Differential Response to Water Deficit in Chili Pepper (Capsicum annuum L.) Growing in Two Types of Soil Under Different Irrigation Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Establishment of the Experiment
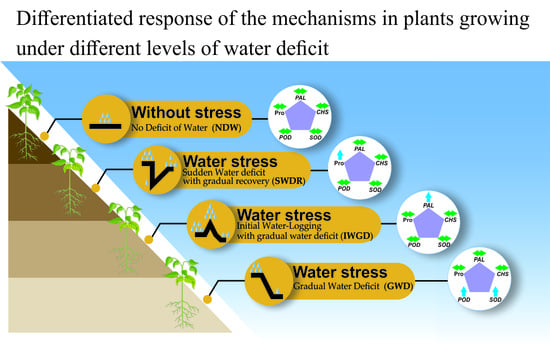
2.3. Calculation of Stress Type
2.4. Irrigation System
2.5. Measurement of the Morphological Variables
2.6. Determination of Proline Concentration in Capsicum annuum L. Leaves
2.7. RNA Extraction and cDNA Synthesis
2.8. RT-qPCR Conditions and Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of Soils on Morphological and Biochemical Variables of Capsicum annuum L.
3.2. Effects of the Type of Drought Treatment on Some Morphological and Biochemical Variables of Capsicum annuum L.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heiser, C.B.; Pickersgill, B. Names for the cultivated capsicum species (Solanaceae). Int. Assoc. Plant Taxon. 1969, 18, 277–283. [Google Scholar] [CrossRef]
- Orobiyi, A.; Dansi, M.; Assogba, P.; Loko, L.Y.; Vodouhe, R.S.; Akouegninou, A.; Sanni, A. Chili (Capsicum annuum L.) in southern Benin: Production constraints, varietal diversity, preference criteria and participatory evaluation. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 2244–2251. [Google Scholar]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.; Pérez-Morales, R.; Ortiz, J.; García-Hernández, J. Characterization of different capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.D.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.D.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants, Vol 1; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–16. ISBN 978-3-319-28899-4. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, F.; Dong, L.; Wu, J.; Cheng, Q.; Qi, D.; Yan, X.; Jiang, L.; Fan, S.; et al. Phenylalanine ammonia-lyase2.1 contributes to the soybean response towards Phytophthora sojae infection. Sci. Rep. 2017, 7, 7242. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yu, Q.; Shen, W.; El Mohtar, C.A.; Zhao, X.; Gmitter, F.G. Functional study of CHS gene family members in citrus revealed a novel CHS gene affecting the production of flavonoids. BMC Plant Biol. 2018, 18, 189. [Google Scholar] [CrossRef]
- Lenk, I.; Fisher, L.; Vickers, M.; Akinyemi, A.; Didion, T.; Swain, M.; Jensen, C.; Mur, L.; Bosch, M. Transcriptional and Metabolomic Analyses Indicate that Cell Wall Properties are Associated with Drought Tolerance in Brachypodium distachyon. Int. J Mol. Med. 2019, 20, 1758. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Yao, H.; Peng, X.; Wang, R.; Li, F.; Wang, Z.; Zhao, M.; Jin, L. Overexpression of Chalcone Synthase Improves Flavonoid Accumulation and Drought Tolerance in Tobacco. Plant Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Gökmen, E.; Ceyhan, E. Effects of drought stress on growth parameters, enzyme activities and proline content in chickpea genotypes. Bangladesh J. Bot. 2018, 44, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Ponce de León, J.; García, F.; Durán, A. Aspectos Metodologicos en la Determinacion de la Capacidad de Retener Agua de los Suelos del Uruguay; Universidad de la Republica/Facultad de Agronomia: Montevideo, Uruguay, 1988; p. 20. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- De Beenhouwer, M.; Van Geel, M.; Ceulemans, T.; Muleta, D.; Lievens, B.; Honnay, O. Changing soil characteristics alter the arbuscular mycorrhizal fungi communities of Arabica coffee (Coffea arabica) in Ethiopia across a management intensity gradient. Soil Biol. Biochem. 2015, 91, 133–139. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Fiasconaro, M.L.; Lovato, M.E.; Antolín, M.C.; Clementi, L.A.; Torres, N.; Gervasio, S.; Martín, C.A. Role of proline accumulation on fruit quality of pepper (Capsicum annuum L.) grown with a K-rich compost under drought conditions. Sci. Hortic. 2019, 249, 280–288. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 17, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, Q.; Zheng, S.; Chen, M.; Zhao, F.; Xu, S. Atrazine exposure triggers common carp neutrophil apoptosis via the CYP450s/ROS pathway. Fish Shellfish Immunol. 2019, 84, 551–557. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2014, 22, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.; Guo, M.; Wang, H.; Lu, J.; Liu, J.; Zhang, C.; Gong, Z.; Lu, M. Autophagy, a Conserved Mechanism for Protein Degradation, Responds to Heat, and Other Abiotic Stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant. Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, X.; Guan, H.; Li, X.; Du, Y.; Wang, P.; Mou, H. Promotive effects of alginate-derived oligosaccharides on the inducing drought resistance of tomato. J. Ocean Univ. China 2009, 8, 303–311. [Google Scholar] [CrossRef]
- Ximénez-Embún, M.G.; González-Guzmán, M.; Arbona, V.; Gómez-Cadenas, A.; Ortego, F.; Castañera, P. Plant-mediated effects of water deficit on the performance of tetranychus evansi on tomato drought-adapted accessions. Front. Plant Sci. 2018, 9, 1490. [Google Scholar] [CrossRef] [Green Version]










| Characteristic | Sandy | Clay |
|---|---|---|
| Clay | 21 | 55 |
| Sandy | 59 | 25 |
| Silt | 20 | 20 |
| Organic matter | 8.71 | 1.81 |
| DAP (g/cm3) | 1.55 | 1.4 |
| Characteristic | Sandy | Clay |
|---|---|---|
| Nitrogen | 248 KN/Ha | 39 KN/Ha |
| Phosphorus | 17.6 mg/kg | 1.66 mg/kg |
| Calcium | 6387.02 mg/kg | 5091.11 mg/kg |
| Magnesium | 1317.04 mg/kg | 1301.47 mg/kg |
| Potassium | 328.81 mg/kg | 990.19 mg/kg |
| Sodium | 9.97 mg/kg | 263.78 mg/kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macias-Bobadilla, I.; Vargas-Hernandez, M.; Guevara-Gonzalez, R.G.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Torres-Pacheco, I. Differential Response to Water Deficit in Chili Pepper (Capsicum annuum L.) Growing in Two Types of Soil Under Different Irrigation Regimes. Agriculture 2020, 10, 381. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10090381
Macias-Bobadilla I, Vargas-Hernandez M, Guevara-Gonzalez RG, Rico-Garcia E, Ocampo-Velazquez RV, Torres-Pacheco I. Differential Response to Water Deficit in Chili Pepper (Capsicum annuum L.) Growing in Two Types of Soil Under Different Irrigation Regimes. Agriculture. 2020; 10(9):381. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10090381
Chicago/Turabian StyleMacias-Bobadilla, Israel, Marcela Vargas-Hernandez, Ramon G. Guevara-Gonzalez, Enrique Rico-Garcia, Rosalia V. Ocampo-Velazquez, and Irineo Torres-Pacheco. 2020. "Differential Response to Water Deficit in Chili Pepper (Capsicum annuum L.) Growing in Two Types of Soil Under Different Irrigation Regimes" Agriculture 10, no. 9: 381. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10090381