(R,S)-5-(4-Chlorophenyl)-3-{(E)-2-[(R,S)-2,6,6-trimethylcyclohex-2-en-1-yl]vinyl}-4,5-dihydro-1H-pyrazole-1-carboximidamide Hydrochloride
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
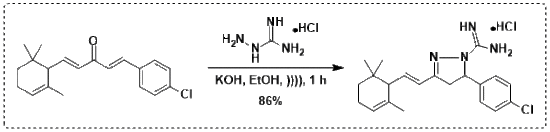
3.2. Procedure for the Synthesis of Terpenyl Pyrazoline 3
3.3. Characterization and Experimental Data of Terpenyl Pyrazoline 3
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marin, J.; Kumar, R. 4,5-Dihydro-1H-pyrazole: An indispensable scaffold. J. Enzyme Inhib. Med. Chem. 2014, 29, 427–442. [Google Scholar] [CrossRef]
- Silva, C.C.; Martins, R.; Lund, R.; Pizzuti, L.; Pereira, C.M.P. Recent Highlights on the Synthesis of Pyrazoles with Antimicrobial Activity. Curr. Bioact. Comp. 2018, in press. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, S.; Suryawanshi, S.N.; Mittal, M.; Vishwakarma, P.; Gupta, S. Chemotherapy of leishmaniasis part X: Synthesis and bioevaluation of novel terpenyl heterocycles. Bioorg. Med. Chem. Lett. 2013, 23, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, D.; Zabinski, J.; Kazmierczak, P.; Rezler, M.; Krassowska-Swiebocka, B.; Collins, M.S.; Cushion, M.T. Analogs of pentamidine as potential anti-Pneumocystis chemotherapeutics. Eur. J. Med. Chem. 2012, 48, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebeschuetz, J.W.; Jones, S.D.; Morgan, P.J.; Murray, C.W.; Rimmer, A.D.; Roscoe, J.M.E.; Waszkowycz, B.; Welsh, P.M.; Wylie, W.A.; Young, S.C.; et al. PRO_SELECT: Combining Structure-Based Drug Design and Array-Based Chemistry for Rapid Lead Discovery. 2. The Development of a Series of Highly Potent and Selective Factor Xa Inhibitors. J. Med. Chem. 2002, 45, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, H.; Yang, S.; Chreifi, G.; Martásek, P.; Roman, L.J.; Meyskens, F.L.; Poulos, T.L.; Silverman, R.B. Potent and Selective Double-Headed Thiophene-2-carboximidamide Inhibitors of Neuronal Nitric Oxide Synthase for the Treatment of Melanoma. J. Med. Chem. 2014, 57, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Jana, G.H.; Jain, S.; Arora, S.K.; Sinha, N. Synthesis of some diguanidino 1-methyl-2,5-diaryl-1H-pyrroles as antifungal agents. Bioorg. Med. Chem. Lett. 2005, 15, 3592–3595. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Model List of Essential Medicines. 2017. Available online: http://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 27 December 2018).
- Pizzuti, L.; Martins, P.L.G.; Ribeiro, B.A.; Quina, F.H.; Pinto, E.; Flores, A.F.C.; Venzke, D.; Pereira, C.M.P. Efficient sonochemical synthesis of novel 3,5-diaryl-4,5-dihydro-1H-pyrazole-1-carboximidamides. Ultrason. Sonochem. 2010, 17, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.F.S.; Cury, N.M.; Nascimento, T.A.; Raminelli, C.; Casagrande, G.A.; Pereira, C.M.P.; Simionatto, E.; Yunes, J.A.; Pizzuti, L. Ultrasound-Promoted Synthesis of 3-(Thiophen-2-yl)-4,5-dihydro-1H-pyrazole-1-carboximidamides and Anticancer Activity Evaluation in Leukemia Cell Lines. J. Braz. Chem. Soc. 2017, 28, 217–224. [Google Scholar] [CrossRef]
- Brasil, S.R.; Capiotto, A.C.; Flores, A.F.C.; Back, D.F.; Faoro, E.; Pizzuti, L. Unexpected Formation of Bis(hydrazinecarboximidamide) via Ultrasound Promoted Rearrangement of Epoxy Ketone. Orbital Electron. J. Chem. 2017, 9, 204–209. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, G.; Batist, G.; Wua, J.H. Syntheses and potential anti-prostate cancer activities of ionone-based chalcones. Bioorg. Med. Chem. Lett. 2009, 19, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Bristow, A.W.T.; Webb, K.S. Intercomparison study on accurate mass measurement of small molecules in mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1086–1098. [Google Scholar] [CrossRef] [Green Version]
- Knolhoff, A.M.; Callahan, J.H.; Croley, T.R. Mass accuracy and isotopic abundance measurements for HR-MS instrumentation: Capabilities for non-targeted analyses. J. Am. Soc. Mass Spectrom. 2014, 25, 1285–1294. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, M.C.; Pizzuti, L. (R,S)-5-(4-Chlorophenyl)-3-{(E)-2-[(R,S)-2,6,6-trimethylcyclohex-2-en-1-yl]vinyl}-4,5-dihydro-1H-pyrazole-1-carboximidamide Hydrochloride. Molbank 2019, 2019, M1046. https://0-doi-org.brum.beds.ac.uk/10.3390/M1046
Carvalho MC, Pizzuti L. (R,S)-5-(4-Chlorophenyl)-3-{(E)-2-[(R,S)-2,6,6-trimethylcyclohex-2-en-1-yl]vinyl}-4,5-dihydro-1H-pyrazole-1-carboximidamide Hydrochloride. Molbank. 2019; 2019(1):M1046. https://0-doi-org.brum.beds.ac.uk/10.3390/M1046
Chicago/Turabian StyleCarvalho, Michele Cristina, and Lucas Pizzuti. 2019. "(R,S)-5-(4-Chlorophenyl)-3-{(E)-2-[(R,S)-2,6,6-trimethylcyclohex-2-en-1-yl]vinyl}-4,5-dihydro-1H-pyrazole-1-carboximidamide Hydrochloride" Molbank 2019, no. 1: M1046. https://0-doi-org.brum.beds.ac.uk/10.3390/M1046