described by Yu et al. (2014). For the amplification of ITS, IGS, nLSU, and mtSSU, the PCR conditions consisted of an initial denaturation at 94 °C for 2 min; followed by 30 cycles of denaturation at 94 °C for 35 s, annealing at 45 °C for 35 s, and extension at 72 °C for 1 min; and a final extension at 72 °C for 10 min. For tef1, the PCR protocol consisted of initial denaturation at 94 °C for 2 min; followed by 10 cycles at 94 °C for 35 s, 57 °C for 35 s (decreasing 0.3 °C per cycle), and 72 °C for 1 min; followed by 29 cycles at 94 °C for 35 s, 54 °C for 35 s, and 72 °C for 1 min; and a final extension at 72 °C for 10 min. PCR products were checked on a 1.0% agarose gel and visualized by staining with ethidium bromide. Sequencing was performed on an ABI Prism 3730 genetic analyzer (PE Applied Biosystems, Foster City, CA, USA). The sequences generated from this study are listed in Table 1.
| Regions | Primer | Sequence (5′-3′) | Reference |
|---|---|---|---|
| ITS | ITS5 | GGA AGT AAA AGT CGT AAC AAG G | White et al. (1990) |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | White et al. (1990) | |
| IGS | CNL12 | CTG AAC GCC TCT AAG TCA G | White et al. (1990) |
| 5SA | CAG AGT CCT ATG GCC GTG AT | White et al. (1990) | |
| nLSU | LROR | ACC CGC TGA ACT TAA GC | Rehner & Samuels (1994) |
| LR7 | TAC TAC CAC CAA GAT CT | Vilgalys & Hester (1990) | |
| mtSSU | MS1 | CAG CAG TCA AGA ATA TTA GTC AAT G | White et al. (1990) |
| MS2 | GCG GAT TAT CGA ATT AAA TAA C | White et al. (1990) | |
| tef1 | tefF | TAC AAR TGY GGT GGT ATY GAC A | Morehouse et al. (2003) |
| tefR | ACN GAC TTG ACY TCA GTR GT | Morehouse et al. (2003) |
Data analyses
Sequences of each region were aligned with Clustal X (Thompson et al., 1997) and then manually edited with BioEdit 5.0.6 (Hall et al., 2003). The aligned sequences of each region were analyzed using DNAstar 7.1.0 (Lasergene, WI, USA) to calculate the similarity matrices. The intra- and inter-specific variations of the candidate barcode loci for each species were then assessed using TaxonGap 2.4.1 (Slabbinck et al., 2008). Finally, the results were processed and showed by GSview 4.9.
Genetic pairwise distances for evaluating the sequence variations within and between species of the potential barcode regions were computed using MEGA 7.0 (Kumar, Stecher & Tamura, 2016) based on the Kimura 2-Parameter (K2P) model (Kimura, 1980). Barcoding gaps comparing the distributions of the pairwise intra- and inter-specific distances for each candidate barcode with distance intervals of 0.004 (ITS, nLSU, and mtSSU) or 0.008 (IGS and tef1) were estimated in Microsoft Excel 2016.
Neighbor-joining tree reconstruction
To show the relationships among the eight Lepista species, a neighbor-joining tree was constructed based on the ITS region using MEGA with the K2P substitution model. Branch support was calculated by a bootstrap analysis with 1,000 replicates, and Tricholoma matsutake (AB699640) was used as the outgroup. For comparison, the combined dataset of five regions was used to construct a neighbor-joining tree. Alignments have been deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S24378).
Results
PCR amplification and sequencing
A total of 134 sequences of the five candidate DNA barcode regions were obtained from the eight Lepista species (Table 1). The five regions were then evaluated for their potential as barcoding markers (Table 3). Sequence lengths ranged from 400 bp for IGS to 1,000 bp for nLSU, that is, all five regions were sufficiently short to be used as barcode markers. The amplification success rate exceeded 90% for all regions except tef1, and the sequencing success rate was highest (100%) for ITS.
| Region | Region length (bp) | Total number of samples | No. of PCR successes | PCR success rate (%) | No. of sequencing successes | Sequencing success rate (%) |
|---|---|---|---|---|---|---|
| ITS | 605–615 | 34 | 34 | 100 | 34 | 100 |
| IGS | 415–440 | 34 | 34 | 100 | 27 | 79 |
| nLSU | 934–939 | 34 | 33 | 97 | 24 | 71 |
| mtSSU | 662–740 | 34 | 32 | 94 | 28 | 82 |
| tef1 | 861–920 | 34 | 23 | 68 | 21 | 62 |
Intra- and inter-specific variation
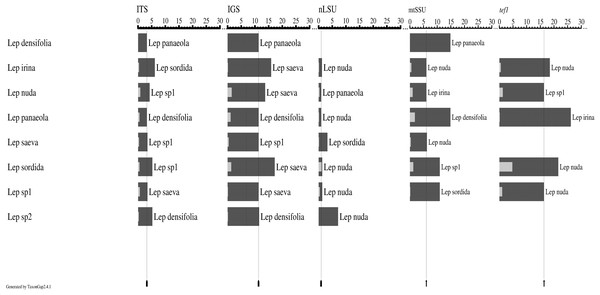
According to TaxonGap analyses of the intra- and inter-specific variations of the candidate DNA barcode regions, ITS, IGS, tef1, and mtSSU provided a somewhat better resolution of the eight species than nLSU. Except for nLSU, the other four regions showed significant inter- and intra-specific variation (Fig. 1).
Figure 1: Intra- and inter-specific variations among the candidate barcode regions (ITS, IGS, nLSU, mtSSU, and tef1) from eight Lepista species.
Graphs were generated by TaxonGap software. The black and gray bars represent the level of inter- and intra-specific variations, respectively. The thin black lines indicate the lowest inter-specific variation for each candidate barcode. Taxon names next to the dark bars indicate the most closely related species among the species listed on the left. Four regions, that is, ITS, IGS, tef1, and mtSSU, showed significant inter- and intra-specific variation.Barcoding gaps
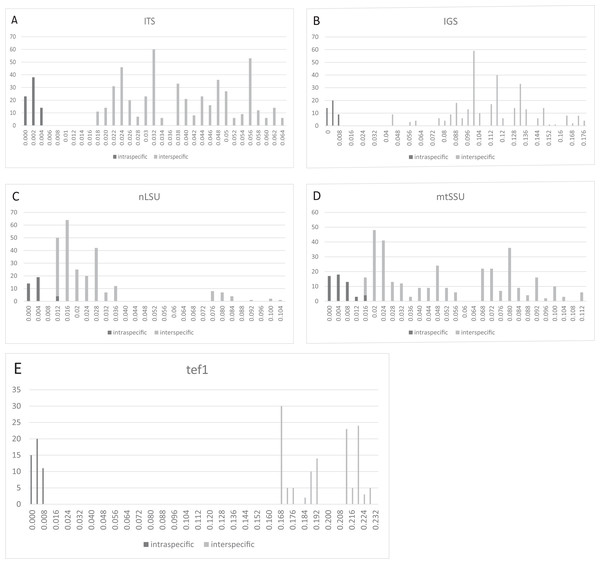
Three regions, that is, ITS (Fig. 2A), IGS (Fig. 2B), and tef1 (Fig. 2E), had relatively clear barcoding gaps. The two remaining candidate barcodes (mtSSU and nLSU) had overlaps between their intra- and inter-specific distances (Figs. 2C and 2D).
Figure 2: Frequency distributions of intra- and inter-specific Kimura-2-Parameter pairwise distances among ITS, IGS, nLSU, mtSSU, and tef1 datasets from eight Lepista spp.
The black and gray bars represent the level of intra- and inter-specific variations, respectively. Three regions, that is, ITS, IGS, and tef1, had relatively clear barcoding gaps. (A) ITS. (B) IGS. (C) nLSU. (D) mtSSU. (E) tef1.Neighbor-joining analysis
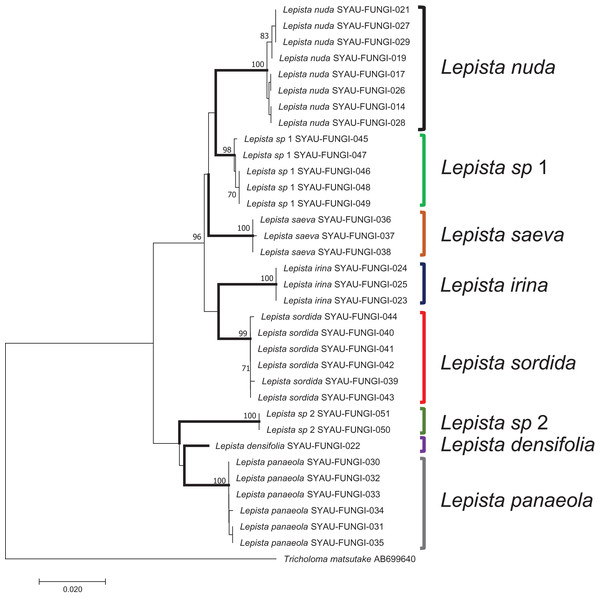
In a tree generated by a neighbor-joining analysis of the ITS region, the eight species were well-separated from each other and formed independent terminal branches (Fig. 3). Sequences from different samples of the same species showed high bootstrap values. Two clades, named Lepista sp 1 and L. sp 2, were supported by high bootstrap values and were inferred to represent new species of Lepista. The topology of the combined dataset tree was similar to that produced by ITS region (Fig. S1).
Figure 3: A neighbor-joining tree generated by analysis of ITS from eight Lepista spp.
Bootstrap values ≥70% are shown above the relevant branches. The eight Lepista spp. are highlighted in bold.Discussion
There are two important factors for evaluating candidate DNA barcodes: a high success rate of PCR amplification and sequencing, and substantially greater inter-specific than intra-specific variation. In the current study, the ITS region had high success rates of amplification and sequencing, substantially greater inter-specific than intra-specific variation, as well as clear barcoding gaps among the Lepista species. Based on the criteria, we therefore conclude that the ITS region would be useful for the identification of Lepista species and determine that the ITS is a suitable DNA barcode for the genus Lepista.
The ITS region has been proposed as a universal barcode for fungi (Schoch et al., 2012). The region is present in several chromosomes and is arranged in tandem repeats that are thousands of copies long (Ajmal Ali et al., 2014). Because of the high copy number, the ITS region is easy to amplify and sequence, even with samples from very old specimens (Larsson & Jacobsson, 2004). ITS has been found to be a suitable barcode for some groups in the Agaricales, including the genus Cortinarius (Liimatainen et al., 2014; Stefani, Jones & May, 2014) and the family Lyophyllaceae (Bellanger et al., 2015).
Although IGS had a high PCR success rate (100%) and suitable inter- and intra-specific variation, its sequencing success rate was relatively low (82%), which made it the second best marker after ITS for identification of Lepista species. IGS has been previously used to differentiate among species and even among strains within the same species in yeasts (Fell et al., 2000; Scorzetti et al., 2002). In the current study, the regions of nLSU and mtSSU lacked barcoding gaps in the analysis of intra- and inter-specific distance. tef1 showed clear barcoding gaps, but its amplification and sequencing success rates were low.
In preliminary studies, we also assessed the largest subunit of RNA polymerase II (RPB1) and RPB2, but we obtained only six sequences of RPB2 and one sequence of RPB1. These numbers of RPB1 and RPB2 sequences were too small for analysis of barcoding, and the two regions were therefore not included in this study.
According to the phylogenetic analysis based on the ITS region, the eight Lepista species received high support (≥98%), which demonstrates that ITS could be used for the identification of Lepista species. The two new clades identified in the present study may represent two new species. Identification of cryptic species by DNA barcoding has been reported in the other groups, such as Amillariella (Guo et al., 2016) and Pleurotus (Li et al., 2017). In future research, the morphological characteristics of Lepista sp 1 and L. sp 2 should be described, and the utility of ITS as a barcode for identification of additional Lepista species should be evaluated.
Conclusions
In this study, we assessed five regions for identifying a DNA barcode for eight Lepista species. Only the ITS region had the highest success rates of amplification and sequencing, substantially greater inter-specific than intra-specific variation. Therefore, we propose that the ITS region could be used as a suitable DNA barcode for the genus Lepista. And the ITS region also could separate all the tested Lepista species in the phylogenetic analyses. Overall, the ITS region was proved as a reference marker for the other species.
Supplemental Information
A neighbor-joining tree generated by analysis of five regions from eight Lepista spp.
Figure S1. A neighbor-joining tree generated by analysis of five regions from eight Lepista spp. Bootstrap values ≥70% are shown above the relevant branches. The eight Lepista spp. are highlighted in bold.