Effects of Rice Husk Biochar on Carbon Release and Nutrient Availability in Three Cultivation Age of Greenhouse Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Soil, Biochar, and Compost
2.2. Characterization of Soil, Biochar, and Compost
2.3. Soil–Biochar Incubation
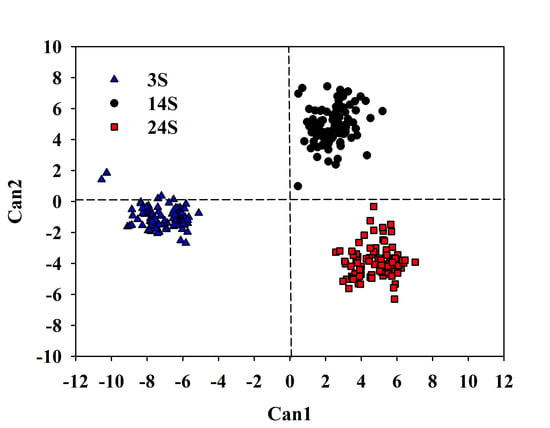
2.4. Statistical Analysis
3. Results
3.1. Initial Soil and RHB Characterization
3.2. Carbon Mineralization and Nutrient Availability
4. Discussion
4.1. Cumulative C (CO2) Release, pH, and EC
4.2. TC, TN, TP, and CEC
4.3. Available P, K, Ca, and Mg
4.4. Available Cu, Pb, and Zn
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, L.-H.; Qu, M.; Ren, H.; Sui, X.; Chen, Q.-Y.; Zhang, Z. Structure, Function, Application, and Ecological Benefit of a Single-slope, Energy-efficient Solar Greenhouse in China. HortTechnology 2010, 20, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.D.; Kang, S.H.; Kim, K.Y.; Kim, H.T.; Kang, S.J. White Revolution of Agriculture in Korea: The achievement of Year-Round Production and Distribution of Horticultural Crops by the Expansion of Greenhouse Cultivation; Korea Development Institute (KDI): Seoul, Korea, 2013; pp. 26–34. [Google Scholar]
- Han, J.; Luo, Y.; Yang, L.; Liu, X.; Wu, L.; Xu, J. Acidification and salinization of soils with different initial pH under greenhouse vegetable cultivation. J. Soils Sediments 2014, 14, 1683–1692. [Google Scholar] [CrossRef]
- Kong, X.; Cao, J.; Tang, R.; Zhang, S.; Dong, F. Pollution of intensively managed greenhouse soils by nutrients and heavy metals in the Yellow River Irrigation Region, Northwest China. Environ. Monit. Assess. 2014, 186, 7719–7731. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Kou, C.; Zhang, F.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, H.; Wang, Z.; Zhou, L. Accumulation Characteristics of Copper and Cadmium in Greenhouse Vegetable Soils in Tongzhou District of Beijing. Procedia Environ. Sci. 2011, 10, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tian, T.; Gao, L.; Tian, Y. Nutrients, heavy metals and phthalate acid esters in solar greenhouse soils in Round-Bohai Bay-Region, China: Impacts of cultivation year and biogeography. Environ. Sci. Pollut. Res. 2016, 23, 13076–13087. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mi, W.; Song, P.; Xie, H.; Zhu, L.; Wang, J. Cultivation Ages Effect on Soil Physicochemical Properties and Heavy Metal Accumulation in Greenhouse Soils. Chin. Geogr. Sci. 2018, 28, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Ju, X.; Kou, C.; Christie, P.; Dou, Z.; Zhang, F. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Yang, H.L.; Li, Y.L.; Li, X.Z.; Zhou, J.G. Variation characteristics of soil water-soluble salts of large plastic house vegetable field for different cultivation year. J. Soil Water Conserv. 2012, 26, 241–245, (In Chinese with English abstract). [Google Scholar]
- Bernhart, M.; Fasina, O.; Fulton, J.; Wood, C. Compaction of poultry litter. Bioresour. Technol. 2010, 101, 234–238. [Google Scholar] [CrossRef]
- Cheng, M.-P.; Chung, C.-H.; Su, T.-M.; Hung, C.-C.; Lee, C.-F.; Hsiao, T.-H. Investigation on the litter production and composition for native chicken. Taiwan Livest. Res. 2015, 48, 288–296, (In Chinese with English abstract). [Google Scholar]
- Tsai, C.C.; Chen, Z.S.; Hseu, Z.Y.; Guo, H.Y. Representative soils selected from arable and slope soils in Taiwan and their database establishment. Soil Environ. 1998, 1, 73–88, (In Chinese, with English abstract). [Google Scholar]
- Kyveryga, P.M.; Blackmer, A.M.; Ellsworth, J.W.; Isla, R. Soil pH effects on nitrification of fall-applied anhydrous ammonia. Soil Sci. Soc. Am. J. 2004, 68, 545–551. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Jones, D.L. Soil acidification used as a management strategy to reduce nitrate losses from agricultural land. Soil Boil. Biochem. 2005, 37, 867–875. [Google Scholar] [CrossRef]
- Lo, C.-S. Technology for Organic Vegetable Production in Plastic House. In Proceedings of the Symposium on Organic Crop Culture Technology, Taichung, Taiwan, 26 June 2008; Agricultural Research Institute, Council of Agriculture, Executive Yuan: Taichung, Taiwan, 2008; pp. 47–60, (In Chinese with English abstract). [Google Scholar]
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable gasification-biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.M.; Haszeldine, S. Sustainable gasification–biochar systems? A case-study of rice-husk gasification in Cambodia, Part II: Field trial results, carbon abatement, economic assessment and conclusions. Energy Policy 2012, 41, 618–623. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Li, S.; Liang, C.; Shangguan, Z. Effects of apple branch biochar on soil C mineralization and nutrient cycling under two levels of N. Sci. Total. Environ. 2017, 607, 109–119. [Google Scholar] [CrossRef]
- Shi, J.; Fan, X.; Tsang, D.C.; Wang, F.; Shen, Z.; Hou, D.; Alessi, D.S. Removal of lead by rice husk biochars produced at different temperatures and implications for their environmental utilizations. Chemosphere 2019, 235, 825–831. [Google Scholar] [CrossRef]
- Oldfield, T.L.; Sikirica, N.; Mondini, C.; López, G.; Kuikman, P.J.; Holden, N.M. Biochar, compost and biochar-compost blend as options to recover nutrients and sequester carbon. J. Environ. Manag. 2018, 218, 465–476. [Google Scholar] [CrossRef]
- Trupiano, D.; Cocozza, C.; Baronti, S.; Amendola, C.; Vaccari, F.P.; Lustrato, G.; Di Lonardo, S.; Fantasma, F.; Tognetti, R.; Scippa, G. The Effects of Biochar and Its Combination with Compost on Lettuce (Lactuca sativa L.) Growth, Soil Properties, and Soil Microbial Activity and Abundance. Int. J. Agron. 2017, 2017, 3158207. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Schulz, H.; Brandl, S.; Miehtke, H.; Huwe, B.; Glaser, B. Short-term effect of biochar and compost on soil fertility and water status of a Dystric Cambisol in NE Germany under field conditions. J. Plant Nutr. Soil Sci. 2012, 175, 698–707. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bird, M.I.; Nelson, P.N.; Bass, A. The ameliorating effects of biochar and compost on soil quality and plant growth on a Ferralsol. Soil Res. 2015, 53, 1–12. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-F. Carbon Dynamics and Fertility in Biochar-Amended Soils with Excessive Compost Application. Agronomy 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-C.; Chang, Y.-F. Nitrogen Availability in Biochar-Amended Soils with Excessive Compost Application. Agronomy 2020, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-C.; Chang, Y.-F. Effects of Biochar to Excessive Compost-Fertilized Soils on the Nutrient Status. Agronomy 2020, 10, 683. [Google Scholar] [CrossRef]
- Chien, C.C.; Huang, Y.P.; Sah, J.G.; Cheng, W.J.; Chang, R.Y.; Lu, Y.S. Application of Rice Husk Charcoal on Remediation of Acid Soil. Mater. Sci. Forum 2011, 685, 169–180. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3. Chemical Methods; Bigham, J.M., Ed.; Agronomy Society of America and Soil Science Society of America: Madison, WI, USA, 1986; pp. 475–489. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3. Chemical Methods; Bigham, J.M., Ed.; Agronomy Society of America and Soil Science Society of America: Madison, WI, USA, 1986; pp. 417–435. [Google Scholar]
- Poppe, L.; Eliason, A.; Fredericks, J. A computerized particle-size analysis system. Comput. Geosci. 1986, 12, 93–96. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Automated instruments for determination of total carbon, nitrogen, and sulfur in soils by combustion techniques. In Soil Analysis: Modern Instrumental Techniques; Smith, K.A., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 261–289. [Google Scholar]
- Environmental Protection Administration (EPA), Taiwan. Total Concentration of Metals in Soils; Method Code No: NIEA S321.63B; Environmental Protection Administration of Taiwan: Taipei, Taiwan, 2003; 11p.
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis-Mineralogical Organic and Inorganic Methods; Springer: Heidelberg, Germany, 2006; pp. 677–686. [Google Scholar]
- Mulvaney, R.L. Nitrogen-inorganic forms. In Methods of Soil Analysis, Part 3 Chemical Method, 1st ed.; Book Series No.5; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1123–1185. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, and NH4. Short Test Methods Used in Soil Testing Division, Department of Agriculture, Raleigh, North Carolina; North Carolina Department of Agriculture: Raleigh, NC, USA, 1953; 8p.
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America and Soil Science Society of America: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Jones, J.B.; Case, V.W.; Westerman, R. Sampling, Handling, and Analyzing Plant Tissue Samples. In Methods of Soil Analysis, Part 5-Mineralogical Methods; Soil Science Society of America: Madison, WI, USA, 2018; pp. 389–427. [Google Scholar]
- Wang, X.; Cook, R.; Tao, S.; Xing, B. Sorption of organic contaminants by biopolymers: Role of polarity, structure and domain spatial arrangement. Chemosphere 2007, 66, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Zibilske, L.M. Carbon Mineralization. In Methods of Soil Analysis, Part 2, Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomly, P., Eds.; Soil Science of America: Madison, WI, USA, 1994; pp. 835–863. [Google Scholar]
- Ribeiro, H.; Fangueiro, D.; Alves, F.; Vasconcelos, E.; Coutinho, J.; Bol, R.; Cabral, F. Carbon-mineralization kinetics in an organically managed Cambic Arenosol amended with organic fertilizers. J. Plant Nutr. Soil Sci. 2009, 173, 39–45. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.; Van Der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Mukome, F.N.; Six, J.; Parikh, S.J. The effects of walnut shell and wood feedstock biochar amendments on greenhouse gas emissions from a fertile soil. Geoderma 2013, 200, 90–98. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Boil. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.M.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.; Watts, D.; Laird, D.; Ahmedna, M.; Niandou, M. Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Boil. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purakayastha, T.J.; Kumari, S.; Pathak, H. Characterisation, stability, and microbial effects of four biochars produced from crop residues. Geoderma 2015, 239, 293–303. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on the decomposition of 14C-labelled maize residues and on their stabilization insoil aggregates. Eur. J. Soil Sci. 2013, 64, 488–499. [Google Scholar] [CrossRef]
- El-Naggar, A.H.; Usman, A.R.; Al-Omran, A.M.; Ok, Y.S.; Ahmad, M.; Al-Wabel, M.I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar] [CrossRef]
- Ducey, T.F.; Ippolito, J.A.; Cantrell, K.B.; Novak, J.M.; Lentz, R.D. Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl. Soil Ecol. 2013, 65, 65–72. [Google Scholar] [CrossRef]
- Luo, X.; Chen, L.; Zheng, H.; Chang, J.; Wang, H.; Wang, Z.; Xing, B. Biochar addition reduced net N mineralization of a coastal wetland soil in the Yellow River Delta, China. Geoderma 2016, 282, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total. Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Dodson, J. Wheat Straw Ash and its Use as a Silica Source. Ph.D. Thesis, University of York, Heslington, York, UK, September 2011. [Google Scholar]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Ippolito, J.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood Biochar Influences Calcareous Soil Physicochemical and Microbiological Status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark ofAcacia mangiumon the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Berek, A.K.; Hue, N.V.; Radovich, T.J.K.; Ahmad, A.A. Biochars Improve Nutrient Phyto-Availability of Hawai’i’s Highly Weathered Soils. Agronomy 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Mollinedo, J.; Schumacher, T.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2013, 60, 393–404. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hosseini, B.S.; Xu, Z.; Blumfield, T.J.; Zhao, H.; Wang, H.; Wallace, H.M.; Van Zwieten, L. Biochar application increases soil available nitrogen and plantto-soil carbon input. In Proceedings of the 2nd International Conference on Biochar and Green Agriculture, Nanjing, China, 14–18 April 2015. [Google Scholar]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.; Pfeiffer, E.-M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.; Murphy, D. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Boil. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Anawar, H.M. Application of Biochars for Soil Constraints: Challenges and Solutions. Pedosphere 2015, 25, 631–638. [Google Scholar] [CrossRef]
- Sackett, T.; Basiliko, N.; Noyce, G.L.; Winsborough, C.; Schurman, J.; Ikeda, C.; Thomas, S.C. Soil and greenhouse gas responses to biochar additions in a temperate hardwood forest. GCB Bioenergy 2014, 7, 1062–1074. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total. Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Eduah, J.; Nartey, E.; Abekoe, M.; Breuning-Madsen, H.; Andersen, M. Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 2019, 341, 10–17. [Google Scholar] [CrossRef]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Xu, X.; Harris, W. Phosphorus Release from Dairy Manure, the Manure-Derived Biochar, and Their Amended Soil: Effects of Phosphorus Nature and Soil Property. J. Environ. Qual. 2014, 43, 1504–1509. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2015, 46, 406–433. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]


| Soil Characteristics | 3S | 14S | 24S |
|---|---|---|---|
| Sand (%) | 12.8 | 18.0 | 20.1 |
| Clay (%) | 32.2 | 31.2 | 35.1 |
| Silt (%) | 55.0 | 50.8 | 44.7 |
| Soil Texture | Silty clay loam | Silty clay loam | Clay loam |
| pH | 4.42 | 4.56 | 5.62 |
| Electrical conductivity (EC) (dS m−1) | 0.40 | 0.51 | 0.48 |
| Total C (%) | 2.49 | 1.75 | 2.08 |
| Total N (%) | 0.39 | 0.29 | 0.34 |
| C/N | 6.38 | 6.03 | 6.12 |
| Total P (g kg−1) | 1.58 | 3.14 | 4.00 |
| Total Cu (mg kg−1) | 20.0 | 32.3 | 35.2 |
| Total Pb (mg kg−1) | 10.5 | 9.59 | 8.91 |
| Total Zn (mg kg−1) | 422 | 724 | 749 |
| Available NH4+-N (mg kg−1) | 14.2 | 11.4 | 19.9 |
| Available NO3−-N (mg kg−1) | 148 | 253 | 119 |
| Available P (mg kg−1) | 424 | 822 | 912 |
| Available K (mg kg−1) | 131 | 265 | 230 |
| Exchangeable K (coml(+) kg−1 soil−1) | 0.88 | 1.82 | 0.88 |
| Exchangeable Na (coml(+) kg−1 soil−1) | 0.52 | 0.58 | 0.27 |
| Exchangeable Ca (coml(+) kg−1 soil−1) | 3.26 | 4.28 | 4.64 |
| Exchangeable Mg (coml(+) kg−1 soil−1) | 0.75 | 1.14 | 0.89 |
| Cation exchange capacity (CEC) (coml(+) kg−1 soil−1) | 12.2 | 13.0 | 12.9 |
| Base saturation (BS) (%) | 44 | 60 | 52 |
| Mehlich-3 extraction P (mg kg−1) | 404 | 752 | 944 |
| Mehlich-3 extraction K (mg kg−1) | 75.5 | 179 | 155 |
| Mehlich-3 extraction Na (mg kg−1) | 34.9 | 48.8 | ND 1 |
| Mehlich-3 extraction Ca (mg kg−1) | 1722 | 1675 | 3087 |
| Mehlich-3 extraction Mg (mg kg−1) | 282 | 396 | 427 |
| Mehlich-3 extraction Fe (mg kg−1) | 313 | 342 | 319 |
| Mehlich-3 extraction Al (mg kg−1) | 686 | 744 | 536 |
| Mehlich-3 extraction Mn (mg kg−1) | 38.2 | 40.4 | 28.9 |
| Mehlich-3 extraction Cu (mg kg−1) | 4.21 | 6.17 | 7.79 |
| Mehlich-3 extraction Pb (mg kg−1) | 6.68 | 0.88 | 2.87 |
| Mehlich-3 extraction Zn (mg kg−1) | 40.0 | 55.6 | 62.0 |
| Characteristics | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| pH (1:5) | 8.35 | 7.87 | 8.07 | 7.40 | 7.23 | 8.38 | |
| Electrical conductivity (EC) (1:10, 30min) (dS m−1) | 0.40 | 0.19 | 0.17 | 0.58 | 0.67 | 0.50 | |
| Electrical conductivity (EC) (1:10, 24Hr) (dS m−1) | 0.78 | 0.40 | 0.38 | 0.84 | 0.99 | 0.85 | |
| Total content (g kg−1) | P | 1.71 | 1.21 | 0.95 | 0.95 | 1.51 | 1.20 |
| K | 19.6 | 16.7 | 15.4 | 12.0 | 13.2 | 20.9 | |
| Ca | 3.34 | 2.18 | 2.05 | 1.58 | 3.27 | 2.91 | |
| Mg | 1.68 | 0.65 | 0.51 | 0.99 | 1.57 | 1.03 | |
| Cu | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Zn | 0.05 | 0.03 | 0.02 | 0.03 | 0.04 | 0.04 | |
| Cation exchangeable capacity (CEC)(cmol(+) kg−1 soil−1) | 3.10 | 4.11 | 4.34 | 5.86 | 4.37 | 7.08 | |
| Exchangeable bases (coml(+) kg−1 soil−1) | K | 0.87 | 1.59 | 2.06 | 1.86 | 1.33 | 5.00 |
| Na | 0.08 | 0.01 | 0.04 | 0.02 | 0.05 | 0.47 | |
| Ca | 0.02 | 0.07 | 0.10 | 0.03 | 0.04 | 0.19 | |
| Mg | 0.01 | 0.02 | 0.03 | 0.03 | 0.03 | 0.08 | |
| Base saturation (BS) (%) | 32 | 42 | 51 | 33 | 33 | 81 | |
| Mehlich-3 extraction (mg kg−1) | P | 170 | 252 | 239 | 315 | 489 | 361 |
| K | 1209 | 638 | 648 | 2269 | 2053 | 2007 | |
| Na | ND 1 | ND | ND | ND | ND | 96.1 | |
| Ca | 184 | 215 | 244 | 277 | 1000 | 422 | |
| Mg | 91.8 | 93.3 | 87.0 | 218 | 336 | 167 | |
| Fe | 32.1 | 16.7 | 19.0 | 32.7 | 156 | 16.7 | |
| Al | 12.6 | 1.69 | 2.10 | 2.44 | 214 | 3.36 | |
| Mn | 48.6 | 74.1 | 64.7 | 64.8 | 68.9 | 100 | |
| Cu | 0.10 | 0.16 | 0.20 | 0.15 | 0.41 | 0.17 | |
| Pb | 0.16 | 0.11 | 0.10 | 0.19 | 0.21 | 0.14 | |
| Zn | 6.43 | 3.50 | 3.90 | 7.40 | 9.76 | 5.39 | |
| BET surface area (m2 g−1) | 2.66 | 2.77 | 2.81 | 2.45 | 2.45 | 2.80 | |
| Elemental analysis (%) | C | 49.2 | 51.9 | 51.1 | 42.2 | 40.4 | 52.2 |
| H | 2.29 | 2.48 | 2.55 | 2.89 | 3.29 | 2.35 | |
| N | 0.71 | 0.63 | 0.62 | 0.54 | 0.63 | 0.65 | |
| O | 13.9 | 15.2 | 15.0 | 22.0 | 24.1 | 14.7 | |
| C/N mass ratio | 68.8 | 82.6 | 82.7 | 78.9 | 64.1 | 80.8 | |
| (O+N)/C atomic ratio | 0.224 | 0.229 | 0.231 | 0.401 | 0.461 | 0.221 | |
| O/C atomic ratio | 0.212 | 0.219 | 0.220 | 0.391 | 0.448 | 0.211 | |
| H/C atomic ratio | 0.558 | 0.574 | 0.599 | 0.820 | 0.979 | 0.540 | |
| Distribution of C chemical shift, ppm (Integrated results of solid-state 13C NMR spectra) (%) | paraffinic C (0–50 ppm) | 18.4 | 20.1 | 21.2 | 16.6 | 18.9 | 19.1 |
| substituted aliphatic C including alcohol, amines, carbohydrates, ethers, and methyl and acetal C (50–109 ppm) | 10.1 | 9.03 | 10.9 | 41.1 | 43.4 | 8.40 | |
| aromatic C (109–145 ppm) | 58.9 | 54.9 | 52.6 | 31.3 | 26.4 | 57.3 | |
| phenolic C (145–163 ppm) | 6.96 | 9.72 | 8.97 | 6.90 | 5.97 | 9.16 | |
| carboxyl C (163–190 ppm) | 2.53 | 3.47 | 3.21 | 1.88 | 2.52 | 3.05 | |
| carbonyl C (190–220 ppm) | 3.16 | 2.78 | 3.21 | 2.19 | 2.83 | 3.05 | |
| Aliphatic C 2 | 28.5 | 29.2 | 32.1 | 57.7 | 62.3 | 27.5 | |
| Polar C 2 | 22.8 | 25.0 | 26.3 | 52.0 | 54.7 | 23.7 | |
| Aliphatic polar C 2 | 10.1 | 9.03 | 10.9 | 41.1 | 43.4 | 8.40 | |
| Soil | Source | Df 1 | Cumulative C Release | pH | EC | TC | TN | TP | CEC | P | K | Ca | Mg | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3S | Rate | 4 | ***2 | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** |
| Treat | 7 | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | |
| Rate × Treat | 20 | *** | *** | ns | *** | ns | *** | ns | ns | *** | ns | *** | *** | *** | *** | |
| 14S | Rate | 4 | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** |
| Treat | 7 | ***2 | *** | *** | *** | *** | ns | ns | *** | *** | ns | ns | ns | ns | *** | |
| Rate × Treat | 20 | *** | *** | *** | *** | ns | ns | ns | ns | *** | ns | ns | ns | ns | ns | |
| 24S | Rate | 4 | *** | *** | ns | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Treat | 7 | ***2 | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | |
| Rate × Treat | 20 | *** | *** | *** | *** | ns | *** | ns | *** | *** | *** | ns | *** | *** | *** | |
| RHB | Source | Df 1 | Cumulative C Release | pH | EC | TC | TN | TP | CEC | P | K | Ca | Mg | Cu | Pb | Zn |
| RHB-A | Soil | 2 | *** | *** | *** | ns | ns | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Rate | 4 | *** | *** | ns | *** | *** | *** | ns | *** | *** | ns | *** | *** | *** | *** | |
| Soil × Rate | 8 | *** | ns | *** | ns | ns | ns | ns | *** | ns | *** | ns | ns | *** | *** | |
| RHB-B | Soil | 2 | *** | *** | *** | ns | ns | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Rate | 4 | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Soil × Rate | 8 | *** | ns | ns | ns | ns | *** | ns | *** | ns | ns | ns | *** | *** | *** | |
| RHB-C | Soil | 2 | *** | *** | *** | ns | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Rate | 4 | ns | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | |
| Soil × Rate | 8 | ns | *** | *** | ns | ns | *** | ns | *** | *** | ns | *** | *** | *** | *** | |
| RHB-D | Soil | 2 | *** | *** | *** | ns | ns | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Rate | 4 | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | |
| Soil × Rate | 8 | *** | *** | *** | ns | ns | ns | ns | *** | *** | ns | *** | *** | *** | *** | |
| RHB-E | Soil | 2 | *** | *** | *** | ns | ns | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Rate | 4 | *** | *** | *** | *** | *** | ns | ns | *** | *** | *** | *** | *** | *** | *** | |
| Soil × Rate | 8 | *** | ns | *** | ns | ns | ns | ns | *** | *** | *** | *** | *** | *** | *** | |
| RHB-F | Soil | 2 | *** | *** | *** | ns | ns | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Rate | 4 | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | |
| Soil × Rate | 8 | *** | ns | *** | ns | ns | ns | ns | *** | *** | ns | ns | *** | *** | *** |
| Rate | n | Cumulative C Release | pH | EC 1 | TC | TN | TP | CEC | P | K | Ca | Mg | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg CO2-C kg−1 soil−1 | dS m−1 | % | g kg−1 | g kg−1 | coml (+) kg−1 soil−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||
| 3S | |||||||||||||||
| Control | 3 | 1622 F 2 | 4.20 F | 0.93 C | 2.23 E | 0.23 C | 1.40 C | 15.5 C | 0.60 C | 0.20 F | 1.80 B | 0.20 D | 4.17 B | 4.23 D | 35.5 B |
| Compost | 3 | 2728 E | 4.83 E | 1.20 A | 2.87 D | 3.30 AB | 2.43 A | 16.2 BC | 1.20 A | 0.57 E | 2.63 A | 0.50 AB | 6.70 A | 11.0 B | 53.1 A |
| 0.5% | 18 3 | 2805 E | 4.86 DE | 1.21 A | 3.03 D | 3.42 A | 2.43 A | 16.4 B | 1.22 A | 0.59 DE | 2.69 A | 0.48 B | 6.71 A | 11.1 AB | 54.0 A |
| 1.0% | 18 | 2943 D | 4.91 D | 1.18 A | 3.19 D | 3.30 AB | 2.49 A | 16.3 BC | 1.23 A | 0.64 D | 2.69 A | 0.48 AB | 6.86 A | 11.3 AB | 54.8A |
| 4.0% | 18 | 3228 C | 4.97 C | 1.22 A | 4.39 C | 3.08 B | 2.47 A | 16.7 B | 1.25 A | 0.94 C | 2.52 A | 0.51 A | 6.91 A | 11.5 A | 54.8 A |
| 10% | 18 | 3488 B | 5.27 B | 1.08 B | 6.28 B | 0.36 C | 1.66 B | 16.9 AB | 0.82 B | 1.08 B | 2.66 A | 0.38 C | 3.44 C | 4.39 CD | 32.9 C |
| 20% | 18 | 4390 A | 5.48 A | 1.07 B | 9.32 A | 0.37 C | 1.86 B | 17.7 A | 0.81 B | 1.58 A | 2.55 A | 0.37 C | 3.34 C | 4.67 C | 32.1 C |
| 14S | |||||||||||||||
| Control | 3 | 1461 E | 4.73 E | 2.50 A | 1.83 E | 0.30 B | 3.13 B | 17.9 ABC | 1.60 B | 0.30 G | 5.03 BC | 0.60 C | 3.47 B | 0.20 A | 64.3 B |
| Compost | 3 | 2332 D | 5.10 CD | 1.93 B | 2.33 DE | 3.03 A | 4.43 A | 16.9 C | 2.83 A | 0.80 F | 4.40 CD | 0.80 A | 5.73 A | 0.77 A | 90.8 A |
| 0.5% | 18 | 2385 D | 5.08 D | 1.89 B | 2.59 D | 2.93 A | 4.35 A | 16.9 C | 2.84 A | 0.91 E | 4.39 CD | 0.81 A | 5.63 A | 0.77 A | 89.4 A |
| 1.0% | 18 | 2367 D | 5.12 CD | 1.86 B | 2.66 D | 3.08 A | 4.40 A | 17.3 BC | 2.87 A | 0.99 D | 4.27 D | 0.82 A | 5.56 A | 0.74 A | 90.3 A |
| 4.0% | 18 | 2692 C | 5.18 BC | 1.86 B | 3.99 C | 2.84 A | 4.32 A | 17.3 BC | 2.75 A | 1.25 B | 3.92 D | 0.74 AB | 5.79 A | 1.26 A | 85.7 A |
| 10% | 18 | 3076 B | 5.27 B | 2.48 A | 6.01 B | 0.49 B | 3.16 B | 18.1 AB | 1.78 B | 1.15 C | 5.84 A | 0.71 AB | 2.97 B | 0.27 A | 55.2 C |
| 20% | 18 | 3842 A | 5.48 A | 2.43 A | 9.37 A | 0.34 B | 3.09 B | 18.6 A | 1.67 B | 1.66 A | 5.48 AB | 0.66 BC | 2.69 B | 0.31 A | 50.5 C |
| 24S | |||||||||||||||
| Control | 3 | 1950 E | 5.67 D | 1.20 A | 1.80 E | 0.30 B | 2.50 C | 15.9 C | 1.70 D | 0.30 F | 5.30 B | 0.40 D | 4.80 D | 0.57 D | 50.5 C |
| Compost | 3 | 3322 D | 5.80 C | 1.20 A | 2.30 DE | 3.10 A | 4.63 A | 17.7 B | 3.17 A | 0.90 E | 5.30 B | 0.77 A | 9.50 A | 3.70 A | 87.1 A |
| 0.5% | 18 | 3505 CD | 5.83 C | 1.17 A | 2.63 D | 3.31 A | 5.12 A | 17.9 B | 3.02 B | 0.91 E | 5.54 B | 0.72 B | 9.49 A | 3.62 A | 88.3 A |
| 1.0% | 18 | 3622 C | 5.82 C | 1.16 A | 2.85 D | 3.19 A | 4.62 A | 17.7 B | 3.03 B | 0.95 D | 5.44 B | 0.72 B | 9.07 B | 3.23 B | 87.4 A |
| 4.0% | 18 | 3944 B | 5.84 C | 1.16 A | 3.91 C | 3.12 A | 4.63 A | 17.8 B | 2.97 B | 1.23 B | 5.28 B | 0.71 B | 8.68 C | 3.17 B | 84.5 B |
| 10% | 18 | 3874 B | 6.18 B | 1.21 A | 6.32 B | 0.36 B | 3.40 B | 18.0 B | 1.77 C | 1.17 C | 6.22 A | 0.53 C | 4.55 DE | 1.15 C | 48.1 D |
| 20% | 18 | 4426 A | 6.30 A | 1.21 A | 9.23 A | 0.34 B | 2.90 BC | 19.0 A | 1.73 CD | 1.63 A | 5.61 B | 0.52 C | 4.25 E | 1.32 C | 45.1E |
| Treat | n | Cumu-lative C Release | pH | EC 1 | TC | TN | TP | CEC | P | K | Ca | Mg | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg CO2-C kg−1 soil−1 | dS m−1 | % | g kg−1 | g kg−1 | coml (+) kg−1 soil−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||
| 3S | |||||||||||||||
| Control | 3 | 1622 E 2 | 4.20 F | 0.93 D | 2.23 D | 0.23 C | 1.40 C | 15.5 C | 0.60 E | 0.20 F | 1.80 D | 0.20 E | 4.17 F | 4.23 E | 35.5 F |
| Compost | 3 | 2728 D | 4.83 E | 1.20 AB | 2.87 C | 3.30 A | 2.43 A | 16.2 BC | 1.20 A | 0.57 E | 2.63 B | 0.50 A | 6.70 A | 11.0 A | 53.1 A |
| RHB-A | 15 3 | 2919 C | 5.11 BC | 1.17 AB | 5.38 A | 2.17 B | 1.97 B | 16.5 AB | 1.00 D | 0.99 B | 2.29 C | 0.43 CD | 5.64 C | 9.24 B | 50.3 B |
| RHB-B | 15 | 2834 CD | 5.10 BC | 1.13 BC | 5.75 A | 2.07 B | 2.15 B | 17.0 AB | 1.03 CD | 0.90 D | 2.49 BC | 0.43 CD | 4.63 E | 7.56 D | 39.8 E |
| RHB-C | 15 | 2863 C | 5.11 B | 1.13 BC | 5.55 A | 2.03 B | 2.46 A | 16.5 AB | 1.09 BC | 0.89 D | 2.70 B | 0.43 CD | 5.15 D | 7.92 D | 40.7 E |
| RHB-D | 15 | 4239 A | 5.05 CD | 1.07 C | 4.47 B | 2.15 B | 2.12 B | 17.0 AB | 1.03 CD | 0.93 CD | 2.92 A | 0.43 D | 6.13 B | 9.02 BC | 46.9 D |
| RHB-E | 15 | 4190 A | 5.03 D | 1.19 AB | 4.52 B | 2.15 B | 2.21 B | 16.9 AB | 1.12 B | 0.96 BC | 2.61 B | 0.47 AB | 5.53 C | 8.72 C | 47.3 CD |
| RHB-F | 15 | 3180 B | 5.19 A | 1.22 A | 5.79 A | 2.09 B | 2.19 B | 17.1 A | 1.11 B | 1.13 A | 2.72 AB | 0.46 BC | 5.64 C | 9.09 BC | 49.3 BC |
| 14S | |||||||||||||||
| Control | 3 | 1461 E | 4.73 D | 2.50 A | 1.83 C | 0.30 D | 3.13 D | 17.9 A | 1.60 C | 0.30 F | 5.03 AB | 0.60 B | 3.47 C | 0.20 A | 64.3 D |
| Compost | 3 | 2332 D | 5.10 C | 1.93 CD | 2.33 C | 3.03 A | 4.43 A | 16.9 A | 2.83 A | 0.80 E | 4.40 BC | 0.80 A | 5.73 A | 0.77 A | 90.8 A |
| RHB-A | 15 | 2574 B | 5.23 AB | 2.42 AB | 5.25 A | 1.95 BC | 3.77 BC | 17.5 A | 2.39 B | 1.25 B | 4.24 C | 0.81 A | 4.72 B | 0.97 A | 79.4 B |
| RHB-B | 15 | 2394 CD | 5.19 BC | 2.29 B | 5.29 A | 1.77 C | 3.90 BC | 17.8 A | 2.33 B | 1.08 D | 4.93 ABC | 0.81 A | 4.15 BC | 0.38 A | 67.8 D |
| RHB-C | 15 | 2530 BC | 5.22 B | 2.05 C | 5.19 A | 1.79 C | 3.91 BC | 18.0 A | 2.23 B | 1.07 D | 5.37 A | 0.71 AB | 4.50 B | 0.71 A | 70.0 CD |
| RHB-D | 15 | 3505 A | 5.19 BC | 1.87 D | 4.29 B | 2.28 B | 3.75 BC | 17.3 A | 2.35 B | 1.17 C | 5.03 AB | 0.70 AB | 5.04 AB | 0.77 A | 75.3 BC |
| RHB-E | 15 | 3558 A | 5.21 B | 1.96 CD | 4.36 B | 2.05 BC | 3.61 C | 17.9 A | 2.50 B | 1.19 BC | 4.67 ABC | 0.77 A | 4.30 BC | 0.52 A | 75.9 BC |
| RHB-F | 15 | 2674 B | 5.32 A | 2.02 CD | 5.15 A | 1.78 C | 4.25 AB | 17.3 A | 2.49 B | 1.39 A | 4.43 BC | 0.70 AB | 4.46 B | 0.67 A | 77.1 B |
| 24S | |||||||||||||||
| Control | 3 | 1950 F | 5.67 E | 1.20 BC | 1.80 C | 0.30 E | 2.50 D | 15.9 B | 1.70 G | 0.30 F | 5.30 CD | 0.40 E | 4.80 E | 0.57 E | 50.5 D |
| Compost | 3 | 3322 DE | 5.80 D | 1.20 BC | 2.30 C | 3.10 A | 4.63 AB | 17.7 A | 3.17 A | 0.90 E | 5.30 CD | 0.77 A | 9.50 A | 3.70 A | 87.1 A |
| RHB-A | 15 | 3280 DE | 6.09 A | 1.18 BCD | 5.17 A | 1.85 CD | 3.79 C | 18.3 A | 2.45 E | 1.23 B | 5.40 BCD | 0.62 D | 7.53 B | 3.01 B | 68.3 C |
| RHB-B | 15 | 3444 CD | 5.99 B | 1.13 DE | 5.12 A | 1.77 D | 4.06 BC | 18.0 A | 2.48 DE | 1.05 D | 5.15 D | 0.61 D | 7.08 C | 2.21 CD | 69.2 C |
| RHB-C | 15 | 3124 E | 5.97 B | 1.23 AB | 5.48 A | 2.31 B | 3.80 C | 18.2 A | 2.35 F | 1.05 D | 6.29 A | 0.62 D | 7.90 B | 3.01 B | 72.7 B |
| RHB-D | 15 | 5180 A | 5.89 C | 1.12 E | 4.21 B | 2.18 B | 3.72 C | 18.2 A | 2.55 C | 1.18 C | 5.19 D | 0.62 D | 6.59 D | 1.92 D | 74.2 B |
| RHB-E | 15 | 4632 B | 5.96 B | 1.16 CDE | 4.31 B | 2.09 BC | 4.53 AB | 18.1 A | 2.51 CD | 1.19 BC | 5.78 ABC | 0.66 C | 7.11 C | 2.36 C | 69.4 C |
| RHB-F | 15 | 3586 C | 6.07 A | 1.27 A | 5.65 A | 2.18 B | 4.89 A | 17.5 A | 2.67 B | 1.37 A | 5.89 AB | 0.70 B | 7.05 C | 2.47 C | 70.1 C |
| Cumu. C Release 1 | pH | EC | TC | TN | TP | CEC | P | K | Ca | Mg | Cu | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3S | ||||||||||||||
| Cumu. C release | 1.00 | 0.49 2 | −0.16 | 0.33 | −0.37 | −0.30 | 0.51 | −0.24 | 0.60 | 0.29 | −0.04 | −0.34 | −0.32 | −0.30 |
| pH | 1.00 | −0.18 | 0.89 | −0.62 | −0.37 | 0.48 | −0.44 | 0.92 | 0.14 | −0.34 | −0.66 | −0.60 | −0.62 | |
| EC | 1.00 | −0.33 | 0.60 | 0.46 | −0.08 | 0.66 | −0.17 | 0.15 | 0.66 | 0.56 | 0.63 | 0.63 | ||
| TC | 1.00 | −0.75 | −0.45 | 0.43 | −0.62 | 0.90 | −0.09 | −0.58 | −0.75 | −0.72 | −0.74 | |||
| TN | 1.00 | 0.72 | −0.38 | 0.90 | −0.66 | 0.15 | 0.82 | 0.92 | 0.95 | 0.90 | ||||
| TP | 1.00 | −0.23 | 0.73 | −0.40 | 0.17 | 0.67 | 0.69 | 0.70 | 0.64 | |||||
| CEC | 1.00 | −0.22 | 0.54 | 0.15 | −0.12 | −0.36 | −0.33 | −0.34 | ||||||
| P | 1.00 | −0.48 | 0.22 | 0.96 | 0.84 | 0.88 | 0.82 | |||||||
| K | 1.00 | −0.01 | −0.36 | −0.66 | −0.60 | −0.60 | ||||||||
| Ca | 1.00 | 0.25 | 0.14 | 0.12 | 0.07 | |||||||||
| Mg | 1.00 | 0.75 | 0.80 | 0.76 | ||||||||||
| Cu | 1.00 | 0.98 | 0.95 | |||||||||||
| Pb | 1.00 | 0.97 | ||||||||||||
| Zn | 1.00 | |||||||||||||
| 14S | ||||||||||||||
| Cumu. C release | 1.00 | 0.39 | 0.23 | 0.48 | −0.44 | −0.44 | 0.36 | −0.44 | 0.61 | 0.34 | −0.25 | −0.42 | −0.13 | −0.46 |
| pH | 1.00 | 0.22 | 0.81 | −0.48 | −0.40 | 0.22 | −0.45 | 0.81 | 0.33 | −0.37 | −0.48 | −0.12 | −0.58 | |
| EC | 1.00 | 0.55 | −0.77 | −0.48 | 0.41 | −0.74 | 0.23 | 0.55 | −0.14 | −0.64 | −0.24 | −0.63 | ||
| TC | 1.00 | −0.77 | −0.59 | 0.41 | −0.76 | 0.78 | 0.53 | −0.47 | −0.71 | −0.22 | −0.82 | |||
| TN | 1.00 | 0.74 | −0.48 | 0.94 | −0.40 | −0.71 | 0.55 | 0.87 | 0.37 | 0.91 | ||||
| TP | 1.00 | −0.50 | 0.74 | −0.28 | −0.49 | 0.49 | 0.68 | 0.26 | 0.72 | |||||
| CEC | 1.00 | −0.44 | 0.23 | 0.40 | −0.14 | −0.38 | −0.07 | −0.41 | ||||||
| P | 1.00 | −0.35 | −0.75 | 0.69 | 0.82 | 0.30 | 0.92 | |||||||
| K | 1.00 | 0.22 | −0.19 | −0.39 | −0.06 | −0.45 | ||||||||
| Ca | 1.00 | −0.52 | −0.62 | −0.31 | −0.72 | |||||||||
| Mg | 1.00 | 0.44 | 0.06 | 0.63 | ||||||||||
| Cu | 1.00 | 0.71 | 0.86 | |||||||||||
| Pb | 1.00 | 0.35 | ||||||||||||
| Zn | 1.00 | |||||||||||||
| 24S | ||||||||||||||
| Cumu. C release | 1.00 | 0.14 | −0.34 | 0.08 | −0.12 | 0.04 | 0.36 | −0.10 | 0.45 | −0.12 | −0.01 | −0.20 | −0.18 | −0.12 |
| pH | 1.00 | 0.17 | 0.90 | −0.78 | −0.55 | 0.34 | −0.77 | 0.74 | 0.32 | −0.64 | −0.75 | −0.57 | −0.83 | |
| EC | 1.00 | 0.29 | −0.16 | −0.10 | −0.22 | −0.21 | 0.19 | 0.24 | −0.07 | −0.11 | 0.02 | −0.13 | ||
| TC | 1.00 | −0.75 | −0.60 | 0.28 | −0.77 | 0.77 | 0.25 | −0.71 | −0.77 | −0.63 | −0.80 | |||
| TN | 1.00 | 0.70 | −0.22 | 0.95 | −0.43 | −0.24 | 0.88 | 0.92 | 0.83 | 0.94 | ||||
| TP | 1.00 | −0.14 | 0.73 | −0.25 | −0.21 | 0.77 | 0.65 | 0.59 | 0.67 | |||||
| CEC | 1.00 | −0.22 | 0.36 | 0.11 | −0.16 | −0.15 | −0.05 | −0.19 | ||||||
| P | 1.00 | −0.40 | −0.29 | 0.93 | 0.90 | 0.79 | 0.95 | |||||||
| K | 1.00 | 0.04 | −0.28 | −0.50 | −0.33 | −0.49 | ||||||||
| Ca | 1.00 | −0.13 | −0.19 | −0.14 | −0.25 | |||||||||
| Mg | −0.13 | 1.00 | 0.86 | 0.81 | 0.88 | |||||||||
| Cu | 1.00 | 0.95 | 0.95 | |||||||||||
| Pb | 1.00 | 0.86 | ||||||||||||
| Zn | 1.00 | |||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Chang, Y.-F. Effects of Rice Husk Biochar on Carbon Release and Nutrient Availability in Three Cultivation Age of Greenhouse Soils. Agronomy 2020, 10, 990. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10070990
Tsai C-C, Chang Y-F. Effects of Rice Husk Biochar on Carbon Release and Nutrient Availability in Three Cultivation Age of Greenhouse Soils. Agronomy. 2020; 10(7):990. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10070990
Chicago/Turabian StyleTsai, Chen-Chi, and Yu-Fang Chang. 2020. "Effects of Rice Husk Biochar on Carbon Release and Nutrient Availability in Three Cultivation Age of Greenhouse Soils" Agronomy 10, no. 7: 990. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10070990