A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
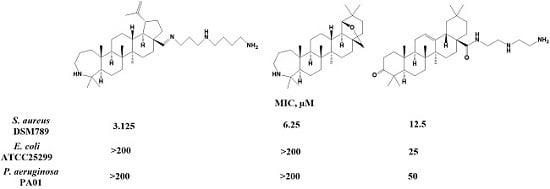
2.2. Biology
3. Materials and Methods
3.1. General Methods and Physical Measurements
3.2. Synthesis of 3-Deoxy-3a-homo-3a-aza-19β,28-epoxy-18α-oleanane (2)
3.3. Synthesis of 5 and 6
3.4. Synthesis of Compounds 7–9
3.5. Synthesis of 3-oxo-urs-12(13)-en-28-N-(2-((2-aminoethyl)amino)ethyl)-2-ethylamide (10)
4. Biology Methods
Determination of Minimal Inhibitory Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent developments in the functionalization of betulinic acid and its natural analogues: A route to new bioactive compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Kvasnica, M.; Urban, M.; Dickinson, N.J.; Sarek, J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. Rep. 2015, 32, 1303–1330. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.E.; Kazakova, O.B. Structure – Anti-influenza Type a Activity Relationship among a Series of Nitrogen Lupane Triterpenoids. Nat. Prod. Commun. 2018, 13, 1267–1270. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Rarova, L.; Janovska, L.; Saman, D.; Wimmer, Z. Enhancing effect of cystamine in its amides with betulinic acid as antimicrobial and antitumor agent in vitro. Steroids 2019, 148, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Apryshko, G.N.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. The synthesis and selective cytotoxicity of new Mannich bases derivatives of 19- and 28-alkynyltriterpenoids. Russ. J. Bioorg. Chem. 2018, 1, 123–127. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Kaletova, E.; Saman, D.; Sievanen, E.; Kolehmainen, E.T.; Slouf, M.; Wimmer, Z. Spectral and microscopic study of self-assambly of novel cationic spermine amides of betulinic acid. Steroids 2017, 117, 90–96. [Google Scholar] [CrossRef]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Synthesis and cytotoxicity evaluation of DOTA-conjugates of ursolic acid. Molecules 2019, 24, 2254. [Google Scholar] [CrossRef]
- Foster, T.J. The Staphylococcus aureus “superbug”. J. Clin. Investig. 2004, 114, 1693–1696. [Google Scholar] [CrossRef]
- Boman, H.G. Antibacterial peptides: Key components needed in immunity. Cell 1991, 65, 205–207. [Google Scholar] [CrossRef]
- Zasloff, M. Antibacterial molecules from frogs, sharks and man. In Phylogenetic Perspective in Immunity: The Insect-Host Defense; Hoffmann, J., Natori, S., Janeway, C., Eds.; RG Landes Biomedical Publisher: Austin, TX, USA, 1994; pp. 31–41. [Google Scholar]
- Cho, J.; Kim, Y. Sharks: A potential source of antiangiogenic factors and tumor treatments. Marine Biotechnology 2002, 4, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N., Jr.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M.; Letourneux, Y. Recent advances in the synthesis of spermine and spermidine analogs of the shark aminosterol squalamine. Eur. J. Org. Chem. 2003, 3897–3907. [Google Scholar] [CrossRef]
- Alhanout, K.; Rolain, J.M.; Brunel, J.M. Squalamine as an example of a new potent antimicrobial agent class: A critical review. Curr. Med. Chem. 2010, 17, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Giniyatullina, G.V.; Medvedeva, N.I.; Tolstikov, G.A. Synthesis of a triterpene-spermidine conjugate. Russ. J. Bioorg. Chem. 2012, 45, 1366–1369. [Google Scholar] [CrossRef]
- Djouhri-Bouktab, L.; Vidal, N.; Rolain, J.M.; Brunel, J.M. Synthesis of new 3,20-bispolyaminosteroid squalamine analogues and evaluation of their antimicrobial activities. J. Med. Chem. 2011, 54, 7417–7421. [Google Scholar] [CrossRef]
- Blanchet, M.; Borselli, D.; Rodallec, A.; Peiretti, F.; Vidal, N.; Bolla, J.M.; Digiorgio, C.; Morrison, K.R.; Wuest, W.M.; Brunel, J.M. Claramines: A new class of broad-spectrum antimicrobial agents with bimodal activity. Chem. Med. Chem. 2018, 13, 1018–1027. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Vida, N.; Rarova, L.; Kolar, M.; Saman, D.; Havlicek, L.; Drasar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Kazakova, O.B.; Lopatina, T.V.; Smirnova, I.E.; Giniyatullina, G.V.; Baikova, I.P.; Kataev, V.E. Synthesis and antimycobacterial activity of triterpenic A-ring azepanes. Eur. J. Med. Chem. 2018, 143, 464–472. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.; Tu, A.L.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Dalla-Vechia, L.; Dassonville-Klimpt, A.; Grellier, P.; Sonnet, P.; Gosmann, G.; Gnoatto, S.C.B. The Beckmann rearrangement applied to ursolic acid with antimalarial activity in medicinal chemistry studies. Lett. Org. Chem. 2012, 9, 92–95. [Google Scholar] [CrossRef]
- Giniyatullina, G.V.; Kazakova, O.B.; Salimova, E.V.; Tolstikov, G.A. Synthesis of new betulonic and oleanolic acid amides. Chem. Nat. Compd. 2011, 47, 68–72. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Ashavina, O.Y.; Boreko, E.I.; Karachurina, L.T.; Pavlova, N.I.; Kabal’nova, N.N.; Savinova, O.V.; Galin, F.Z.; Nikolaeva, S.N.; Zarudii, F.S.; et al. Synthesis of 3-acetylbetulinic acid and betulonic aldehydes according to Svern and the pharmacological activity of related oximes. Pharm. Chem. J. 2002, 36, 303–306. [Google Scholar] [CrossRef]



| Compound | MIC (µM)/MBC (µM) | ||
|---|---|---|---|
| S. aureus DSM789 | E. coli ATCC25299 | P. aeruginosa PA01 | |
| 1 | >200/>200 | >200/>200 | >200/>200 |
| 2 | 6.25/25 | >200/>200 | >200/>200 |
| 5 | 12.5/25 | >200/>200 | >200/>200 |
| 7 | >200/>200 | >200/>200 | >200/>200 |
| 8 | 3.125/6.25 | >200/>200 | >200/>200 |
| 9 | 12.5/25 | 200/>200 | 200/>200 |
| 10 | 12.5/25 | 200/>200 | 200/>200 |
| 11 | 12.5/25 | 25/25 | 50/>200 |
| Ciprofloxacine | 0.20 | 0.20 | 0.20 |
| Vancomycine | 0.80 | >200 | >200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakova, O.B.; Brunel, J.M.; Khusnutdinova, E.F.; Negrel, S.; Giniyatullina, G.V.; Lopatina, T.V.; Petrova, A.V. A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity. Molbank 2019, 2019, M1078. https://0-doi-org.brum.beds.ac.uk/10.3390/M1078
Kazakova OB, Brunel JM, Khusnutdinova EF, Negrel S, Giniyatullina GV, Lopatina TV, Petrova AV. A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity. Molbank. 2019; 2019(3):M1078. https://0-doi-org.brum.beds.ac.uk/10.3390/M1078
Chicago/Turabian StyleKazakova, Oxana B., Jean Michel Brunel, Elmira F. Khusnutdinova, Sophie Negrel, Gulnara V. Giniyatullina, Tatyana V. Lopatina, and Anastasiya V. Petrova. 2019. "A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity" Molbank 2019, no. 3: M1078. https://0-doi-org.brum.beds.ac.uk/10.3390/M1078