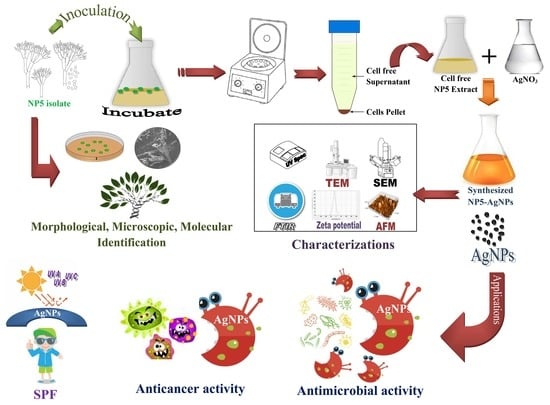
Myco-Nanofabrication of Silver Nanoparticles by Penicillium brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line
Abstract
:1. Introduction
2. Results
2.1. Morphological and Molecular Characterization of NP5 Fungal Strain
2.2. Green Synthesis of NP5-AgNPs and Their Characterization
2.2.1. UV-Visible Spectroscopy of NP5-AgNPs from Penicillium brasilianum NP5
2.2.2. FTIR Analysis of NP5-AgNPs
2.2.3. AFM analysis of NP5-AgNPs
2.2.4. TEM analysis of NP5-AgNPs
2.2.5. EDX analysis of NP5-AgNPs
2.2.6. XRD analysis of NP5-AgNPs
2.2.7. Zeta Potential analysis of NP5-AgNPs
2.2.8. TGA analysis of NP5-AgNPs
2.3. Biological Activity of Synthesized NP5-AgNPs
2.3.1. Antimicrobial Activity of NP5-AgNPs
2.3.2. Anticancer Activity of NP5-AgNPs
2.3.3. Apoptosis Assay of NP5-AgNPs by Flow Cytometry
2.3.4. SPF analysis of the AgNPs Synthesized from Penicillium brasilianum NP5
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Isolation of Fungi from Soil Sample
4.3. Primary Screening of Fungi Isolates
4.4. Morphological and Molecular Characterization of NP5 Isolate
4.5. Preparation of Fungal Extract for Nanoparticle Synthesis
4.6. Synthesis of AgNPs Using NP5 Fungal Extract
4.7. Characterization of Synthesized AgNPs Synthesized from NP5 Fungal Extract
4.7.1. UV-Visible Spectroscopy Analysis of Synthesized NP5-AgNPs
4.7.2. Fourier Transform Infrared Spectroscopy of Synthesized NP5-AgNPs
4.7.3. Atomic Force Microscopic Analysis of Synthesized NP5-AgNPs
4.7.4. Transmission Electron Microscopy of NP5-AgNPs
4.7.5. Energy Dispersive X-ray Analysis of Synthesized NP5-AgNPs
4.7.6. X-ray Diffractometric Analysis of NP5-AgNPs
4.7.7. Zeta Potential Analysis of Synthesized NP5-AgNPs
4.7.8. Thermo Gravimetric Analysis of Synthesized NP5-AgNPs
4.8. Biological Activity of Synthesized NP5-AgNPs
4.8.1. Antimicrobial Activity of Synthesized NP5-AgNPs
4.8.2. Anticancer Activity of Synthesized NP5-AgNPs
4.8.3. Apoptosis Assay of NP5-AgNPs by Flow Cytometry
4.8.4. Determination of SPF Value of Synthesized NP5-AgNPs
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulzer, F.; Orrit, M. Single-molecule optics. Ann. Rev. Phys. Chem. 2004, 55, 585–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Bonde, S.; Golinsk, P.; Trzcinska-Wencel, J.; Gade, A.; Abd-Elsalam, K.A.; Shende, S.; Gaikwad, S.; Ingle, A.P. Fusarium as a novel fungus for the synthesis of nanoparticles: Mechanism and applications. J. Fungi. 2021, 7, 139. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B. 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Ranjani, S.; Shariq, A.M.; Senthil-Kumar, N.; Ruckmani, K.; Hemalatha, S. Synthesis, characterization and applications of endophytic fungal nanoparticles. Inorg. Nano-Metal Chem. 2021, 51, 280–287. [Google Scholar] [CrossRef]
- Nayaka, S.; Chakraborty, B.; Bhat, M.P.; Nagaraja, S.K.; Airodagi, D.; Swamy, P.S.; Rudrappa, M.; Hiremath, H.; Basavarajappa, D.S.; Kanakannanavar, B. Biosynthesis, characterization, and in vitro assessment on cytotoxicity of actinomycete-synthesized silver nanoparticles on Allium cepa root tip cells. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 51. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Sara, M.; Hossein, J.; Ashraf-Asadat, N. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 2022, 34, 1018–3647. [Google Scholar] [CrossRef]
- Fu, M.; Li, Q.; Sun, D.; Lu, Y.; He, N.; Deng, X.; Wang, H.; Huang, J. Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chin. J. Chem. Eng. 2006, 14, 114–117. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Mustafa, D.E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Guliger-Casagrande, M.; Germano-Costa, T.; Pasquoto-Stigliani, T.; Fraceto, L.F.; Lima, R. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci. Rep. 2019, 9, 14351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaus, T.; Joerger, R.; Olsson, F.; Granqvist, C.G. Silver-based crystalline nanoparticles; microbial fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Basu, A.; Kundu, S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina paseolina. Nanoscale Res. Lett. 2014, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Zomorodian, K.; Pourshahid, S.; Sadatsharifi, A.; Mehryar, P.; Pakshir, K.; Rahimi, M.J.; Monfared, A.A. Biosynthesis and characterization of silver nanoparticles by Aspergillus species. BioMed Res. Internat. 2016, 2016, 5435397. [Google Scholar] [CrossRef] [Green Version]
- Bhat, M.A.; Nayak, B.K.; Nanda, A. Evaluation of bactericidal activity of biologically synthesized silver nanoparticles from Candida albicans in combination with ciprofloxacin. Mat. Today Proc. 2015, 2, 4395–4401. [Google Scholar] [CrossRef]
- Vijayan, S.; Divya, K.; George, T.K.; Jisha, M.S. Biogenic synthesis of silver nanoparticles using endophytic fungi Fusarium oxysporum isolated from Withania somnifera, its antimicrobial and cytotoxic activity. J. Bionanosci. 2016, 10, 369–376. [Google Scholar] [CrossRef]
- Rajput, S.; Werezuk, R.; Lange, R.M.; Mcdermott, M.T. Fungal isolate optimized for biogenesis of silver nanoparticles with enhanced colloidal stability. Langmuir 2016, 32, 8688–8697. [Google Scholar] [CrossRef]
- Burdusel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Res. Internat. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef] [Green Version]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, N.S.; Koli, S.H.; Mohite, B.V.; Patil, V.S.; Patil, S.V. Xanthomonadin mediated synthesis of biocidal and photo-protective silver nanoparticles (XP-AgNPs). Res. Chem. 2022, 4, 100663. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Subashini, G.; Bhuvaneswari, S. Nanoparticles from Fungi (Myconanoparticles). In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Mohammadi, B.; Salouti, M. Extracellular bioynthesis of silver nanoparticles by Penicillium chrysogenum and Penicillium expansum. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2015, 45, 844–847. [Google Scholar] [CrossRef]
- Honary, H.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop. J. Pharm. Res. 2013, 1, 7–11. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environ. Dev. Sustain. 2015, 17, 731–745. [Google Scholar] [CrossRef]
- Taha, Z.K.; Hawar, S.N.; Sulaiman, G.M. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol. Lett. 2019, 41, 899–914. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, X.; Wei, J. Localized surface plasmon resonance of silver nano triangles synthesized by a versatile solution reaction. Nanoscale Res. Lett. 2015, 10, 354. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Bakr, O.M.; Stellacci, F.A. Study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.M.A.; Almunqedhi, B.M.A. Biosynthesis of silver nanoparticles using Penicillium verrucosum and analysis of their antifungal activity. Saud. J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Ballottin, D.; Fulaz, S.; Souza, M.L.; Corio, P.; Rodrigues, A.G.; Souza, A.O.; Gaspari, P.M.; Gomes, A.F.; Gozzo, F.; Tasic, L. Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Res. Lett. 2016, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Internat. J. Nanomed. 2015, 10, 4203–4223. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Gen. Eng. Biotech. 2017, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasa, N.; Meghashyama, B.P.; Pallavi, S.S.; Bidhayak, C.; Dattatraya, A.; Muthuraj, R.; Shashiraj, K.N.; Halaswamy, H.; Dhanyakumara, S.B.; Vaishnavi, M.D. Biogenic synthesis of silver nanoparticles using Paenibacillus sp. in-vitro and their antibacterial, anticancer activity assessment against human colon tumour cell line. J. Environ. Biol. 2021, 42, 118–127. [Google Scholar] [CrossRef]
- Rudrappa, M.; Rudayni, H.A.; Assiri, R.A.; Bepari, A.; Basavarajappa, D.S.; Nagaraja, S.K.; Chakraborty, B.; Swamy, P.S.; Agadi, S.N.; Niazi, S.K.; et al. Plumeria alba-mediated green synthesis of silver nanoparticles exhibits antimicrobial effect and anti-oncogenic activity against Glioblastoma U118 MG cancer cell line. Nanomaterials 2022, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Khan, S.; Fu, P.C. Fungus- (Alternaria sp.) mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green synthesis of silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Saud. J. Biol. Sci. 2022, 29, 228–238. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Su, W.; Liu, J.; Zeng, X.; Huang, Z.; Li, W.; Liu, Z.; Tang, J. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mat. Sci. Eng. C 2017, 77, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Sytu, M.R.C.; Camacho, D.H. Green synthesis of silver nanoparticles (AgNPs) from Lenzites betulina and the potential synergistic effect of AgNP and capping biomolecules in enhancing antioxidant activity. BioNanoScience 2018, 8, 835–844. [Google Scholar] [CrossRef]
- Fafal, T.; Taştan, P.; Tüzün, B.S.; Ozyazici, M.; Kivcak, B. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Asphodelus aestivus Brot. aerial part extract. S. Afr. J. Bot. 2017, 112, 346–353. [Google Scholar] [CrossRef]
- Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Kotresha, D.; Rudrappa, M.; Pallavi, S.S.; Hiremath, H.; Perumal, K.; Nayaka, S. Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J. King Saud Univ. Sci. 2021, 33, 101660. [Google Scholar] [CrossRef]
- Swamy, P.S.; Bhat, M.P.; Nayaka, S. Amycolatopsis sp. strain MN235945 mediated biosynthesis of silver nanoparticles: Characterization, antimicrobial and anticancer activity against HeLa and MCF-7 cell lines. Indian J. Pharm. Sci. 2022, 84, 1178–1188. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kumar, R.S.; Rudrappa, M.; Basavarajappa, D.S.; Swamy, P.S.; Almansour, A.I.; Perumal, K.; Nayaka, S. Bio-inspired silver nanoparticles from Artocarpus lakoocha fruit extract and evaluation of their antibacterial activity and anticancer activity on human prostate cancer cell line. Appl. Nanosci. 2022, 13, 3041–3051. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against Gram-positive and Gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef] [Green Version]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.K.; Niazi, S.K.; Bepari, A.; Assiri, R.A.; Nayaka, S. Leonotis nepetifolia flower bud extract mediated green synthesis of silver nanoparticles, their characterization, and in vitro evaluation of biological applications. Materials 2022, 15, 8990. [Google Scholar] [CrossRef]
- Edetsberger, M.; Gaubitzer, E.; Valic, E.; Waigmann, E.; Kohler, G. Detection of nanometer-sized particles in living cells using modern fluorescence fluctuation methods. Biochem. Biophys. Res. Commun. 2005, 332, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, C.; Guo, Z.; Wang, Z.; Liu, L. Silver nanocrystals mediated combination therapy of radiation with magnetic hyperthermia on glioma cells. J. Nanosci. Nanotechnol. 2012, 12, 8276–8281. [Google Scholar] [CrossRef] [PubMed]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Ali, A.A.A.L.A. Effect of Ag nanoparticles on viability of MCF-7 and Vero cell lines and gene expression of apoptotic genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, V.K.; Das, S.K.; Mishra, N.; Bisht, L.; Joshi, A.; Sharma, N. Characterization and anti-cancerous effect of Putranjiva roxburghii seed extract mediated silver nanoparticles on human colon (HCT-116), pancreatic (PANC-1) and breast (MDA-MB 231) cancer cell lines: A comparative study. Int. J. Nanomed. 2020, 15, 573–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajiri, M.S.; Aminsalehi, M.; Shahbandeh, M.; Maleki, A.; Jonoubi, P.; Rad, A.C. Anticancer and therapeutic potential of Delonix regia extract and silver nanoparticles (AgNPs) against pancreatic (Panc-1) and breast (MCF-7) cancer cell. Toxicol. Environ. Health Sci. 2021, 13, 45–56. [Google Scholar] [CrossRef]
- Mello, D.F.; Trevisan, R.; Rivera, N.; Geitner, N.K.; Di Giulio, R.T.; Wiesner, M.R.; Hsu-Kim, H.; Meyer, J.N. Caveats to the use of MTT, neutral red, Hoechst and Resazurin to measure silver nanoparticle cytotoxicity. Chem. Biol. Interact. 2020, 5, 315. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver nanoparticle catalysed redox reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S025405840500550X (accessed on 4 March 2023). [CrossRef]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhrandnia, S.; Nystrom, B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Otohinoyi, D.A. Inorganic nanoparticles restrict viability of metastatic breast cancer cells in vitro. Comp. Clin. Pathol. 2019, 28, 949–954. [Google Scholar] [CrossRef]
- Yang, S.I.; Liu, S.; Brooks, G.J.; Lanctot, Y.; Gruber, J.V. Reliable and simple spectrophotometric determination of sun protection factor: A case study using organic UV filter-based sunscreen products. J. Cos. Dermat. 2018, 17, 518–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawwaz, M.; Saleh, A.; Makalalag, M.I. Sun protection factor activity of unregistered facial cream in Makassar City. Internat. J. Chem. Conc. 2017, 3, 342–346. [Google Scholar]
- Salem, S.S.; Fouda, M.M.G.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.M.; Shaheen, T.I. Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J. Clust. Sci. 2021, 32, 351–361. [Google Scholar] [CrossRef]
- Nayaka, S.; Chakraborty, B.; Airodgi, D.; Bhat, M.P.; Nagaraja, S.K.; Swamy, P.S.; Rudrappa, M.; Hiremath, H.; Basavarajappa, D.S.; Rego, M. In-vitro antimicrobial activity of biological synthesized silver nanoparticles using Stenotrophomonas maltophilia strain NS-24 from non-rhizosphere soil. Int. J. Pharm. Pharm. Sci. 2020, 12, 73–79. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Dupadahalli, K.; Rudrappa, M.; Basavarajappa, D.S.; Swamy, P.S.; Perumal, K.; Nayaka, S. Characterization, antimicrobial activity and anticancer activity of Pyrostegia venusta leaf extract-synthesized silver nanoparticles against COS-7 cell line. Appl. Nanosci. 2023, 13, 2303–2314. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Internat. J. Microbiol. 2019, 8, 8642303. [Google Scholar] [CrossRef] [Green Version]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; Hassan, S.E.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Bepari, A.; Assiri, R.A.; Niazi, S.K.; Nayaka, S.; Rudrappa, M.; Nagaraja, S.K.; Bhat, M.P. Saussurea lappa exhibits anti-oncogenic effect in hepatocellular carcinoma, HepG2 cancer cell line by Bcl-2 mediated apoptotic pathway and mitochondrial cytochrome C release. Cur. Iss. Mol. Biol. 2021, 43, 1114–1132. [Google Scholar] [CrossRef]
- Olayemi, O.; Isimi, C.; Ekere, K.; Gbate, M.A.; Emeje, M. Determination of sun protection factor number: An emerging in–vitro tool for predicting UV protection capabilities. Internat. J. Herb. Med. 2017, 5, 6–9. [Google Scholar]
- Rudrappa, M.; Nayaka, S.; Kumar, S.K. In silico molecular docking approach of melanin against melanoma causing MITF proteins and anticancer, oxidation–reduction, photoprotection, and drug-binding affinity properties of extracted melanin from Streptomyces sp. strain MR28. Appl. Biochem. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]














| Fungal Isolates | Zone of Inhibition in mm | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| NP1 | 14 | 10 | 11 |
| NP2 | 13 | 0 | 12 |
| NP3 | 0 | 0 | 0 |
| NP4 | 11 | 0 | 14 |
| NP5 | 13 | 11 | 15 |
| NP6 | 10 | 0 | 13 |
| NP7 | 12 | 0 | 10 |
| NP8 | 14 | 0 | 9 |
| NP9 | 0 | 0 | 12 |
| NP10 | 0 | 0 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudrappa, M.; Kumar, R.S.; Nagaraja, S.K.; Hiremath, H.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K.; Nayaka, S. Myco-Nanofabrication of Silver Nanoparticles by Penicillium brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line. Antibiotics 2023, 12, 567. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics12030567
Rudrappa M, Kumar RS, Nagaraja SK, Hiremath H, Gunagambhire PV, Almansour AI, Perumal K, Nayaka S. Myco-Nanofabrication of Silver Nanoparticles by Penicillium brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line. Antibiotics. 2023; 12(3):567. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics12030567
Chicago/Turabian StyleRudrappa, Muthuraj, Raju Suresh Kumar, Shashiraj Kareyellappa Nagaraja, Halaswamy Hiremath, Pooja Vidyasagar Gunagambhire, Abdulrahman I. Almansour, Karthikeyan Perumal, and Sreenivasa Nayaka. 2023. "Myco-Nanofabrication of Silver Nanoparticles by Penicillium brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line" Antibiotics 12, no. 3: 567. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics12030567