Effects of Polyacrylamide-Based Super Absorbent Polymer and Corn Straw Biochar on the Arid and Semi-Arid Salinized Soil
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials Collection and Preparation
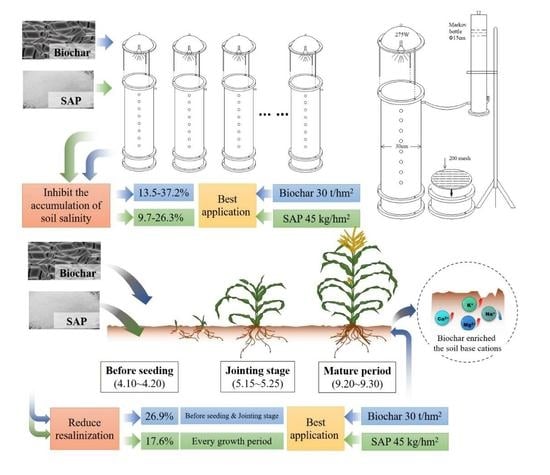
2.2. Lab Soil Simulation Column Experiment
2.3. Field Soil Column Experiment
2.4. Data Analysis
3. Results
3.1. Lab Soil Column Simulation Observations
3.1.1. Effect of SAP on Soil Salinity in Simulated Infiltration Process
3.1.2. Effect of Biochar on Soil Salinity in Simulated Infiltration Process
3.2. Field Soil Column Investigation
3.2.1. Effect of SAP on the Salt Content of Cultivated Soil
3.2.2. Effect of Biochar on the Salt Content of Cultivated Soil
3.3. Effects of Biochar on Base Ion Concentrations in Soil Column
4. Discussion
4.1. Comparative Analysis of Biochar and SAP Effects on Soil Salinity
4.2. Effect of Biochar on Soil Nutrients
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Walker, S.; Kumar, J.; Biswas, B. Assessment of different indices (vegetation, salinity) and salt effected area trend analysis using shannon entropy approach–a case study in a semi-arid region of india using rs/gis. Plant Arch. 2019, 19, 3457–3466. [Google Scholar]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Nachshon, U. Cropland soil salinization and associated hydrology: Trends, processes and examples. Water 2018, 10, 1030. [Google Scholar]
- Sahaar, S.A.; Niemann, J.D. Impact of regional characteristics on the estimation of root-zone soil moisture from the evaporative index or evaporative fraction. Agric. Water Manag. 2020, 2381, 106225. [Google Scholar] [CrossRef]
- Liu, A.; Qu, Z.; Nachshon, U. On the potential impact of root system size and density on salt distribution in the root zone. Agric. Water Manag. 2020, 234, 106118. [Google Scholar] [CrossRef]
- Chang, X.; Gao, Z.; Wang, S.; Chen, H. Modelling long-term soil salinity dynamics using SaltMod in Hetao Irrigation District, China. Comput. Electron. Agric. 2019, 156, 447–458. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Qi, Q.; Tong, S.; An, Y.; Lu, X.; Liu, Y. The restoration feasibility of degraded Carex Tussock in soda-salinization area in arid region. Ecol. Indic. 2019, 98, 131–136. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, G.; Li, T.; Zhou, X.; Li, J. Soil salinization of cultivated land in Shandong Province, China-Dynamics during the past 40 years. Land Degrad. Dev. 2019, 30, 426–436. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yang, Y.; Chen, Z.; Guo, C.; Li, S. Influence of super absorbent polymer on soil water retention, seed germination and plant survivals for rocky slopes eco-engineering. Ecol. Eng. 2014, 62, 27–32. [Google Scholar] [CrossRef]
- Herath, H.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209, 188–197. [Google Scholar] [CrossRef]
- Guo, M.; He, Z.; Uchimiya, S.M. (Eds.) Introduction to Biochar as an Agricultural and Environmental Amendment. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; SSSA Special Publication 63; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 1–14. [Google Scholar]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohyd. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Khodadadi Dehkordi, D. Effect of superabsorbent polymer on soil and plants on steep surfaces. Water Environ. J. 2017, 32, 158–163. [Google Scholar] [CrossRef]
- Hou, X.; Li, R.; He, W.; Dai, X.; Ma, K.; Liang, Y. Superabsorbent polymers influence soil physical properties and increase potato tuber yield in a dry-farming region. J. Soil. Sediment. 2018, 18, 816–826. [Google Scholar] [CrossRef]
- Lejcuś, K.; Śpitalniak, M.; Dąbrowska, J. Swelling behaviour of superabsorbent polymers for soil amendment under different loads. Polymers. 2018, 10, 271. [Google Scholar] [CrossRef]
- Guo, J.; Shi, W.; Wen, L.; Shi, X.; Li, J. Effects of a super-absorbent polymer derived from poly-γ-glutamic acid on water infiltration, field water capacity, soil evaporation, and soil water-stable aggregates. Arch. Agron. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Liao, R.; Yang, P.; Yu, H.; Wu, W.; Ren, S. Establishing and validating a root water uptake model under the effects of superabsorbent polymers. Land Degrad. Dev. 2018, 29, 1478–1488. [Google Scholar] [CrossRef]
- Satriani, A.; Catalano, M.; Scalcione, E. The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: A case-study in Southern Italy. Agric. Water Manag. 2018, 195, 114–119. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. R. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef]
- Smebye, A.; Alling, V.; Vogt, R.D.; Gadmar, T.C.; Mulder, J.; Cornelissen, G.; Hale, S.E. Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 2016, 142, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Senbayram, M.; Zang, H.; Ugurlar, F.; Aydemir, S.; Brüggemann, N.; Kuzyakov, Y.; Bol, R.; Blagodatskaya, E. Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Appl. Soil Ecol. 2018, 129, 121–127. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xu, X.; Lü, L.; Ren, D.; Ke, J.; Xiong, Y.; Huo, Z.; Huang, G. Soil salinity distribution based on remote sensing and its effect on crop growth in Hetao Irrigation District. Trans. Chin. Soc. Agric. Eng. 2018, 34, 102–109. (In Chinese) [Google Scholar]
- Lei, T.; Issac, S.; Yuan, P.; Huang, X.; Yang, P. Strategic considerations of efficient irrigation and salinity control on Hetao Plain in inner Mongolia. Trans. Chin. Soc. Agric. Eng. 2001, 17, 48–52. (In Chinese) [Google Scholar]
- Qi, Z.; Feng, H.; Zhao, Y.; Zhang, T.; Yang, A.; Zhang, Z. Spatial distribution and simulation of soil moisture and salinity under mulched drip irrigation combined with tillage in an arid saline irrigation district, northwest China. Agric. Water Manag. 2018, 201, 219–231. [Google Scholar] [CrossRef]
- Olson, G.; Walsh, J.F. Superabsorbent Polymer Seed Coatings and Associated Methods. U.S. Patents 10,021,824 B2, 17 July 2018. [Google Scholar]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef]
- Paetsch, L.; Mueller, C.W.; Rumpel, C.; Angst, Š.; Wiesheu, A.C.; Girardin, C.; Ivleva, N.P.; Niessner, R.; Kögel-Knabner, I. A multi-technique approach to assess the fate of biochar in soil and to quantify its effect on soil organic matter composition. Org. Geochem. 2017, 112, 177–186. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Li, T.; Li, M.; He, L.; Ma, Z. Cotransport and deposition of biochar with different sized-plastic particles in saturated porous media. Sci. Total Environ. 2020, 713, 136387. [Google Scholar] [CrossRef]
- Egilla, J.N.; Daviesjr, F.T.; Drew, M.C. Effect of potassium on drought resistance of Hibiscus rosa-sinensis cv. Leprechaun: Plant growth, leaf macro- and micronutrient content and root longevity. Plant Soil. 2001, 229, 213–224. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.Y.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Poormansour, S.; Razzaghi, F.; Sepaskhah, A.R. Wheat Straw Biochar Increases Potassium Concentration, Root Density, and Yield of Faba Bean in a Sandy Loam Soil. Commun. Soil Sci. Plan. 2019, 50, 1799–1810. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Brownsort, P.; Rovere, M.; Tagliaferro, A.; Zhao, L.; Cao, X.; Xu, G. Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Hepler, P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell. 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Effect of biochar on growth and ion contents of bean plant under saline condition. Environ. Sci. Pollut. R. 2018, 25, 11556–11564. [Google Scholar] [CrossRef]
- Bhat, M.A.; Lone, H.A.; Mehraj, S.S. Nutrient Remobilization during Senescence. In Senescence Signalling and Control in Plants; Sarwat, M., Tuteja, N., Eds.; Academic Press: London, UK, 2019; pp. 227–237. [Google Scholar]
- Williams, W.D. Anthropogenic salinisation of inland waters. Hydrobiologia 2001, 466, 329–337. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, J.; Cheng, Z.; Huang, L.; Sun, P.; Chen, L.; Shen, G. Synthesis of a stable magnesium-impregnated biochar and its reduction of phosphorus leaching from soil. Chemosphere 2018, 199, 402–408. [Google Scholar] [CrossRef]
- Bai, T.; Wang, P.; Hall, S.J.; Wang, F.; Ye, C.; Li, Z.; Li, S.; Zhou, L.; Qiu, Y.; Guo, J.; et al. Interactive global change factors mitigate soil aggregation and carbon change in a semi-arid grassland. Glob. Chang. Biol. 2020, 26, 5320–5332. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fert. Soils. 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Zhu, M.; Kong, F.; Li, Y.; Li, M.; Zhang, J.; Xi, M. Effects of moisture and salinity on soil dissolved organic matter and ecological risk of coastal wetland. Environ. Res. 2020, 187, 109659. [Google Scholar] [CrossRef]
- Gaunt, J.; Cowie, A. Biochar, Greenhouse Gas Accounting and Emissions Trading. In Greenhouse Gas Accounting and Emissions Trading; Lehmann, J., Joseph, S., Eds.; Science and Technology Press: London, UK, 2009; pp. 317–340. [Google Scholar]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef] [Green Version]
- An, N.; Tang, C.-S.; Cheng, Q.; Wang, D.Y.; Shi, B. Application of electrical resistivity method in the characterization of 2D desiccation cracking process of clayey soil. Eng. Geol. 2020, 265, 105416. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, P.; Wei, K.; Huang, X.; Zhang, X. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem. Eng. J. 2020, 385, 123921. [Google Scholar] [CrossRef]
- Yang, F.; Cen, R.; Feng, W.; Liu, J.; Qu, Z.; Miao, M. Effects of Super-Absorbent Polymer on Soil Remediation and Crop Growth in Arid and Semi-arid Areas. Sustainability 2020, 12, 7825. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Gao, J.; Cen, R.; Yang, F.; He, Z.; Wu, J.; Miao, Q.; Liao, H. Effects of Polyacrylamide-Based Super Absorbent Polymer and Corn Straw Biochar on the Arid and Semi-Arid Salinized Soil. Agriculture 2020, 10, 519. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10110519
Feng W, Gao J, Cen R, Yang F, He Z, Wu J, Miao Q, Liao H. Effects of Polyacrylamide-Based Super Absorbent Polymer and Corn Straw Biochar on the Arid and Semi-Arid Salinized Soil. Agriculture. 2020; 10(11):519. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10110519
Chicago/Turabian StyleFeng, Weiying, Jiayue Gao, Rui Cen, Fang Yang, Zhongqi He, Jin Wu, Qingfeng Miao, and Haiqing Liao. 2020. "Effects of Polyacrylamide-Based Super Absorbent Polymer and Corn Straw Biochar on the Arid and Semi-Arid Salinized Soil" Agriculture 10, no. 11: 519. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture10110519