Field Application of ZnO and TiO2 Nanoparticles on Agricultural Plants
Abstract
:1. Introduction
2. ZnO NPs and Application in Agriculture
3. TiO2 NPs and Application in Agriculture
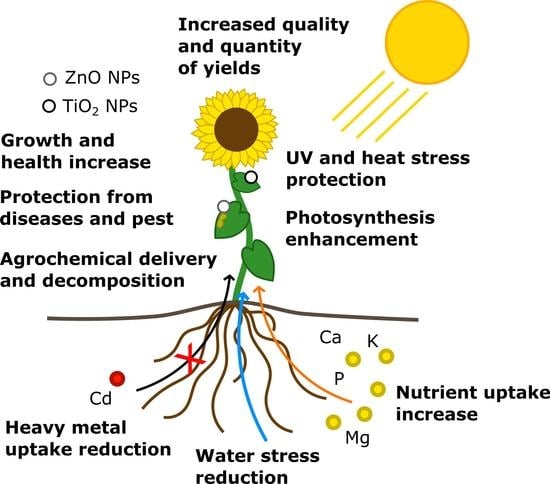
4. Application of ENPs in Field Conditions
4.1. Seed Application and Effects
4.2. Soil Application and Root Path
4.3. Foliar Application and Translocation Path
5. Conclusions and Future Research Needs
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Williams, B.P.; Qi, Z.; Huang, W.; Tsung, C.-K. The impact of synthetic method on the catalytic application of intermetallic nanoparticles. Nanoscale 2020, 12, 18545–18562. [Google Scholar] [CrossRef] [PubMed]
- Sutarlie, L.; Ow, S.Y.; Su, X. Nanomaterials-based biosensors for detection of microorganisms and microbial toxins. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Esfe, M.H.; Kamyab, M.H.; Valadkhani, M. Application of nanofluids and fluids in photovoltaic thermal system: An updated review. Sol. Energy 2020, 199, 796–818. [Google Scholar] [CrossRef]
- Vinitha, V.; Preeyanghaa, M.; Vinesh, V.; Dhanalakshmi, R.; Neppolian, B.; Sivamurugan, V. Two is better than one: Catalytic, sensing and optical applications of doped zinc oxide nanostructures. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Selvakumar, D.; Nagaraju, P.; Arivanandhan, M.; Jayavel, R. Metal oxide–grafted graphene nanocomposites for energy storage applications. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zheng, X.; Jin, H.J.; Li, X. Biomineralization: An Opportunity and Challenge of Nanoparticle Drug Delivery Systems for Cancer Therapy. Adv. Healthc. Mater. 2020, 9, 2001117. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Yang, W.; Chen, X.; Gao, J.; Gong, X.; Wang, H.; Duan, Y.; Wei, D.; Chang, J. Nanoparticle-based diagnostic and therapeutic systems for brain tumors. J. Mater. Chem. B 2019, 7, 4734–4750. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Popelka, A.; Aljarod, O.; Hassan, M.K.; Kasak, P.; Luyt, A.S. Accelerated Weathering Effects on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and PHBV/TiO2 Nanocomposites. Polymers 2020, 12, 1743. [Google Scholar] [CrossRef] [PubMed]
- Changmei, L.; Chaoying, Z.; Junqiang, W.; Guorong, W.; Mingxuan, T. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–171. [Google Scholar]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of Nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Manceau, A.; Nagy, K.L.; Marcus, M.A.; Lanson, M.; Geoffroy, N.; Jacquet, T.; Kirpichtchikova, T. Formation of Metallic Copper Nanoparticles at the Soil−Root Interface. Environ. Sci. Technol. 2008, 42, 1766–1772. [Google Scholar] [CrossRef] [Green Version]
- Lanson, B.; Marcus, M.A.; Fakra, S.; Panfili, F.; Geoffroy, N.; Manceau, A. Formation of Zn–Ca phyllomanganate nanoparticles in grass roots. Geochim. Cosmochim. Acta 2008, 72, 2478–2490. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.H.; Xing, B.S. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Handy, R.D.; von der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Gao, F.; Hong, F.; Liu, C.; Zheng, L.; Su, M.; Wu, X.; Yang, F.; Wu, C.; Yang, P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: Inducing complex of Rubisco-Rubisco activase. Biol. Trace Elem. Res. 2006, 111, 239–253. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Du, W.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Zhu, J.; Peralta-Videa, J.R.; Guo, H. Physiological and Biochemical Changes Imposed by CeO2 Nanoparticles on Wheat: A Life Cycle Field Study. Environ. Sci. Technol. 2015, 49, 11884–11893. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Moaveni, P.; Farahani, H.A.; Maroufi, K. Effect of TiO2 nanoparticles spraying on barley (Hordeum vulgare L.) under field condition. Adv. Environ. Biol. 2011, 5, 2220–2223. [Google Scholar]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Dobročka, E.; Kšiňan, S.; Illa, R.; Qian, Y.; et al. Foliar Application of Low Concentrations of Titanium Dioxide and Zinc Oxide Nanoparticles to the Common Sunflower under Field Conditions. Nanomaterials 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica l.) under field conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef] [Green Version]
- Irshad, M.A.; ur Rehman, M.Z.; Anwar-ul-Haq, M.; Rizwan, M.; Nawaz, R.; Shakoor, M.B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Ali, S. Effect of green and chemically synthesized titanium dioxide nanoparticles on cadmium accumulation in wheat grains and potential dietary health risk: A field investigation. J. Hazard. Mater. 2021, 415, 125585. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Qayyum, M.F.; Nawaz, R.; Ahmad, A.; Asrar, M.; Ahmad, S.R.; Alsahli, A.A.; et al. Combined use of different nanoparticles effectively decreased cadmium (Cd) concentration in grains of wheat grown in a field contaminated with Cd. Ecotoxicol. Environ. Saf. 2021, 215, 112139. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.; Ullah, F.; Shinwari, Z.K.; Mehmood, S. The effect of titanium dioxide nanoparticles and salicylic acid on growth and biodiesel production potential of sunflower (Helianthus annuus L.) under water stress. Pak. J. Bot. 2021, 53, 1987–1995. [Google Scholar] [CrossRef]
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Maier-Flaig, F.; Schneider, D.; Kalt, H. 65 years of ZnO research—Old and very recent results. Phys. Status Solidi 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833. [Google Scholar] [CrossRef] [Green Version]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Agrawal, A.; Raliya, R.; Kumar, P.; Burman, U.; Kaul, R.K. ZnO Nanoparticles Induced Synthesis of Polysaccharides and Phosphatases by Aspergillus Fungi. Adv. Sci. Eng. Med. 2012, 4, 324–328. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.-W.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020, 722, 137808. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.S.; El Din, T.A.S.; Hendawey, M.H.; Borai, I.H.; Mahdi, A.A. Magnetite and Zinc Oxide Nanoparticles Alleviated Heat Stress in Wheat Plants. Curr. Nanomater. 2018, 3, 32–43. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effect of Foliar Spray of Zinc Oxide on Some Antioxidant Enzymes Activity of Sunflower under Salt Stress. J. Agric. Sci. Technol. 2016, 18, 1013–1025. [Google Scholar]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.-H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A.; Shaaban, E.A. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.; Diaz, B.C.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Silva, R.M.; TeeSy, C.; Franzi, L.; Weir, A.; Westerhoff, P.; Evans, J.E.; Pinkerton, K.E. Biological Response to Nano-Scale Titanium Dioxide (TiO2): Role of Particle Dose, Shape, and Retention. J. Toxicol. Environ. Heal. Part A 2013, 76, 953–972. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles—A Review. J. Nanomater. 2015, 2015, 790173. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Lalley, J.; Namboodiri, D.; Cromer, K.; Nadagouda, M.N. Titanium dioxide-based antibacterial surfaces for water treatment. Curr. Opin. Chem. Eng. 2016, 11, 46–51. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, K.; Lu, N.; Xu, H.; Zhang, S.; Takeuchi, N.; Kobayashi, H.; Li, R. The interplay of sulfur doping and surface hydroxyl in band gap engineering: Mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification. Appl. Catal. B Environ. 2017, 218, 20–31. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef]
- Jarosz, M.; Pawlik, A.; Szuwarzyński, M.; Jaskuła, M.; Sulka, G.D. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloids Surf. B Biointerfaces 2016, 143, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Du, W.; Barrios, A.C.; Armendariz, R.; Zuverza-Mena, N.; Ji, Z.; Chang, C.H.; Zink, J.I.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; et al. Surface coating changes the physiological and biochemical impacts of nano-TiO2 in basil (Ocimum basilicum) plants. Environ. Pollut. 2017, 222, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, H.; Ilyas, N.; Akhtar, N.; Raja, N.I.; Zainab, T.; Shah, T.; Ahmad, A.; Ahmad, P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021, 223, 112519. [Google Scholar] [CrossRef]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794. [Google Scholar] [CrossRef] [PubMed]
- Mahdieh, M.; Sangi, M.R.; Bamdad, F.; Ghanem, A. Effect of seed and foliar application of nano-zinc oxide, zinc chelate, and zinc sulphate rates on yield and growth of pinto bean (Phaseolus vulgaris) cultivars. J. Plant Nutr. 2018, 41, 2401–2412. [Google Scholar] [CrossRef]
- Umar, W.; Hameed, M.K.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Rasheed, N. Synthesis, characterization and application of ZnO nanoparticles for improved growth and Zn biofortification in maize. Arch. Agron. Soil Sci. 2021, 67, 1164–1176. [Google Scholar] [CrossRef]
- Elizabath, A.; Bahadur, V.; Misra, P.; Prasad, V.M.; Thomas, T. Effect of different concentrations of iron oxide and zinc oxide nanoparticles on growth and yield of carrot (Daucus carota L.). J. Pharmacogn. Phytochem. 2017, 6, 1266–1269. [Google Scholar]
- Salehi, H.; Chehregani, A.; Lucini, L.; Majd, A.; Gholami, M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 2018, 616–617, 1540–1551. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [Green Version]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997; ISBN 3826331524. [Google Scholar]
- Guan, H.N.; Chi, D.F.; Yu, J.; Zhang, S.Y. Novel photodegradable insecticide W/TiO2/Avermectin nanocomposites obtained by polyelectrolytes assembly. Colloids Surf. B Biointerfaces 2011, 83, 148–154. [Google Scholar] [CrossRef]
- Ma, H.; Wallis, L.K.; Diamond, S.; Li, S.; Canas-Carrell, J.; Parra, A. Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ. Pollut. 2014, 193, 165–172. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar Fertilization of Crop Plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- González-Melendi, P.; Fernández-Pacheco, R.; Coronado, M.J.; Corredor, E.; Testillano, P.S.; Risueño, M.C.; Marquina, C.; Ibarra, M.R.; Rubiales, D.; Pérez-de-Luque, A. Nanoparticles as smart treatment-delivery systems in plants: Assessment of different techniques of microscopy for their visualization in plant tissues. Ann. Bot. 2008, 101, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. CRC Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Sadak, M.S.; Bakry, B.A. Zinc-oxide and nano ZnO oxide effects on growth, some biochemical aspects, yield quantity, and quality of flax (Linum uitatissimum L.) in absence and presence of compost under sandy soil. Bull. Natl. Res. Cent. 2020, 44, 98. [Google Scholar] [CrossRef]
- Velasco, E.A.P.; Galindo, R.B.; Aguilar, L.A.V.; Fuentes, J.A.G.; Urbina, B.A.P.; Morales, S.A.L.; Valdés, S.S. Effects of the Morphology, Surface Modification and Application Methods of ZnO-NPs on the Growth and Biomass of Tomato Plants. Molecules 2020, 25, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel Effects of Nanoparticulate Delivery of Zinc on Growth, Productivity, and Zinc Biofortification in Maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.S. Effect of Titanium Nanoparticles (TiO2) on Growth, Yield and Chemical Constituents of Coriander Plants. Arab J. Nucl. Sci. Appl. 2015, 48, 187–194. [Google Scholar]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Dashti, S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric. Slov. 2016, 107, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Teszlák, P.; Kocsis, M.; Scarpellini, A.; Jakab, G.; Kőrösi, L. Foliar exposure of grapevine (Vitis vinifera L.) to TiO2 nanoparticles under field conditions: Photosynthetic response and flavonol profile. Photosynthetica 2018, 56, 1378–1386. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bouderias, S.; Csepregi, K.; Bognár, B.; Teszlák, P.; Scarpellini, A.; Castelli, A.; Hideg, É.; Jakab, G. Nanostructured TiO2-induced photocatalytic stress enhances the antioxidant capacity and phenolic content in the leaves of Vitis vinifera on a genotype-dependent manner. J. Photochem. Photobiol. B Biol. 2019, 190, 137–145. [Google Scholar] [CrossRef]
- Zierden, M.R.; Valentine, A.M. Contemplating a role for titanium in organisms. Metallomics 2016, 8, 9–16. [Google Scholar] [CrossRef]

| Plant Name | Size of ZnO NPs | Additional Compounds | Concentrations | Type of Application | Number of Times Applied | Effects of ZnO NPs | Reference |
|---|---|---|---|---|---|---|---|
| Arachis hypogaea L. | 25 nm | no | 133 mg Zn∙L−1 | Foliar | 2 | Increased plant height, pods per plant, filled pods per plant | [29] |
| Zea mays L. | 25 nm | no | 50, 100, 200, 400, 600, 800, 1000, 1500, 2000 mg Zn∙L−1 | Foliar | 2 | Increased plant height, leaf area, dry weight, grain yield, cob length | [86] |
| Daucus carota L. | n.a. | no | 50, 100, 150 mg ZnO∙L−1 | Foliar | 1 | Increased plant height, number of leaves | [74] |
| Setaria italica L. | 20 nm | adjuvant SILWET STAR® | 2.6 mg Zn∙L−1 | Foliar | 2 | Increased total nitrogen, content of oil, dry mass, decreased content of starch | [32] |
| Helianthus annuus L. | 20 nm | adjuvant SILWET STAR® | 2.6 mg Zn∙L−1 | Foliar | 2 | Differences in leaf surfaces’ trichomes diversity, ratio, width, and length, increase in head diameter, weight of dry seed head, weight of thousand seeds, grain yield, content of oil | [31] |
| Linum usitatissimum L. | <40 nm | no | 20, 40, 60 mg ZnO∙L−1 | Foliar | 2 | Increased shoot length, fresh and dry weight, root length, fresh and dry weight, number of fruiting branches, capsules, biological yield per plant, seed and straw yield, weight of 1000 seeds, oil content, seed, oil, biological and straw yield | [84] |
| Zea mays L. | 30–70 nm (width), 160 nm (length) | no | Soil: 8 kg Zn∙ha−1 Foliar: 2% Zn solution | Soil or foliar | 1 | Improved maize growth, yield and grain Zn contents, increased chlorophyll contents, maximum value of photosynthetic rate, transpiration rate | [73] |
| Triticum aestivum L. | 20–30 nm | no | 25 mg Zn∙l−1 | Foliar | 3 | Enhanced wheat growth, yield, nutrients uptake, chlorophyll, carotenoids contents and antioxidants activities and reduced electrolyte leakage under Cd stress | [34] |
| Plant Name | Size of TiO2 NPs | Additional Compounds | Concentrations | Type of Application | Number of Times Applied | Effects of TiO2 NPs | Reference |
|---|---|---|---|---|---|---|---|
| Hordeum vulgare L. | n.a. | no | 100, 200, 300 mg∙L−1 | Foliar | 2 | Increased plant height, grain yield, dry biomass, weight of 1000 grains | [30] |
| Coriandrum sativum L. | 20 nm | no | 2, 4, 6 mg∙L−1 | Foliar | 2 | Increased the plant height, number of branches, fruit yield, increase in amino acids, total sugars, total phenols, total indoles, and pigments | [87] |
| Hordeum vulgare L. | <100 nm | no | 2000 mg∙L−1 | Foliar | 2 | Increased days to anthesis, chlorophyll content, straw yield, number of grains per spike | [88] |
| Vitis vinifera L. | 28 nm | no | 1000 mg∙L−1 | Foliar | 1 | Metabolic (nonstomatal) inhibition of the photosynthesis | [89] |
| Vitis vinifera L. | 28 nm | no | 1000 mg∙L−1 | Foliar | 1 | Boosted the total phenolic content and biosynthesis of the leaf flavanols, increased K, Mg, Ca, B, and Mn levels | [90] |
| Helianthus annuus L. | 20–30 nm | adjuvant SILWET STAR® | 2.6 mg∙L−1 | Foliar | 2 | Increased head diameter, dry-seed head weight, yield and thousand seed weight, increased oil content, improvement in physiological parameters | [31] |
| Triticum aestivum L. | 10–13 nm 6–8 nm | Leaf extract surface modification during synthesis | 100 mg∙L−1 | Foliar | 2 | Increased straw and grain yields, chlorophyll contents, plant height, reduced oxidative stress under Cd stress, decreased Cd in wheat straw, roots, grains, better effect by green synthesised NPs | [33] |
| Helianthus annuus L. | <25 nm | no | 25, 50 mg∙L−1 | Foliar | 2 | Alleviated adverse effect of water deficiency stress on growth, achene quality and biodiesel yield | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šebesta, M.; Kolenčík, M.; Sunil, B.R.; Illa, R.; Mosnáček, J.; Ingle, A.P.; Urík, M. Field Application of ZnO and TiO2 Nanoparticles on Agricultural Plants. Agronomy 2021, 11, 2281. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11112281
Šebesta M, Kolenčík M, Sunil BR, Illa R, Mosnáček J, Ingle AP, Urík M. Field Application of ZnO and TiO2 Nanoparticles on Agricultural Plants. Agronomy. 2021; 11(11):2281. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11112281
Chicago/Turabian StyleŠebesta, Martin, Marek Kolenčík, B. Ratna Sunil, Ramakanth Illa, Jaroslav Mosnáček, Avinash P. Ingle, and Martin Urík. 2021. "Field Application of ZnO and TiO2 Nanoparticles on Agricultural Plants" Agronomy 11, no. 11: 2281. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy11112281