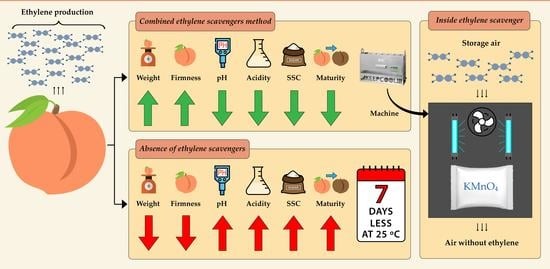
Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
- Weight: 179.5 ± 2.2 g
- Size: 72.0 ± 2 mm
- Firmness: 30.2 ± 1.6 N
- Soluble solids content (SSC): 10.9%
- Total acidity (TA): 3.8%
- Ratio SSC/TA (MI): 2.86
2.2. Experimental Design
- -
- NoES-R (control): No Ethylene Scavenger + Refrigeration temperature.
- -
- ES-R: Ethylene Scavenger + Refrigeration temperature.
- -
- NoES-NoR: No Ethylene Scavenger + No Refrigeration temperature.
- -
- ES-NoR: Ethylene Scavenger + No Refrigeration temperature.
2.3. Conservation Chambers Atmosphere
2.4. Physical Parameters
2.5. Maturity Parameters
2.6. Microbiological Incidence
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in the Conservation Chambers Atmosphere
3.1.1. Ethylene
3.1.2. Carbon Dioxide
3.1.3. Oxygen
3.1.4. Relationship between Ethylene and CO2
3.2. Physical Parameters
3.3. Maturity Parameters
3.4. Microbiological Incidence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Johnson, R.S.; DeJong, T.; Day, K.R. Orchard Factors Affecting Postharvest Stone Fruit Quality. HortScience 1997, 32, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Nazar, R.; Khan MI, R.; Khan, N.A. Variation in photosynthesis and growth of mustard cultivars: Role of ethylene sensitivity. Sci. Hortic. 2012, 135, 1–6. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Molina, F.D.M.; Artés-Hernández, F. Postharvest quality retention of apricots by using a novel sepiolite–loaded potassium permanganate ethylene scavenger. Postharvest Biol. Technol. 2020, 160, 111061. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P.V. Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Sakizci, M. Effect of salt modification and acid activation on ethylene adsorption properties of sepiolite. Adsorption 2013, 19, 1083–1091. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene scavengers for the preservation of fruits and vegetables: A review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium Permanganate-Based Ethylene Scavengers for Fresh Horticultural Produce as an Active Packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Gorinstein, S. Ethylene treatment of ‘Hayward’ kiwifruits (Actinidia deliciosa) during ripening and its influence on ethylene biosynthesis and antioxidant activity. Sci. Hortic. 2006, 108, 22–28. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, G.H.; Kim, S.-W. Ethylene Gas Decomposition Using ZSM-5/WO3-Pt-Nanorod Composites for Fruit Freshness. ACS Sustain. Chem. Eng. 2019, 7, 11250–11257. [Google Scholar] [CrossRef]
- Pathak, N. Photocatalysis and Vacuum Ultraviolet Light Photolysis as Ethylene Removal Techniques for Potential Application in Fruit Storage. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills, R.; Warton, M. Efficacy of Potassium Permanganate Impregnated into Alumina Beads to Reduce Atmospheric Ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.; Martínez-Hernández, G.B. Current Scenario of Adsorbent Materials Used in Ethylene Scavenging Systems to Extend Fruit and Vegetable Postharvest Life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Zapata, P.J.; Guillén, F.; Paladines, D.; Castillo, S.; Valero, D.; Serrano, M. The addition of rosehip oil to Aloe gels improves their properties as postharvest coatings for maintaining quality in plum. Food Chem. 2017, 217, 585–592. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of fruit maturity and its prediction model based on the pericarp index of absorbance difference (IAD) for peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef]
- Le Nguyen, L.P.; Zsom, T.; Sao Dam, M.; Baranyai, L.; Hitka, G. Evaluation of the 1-MCP microbubbles treatment for shelf-life extension for melons. Postharvest Biol. Technol. 2019, 150, 89–94. [Google Scholar] [CrossRef]
- Sammi, S.; Masud, T. Effect of different packaging systems on the quality of tomato (Lycopersicon esculentum var. Rio Grande) fruits during storage. Int. J. Food Sci. Technol. 2009, 44, 918–926. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Q.; Wang, G.-X.; Peng, X.-Y.; Wang, J.-D.; Che, F.-B. Effects of different postharvest treatments on the physiology and quality of ‘Xiaobai’ apricots at room temperature. J. Food Sci. Technol. 2014, 52, 2247–2255. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-W.; Dong, L.; Ben-Arie, R.; Lurie, S. The role of ethylene in the prevention of chilling injury in nectarines. J. Plant Physiol. 2001, 158, 55–61. [Google Scholar] [CrossRef]
- Fan, X.; Shu, C.; Zhao, K.; Wang, X.; Cao, J.; Jiang, W. Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci. Hortic. 2018, 232, 63–70. [Google Scholar] [CrossRef]
- Hayama, H.; Shimada, T.; Fujii, H.; Ito, A.; Kashimura, Y. Ethylene-regulation of fruit softening and softening-related genes in peach. J. Exp. Bot. 2006, 57, 4071–4077. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Garner, D.; Basinal, L.M. Effect of continuous exposure to exogenous ethylene during cold storage on postharvest decay development and quality attributes of stone fruits and table grapes. Postharvest Biol. Technol. 2003, 27, 243–254. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies. Foods 2020, 9, 1452. [Google Scholar] [CrossRef]
- Crisosto, C.; Gugliuzza, G.; Garner, D.; Palou, L. Understanding the role of ethylene in peach cold storage life. Acta Hortic. 2001, 287–288. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. At-harvest fruit maturity affects sucrose metabolism during cold storage and is related to chilling injury in peach. J. Food Sci. Technol. 2020, 57, 2000–2009. [Google Scholar] [CrossRef]
- Emadpour, M.; Ghareyazie, B.; Kalaj, Y.R.; Entesari, M.; Bouzari, N. Effect of the potassium permanganate coated zeolite nanoparticles on the quality characteristic and shelf life of peach and nectarine. J. Agric. Technol. 2015, 11, 1263–1273. [Google Scholar]
- García, J.C.; Balaguera-López, H.E.; Herrera, A.O. Conservación del fruto de banano bocadillo (Musa AA Simmonds) con la aplicación de permanganato de potasio (KMnO4). Rev. Colomb. Cienc. Hortíc. 2012, 6, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Sardabi, F.; Mohtadinia, J.; Shavakhi, F.; Jafari, A.A. The effects of 1-methylcyclopropen (1-MCP) and potassium permanganate coated zeolite nanoparticles on shelf-life extension and quality lossof golden delicious apples. J. Food Process. Preserv. 2014, 38, 2176–2182. [Google Scholar] [CrossRef]
- Chamara, D.; Illeperuma, K.; Galappatty, T.; Sarananda, K. Modified atmosphere packaging of ‘Kolikuttu’ bananas at low temperature. J. Hortic. Sci. Biotechnol. 2000, 75, 92–96. [Google Scholar] [CrossRef]
- Tourky, M.; Tarabih, M.; El-Eryan, E. Physiological studies on the marketability of Williams banana fruits. Am. J. Plant Physiol. 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ramin, A.; Rezaei, A.; Shams, M. Potassium permanganates and short term hypobaric enhances shelf-life of kiwifruits. Acta Hortic. 2010, 849–852. [Google Scholar] [CrossRef]
- Illeperuma, C.K.; Jayasuriya, P. Prolonged storage of ‘Karuthacolomban’ mango by modified atmosphere packaging at low temperature. J. Hortic. Sci. Biotechnol. 2002, 77, 153–157. [Google Scholar] [CrossRef]
- Castro, J.; Conte, R.; Carvalho, C.; Rossetto, C. Effects of postharvest treatments and film packaging on quality of ‘Haden 2H’ mangoes. Acta Hortic. 2010, 864, 295–298. [Google Scholar] [CrossRef]
- Jeronimo, E.M.; Brunini, M.A.; Arruda, M.C.D.; Cruz, J.C.S.; Gava, G.J.D.C.; Silva, M.D.A. Qualidade de mangas ‘Tommy Atkins’ armazenadas sob atmosfera modificada. Ciênc. Agrotecnol. 2007, 31, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Rodríguez-Hernández, A.M.; Castillo-Campohermoso, M.A.; Artés-Hernández, F. An Innovative Ethylene Scrubber Made of Potassium Permanganate Loaded on a Protonated Montmorillonite: A Case Study on Blueberries. Food Bioprocess Technol. 2019, 12, 524–538. [Google Scholar] [CrossRef]
- Katara, G.; Hemvani, N.; Chitnis, S.; Chitnis, V.; Chitnis, D.S. Surface disinfection by exposure to germicidal UV light. Indian J. Med. Microbiol. 2008, 26, 241–242. [Google Scholar] [CrossRef]
- Forges, M.; Bardin, M.; Urban, L.; Aarrouf, J.; Charles, F. Impact of UV-C Radiation Applied during Plant Growth on Pre- and Postharvest Disease Sensitivity and Fruit Quality of Strawberry. Plant Dis. 2020, 104, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Muzzaffar, S.; Bhat, M.M.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Postharvest Biology and Technology of Apricot. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 201–222. [Google Scholar] [CrossRef]
- Vall-Llaura, N.; Giné-Bordonaba, J.; Usall, J.; Larrigaudière, C.; Teixidó, N.; Torres, R. Ethylene biosynthesis and response factors are differentially modulated during the interaction of peach petals with Monilinia laxa or Monilinia fructicola. Plant Sci. 2020, 299, 110599. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Castillejo, N.; Martínez, J.A.; Artés-Hernández, F. Development of an antifungal active packaging containing thymol and an ethylene scavenger. Validation during storage of cherry tomatoes. Food Packag. Shelf Life 2021, 29, 100734. [Google Scholar] [CrossRef]



| Storage Days | Treatment | Weight (g) | Size (mm) | Firmness (N) | Colour (a*) | Colour (b*) |
|---|---|---|---|---|---|---|
| 0 | 170.4 ± 2.1 | 70 ± 2 | 25.9 ± 2.6 | 19.2 ± 0.7 | 59.7 ± 0.9 | |
| 3 | NoES-R | 163.7 ± 2.4 a | 64 ± 2 b | 22.6 ± 1.4 ab | 17.9 ± 1.5 ab | 60.5 ± 1.4 a |
| ES-R | 162.2 ± 1.6 a | 67 ± 2 ab | 27.9 ± 1.6 a | 15.8 ± 1.1 b | 60.7 ± 0.7 a | |
| NoES-NoR | 167.9 ± 6.9 a | 70 ± 1 a | 13.7 ± 1.1 c | 21.3 ± 1.3 a | 56.2 ± 1.5 b | |
| ES-NoR | 158.1 ± 3.4 a | 64 ± 1 b | 17.6 ± 1.4 bc | 19.3 ± 0.8 ab | 57.9 ± 1.1 ab | |
| Ethylene Scavengers (ES) | n.s. | n.s. | *** | n.s. | n.s. | |
| Temperature (T) | n.s. | n.s. | *** | ** | ** | |
| ES × T | n.s. | * | n.s. | n.s. | n.s. | |
| 7 | NoES-R | 144.8 ± 3.4 b | 64 ± 2 a | 22.2 ± 2.2 ab | 20.5 ± 0.8 b | 59.1 ± 1.0 ab |
| ES-R | 150.7 ± 0.6 b | 66 ± 2 a | 26.0 ± 0.6 a | 19.8 ± 0.9 bc | 60.9 ± 2.1 a | |
| NoES-NoR | 172.6 ± 0.0 a | 68 ± 1 a | 11.9 ± 0.5 c | 26.7 ± 0.5 a | 53.6 ± 0.8 c | |
| ES-NoR | 145.5 ± 1.7 b | 65 ± 1 a | 16.8 ± 2.1 bc | 27.2 ± 1.4 a | 54.2 ± 1.1 bc | |
| Ethylene Scavengers (ES) | *** | n.s. | *** | *** | n.s. | |
| Temperature (T) | *** | n.s. | ** | n.s. | *** | |
| ES × T | *** | n.s. | n.s. | *** | n.s. | |
| 10 | NoES-R | 142.0 ± 2.2 a | 62 ± 2 a | 23.9 ± 2.2 a | 19.1 ± 0.9 b | 61.0 ± 1.3 a |
| ES-R | 131.1 ± 2.7 b | 66 ± 1 a | 21.3 ± 1.1 a | 18.4 ± 1.1 b | 62.8 ± 0.8 a | |
| NoES-NoR | - | - | - | - | - | |
| ES-NoR | 135.0 ± 3.1 ab | 66 ± 1 a | 18.3 ± 1.6 a | 32.0 ± 0.8 a | 47.8 ± 0.3 b | |
| One-way ANOVA | * | n.s. | n.s. | *** | *** | |
| 14 | NoES-R | 131.1 ± 3.7 a | 66 ± 2 a | 17.3 ± 1.5 a | 20.8 ± 1.2 b | 55.6 ± 2.4 a |
| ES-R | 115.8 ± 2.8 b | 65 ± 2 a | 15.7 ± 1.3 a | 18.1 ± 1.0 b | 58.6 ± 3.0 a | |
| NoES-NoR | - | - | - | - | - | |
| ES-NoR | 120.8 ± 0.0 ab | 63 ± 1 a | 14.3 ± 1.2 a | 32.7 ± 0.7 a | 44.3 ± 1.3 b | |
| One-way ANOVA | * | n.s. | n.s. | *** | *** | |
| 17 | NoES-R | 114.7 ± 2.3 a | 61 ± 1 a | 20.3 ± 2.3 a | 21.4 ± 1.2 a | 50.0 ± 2.9 b |
| ES-R | 105.7 ± 1.6 b | 61 ± 1 a | 15.0 ± 2.9 a | 18.3 ± 0.8 b | 59.8 ± 1.5 a | |
| NoES-NoR | - | - | - | - | - | |
| ES-NoR | - | - | - | - | - | |
| t-test | * | n.s. | n.s. | * | ** | |
| 22 | NoES-R | 96.7 ± 3.7 a | 57 ± 1 a | 12.1 ± 2.0 a | 21.1 ± 0.5 a | 53.0 ± 1.7 a |
| ES-R | 90.8 ± 3.1 a | 60 ± 2 a | 14.5 ± 1.8 a | 19.1 ± 0.5 b | 51.5 ± 2.7 a | |
| NoES-NoR | - | - | - | - | - | |
| ES-NoR | - | - | - | - | - | |
| t-test | n.s. | n.s. | n.s. | ** | n.s. | |
| 24 | NoES-R | 84.3 ± 2.6 a | 55 ± 2 a | 11.8 ± 0.93 a | 18.0 ± 0.3 a | 47.4 ± 2.0 a |
| ES-R | 82.2 ± 2.7 a | 58 ± 2 a | 12.4 ± 2.1 a | 19.7 ± 1.0 a | 52.1 ± 2.9 a | |
| NoES-NoR | - | - | - | - | - | |
| ES-NoR | - | - | - | - | - | |
| t-test | n.s. | n.s. | n.s. | n.s. | n.s. |
| Storage Days | Treatment | SSC (%) | pH | TA (%) | MI |
|---|---|---|---|---|---|
| 0 | 11.2 ± 0.4 | 3.7 ± 0.03 | 3.7 ± 0.21 | 3.04 ± 0.16 | |
| 3 | NoES-R | 13.0 ± 0.6 a | 3.7 ± 0.05 c | 3.4 ± 0.03 b | 3.82 ± 0.20 ab |
| ES-R | 11.0 ± 1.0 a | 3.6 ± 0.03 c | 3.9 ± 0.07 a | 2.84 ± 0.30 b | |
| NoES-NoR | 11.7 ± 0.9 a | 4.1 ± 0.05 a | 2.4 ± 0.04 d | 4.91 ± 0.41 a | |
| ES-NoR | 11.0 ± 0.6 a | 3.9 ± 0.04 b | 2.8 ± 0.07 c | 3.94 ± 0.19 ab | |
| Ethylene Scavengers (ES) | n.s. | ** | *** | ** | |
| Temperature (T) | n.s. | *** | *** | ** | |
| ES × T | n.s. | n.s. | n.s. | n.s. | |
| 7 | NoES-R | 11.7 ± 0.9 ab | 3.8 ± 0.02 c | 3.8 ± 0.30 a | 3.16 ± 0.38 b |
| ES-R | 14.0 ± 1.0 a | 3.7 ± 0.01 c | 4.3 ± 0.10 a | 3.30 ± 0.31 b | |
| NoES-NoR | 8.3 ± 1.2 c | 4.5 ± 0.05 a | 1.5 ± 0.16 c | 5.88 ± 1.46 a | |
| ES-NoR | 10.7 ± 0.3 bc | 4.1 ± 0.05 b | 2.5 ± 0.25 b | 4.39 ± 0.52 ab | |
| Ethylene Scavengers (ES) | * | *** | ** | n.s. | |
| Temperature (T) | ** | *** | *** | * | |
| ES × T | n.s. | ** | n.s. | n.s. | |
| 10 | NoES-R | 13.6 ± 0.7 a | 3.8 ± 0.03 b | 3.7 ± 0.10 b | 3.73 ± 0.27 ab |
| ES-R | 12.0 ± 0.7 a | 3.7 ± 0.03 b | 4.0 ± 0.09 a | 2.99 ± 0.22 b | |
| NoES-NoR | - | - | - | - | |
| ES-NoR | 12.0 ± 1.0 a | 4.1 ± 0.02 a | 2.1 ± 0.06 c | 4.55 ± 0.47 a | |
| One-way ANOVA | n.s. | *** | *** | *** | |
| 14 | NoES-R | 12.4 ± 1.2 a | 4.0 ± 0.03 b | 4.1 ± 0.16 a | 3.48 ± 0.26 ab |
| ES-R | 13.8 ± 0.9 a | 3.9 ± 0.07 b | 4.0 ± 0.12 a | 3.00 ± 0.19 b | |
| NoES-NoR | - | - | - | - | |
| ES-NoR | 11.6 ± 0.7 a | 4.4 ± 0.05 a | 2.6 ± 0.10 b | 5.79 ± 0.51 a | |
| One-way ANOVA | n.s. | *** | *** | * | |
| 17 | NoES-R | 13.4 ± 1.4 a | 4.1 ± 0.04 a | 3.2 ± 0.11 b | 4.27 ± 0.49 a |
| ES-R | 16.4 ± 2.9 a | 4.0 ± 0.04 a | 3.9 ± 0.15 a | 4.24 ± 0.76 a | |
| NoES-NoR | - | - | - | - | |
| ES-NoR | - | - | - | - | |
| t-test | n.s. | n.s. | ** | n.s. | |
| 22 | NoES-R | 16.8 ± 0.9 a | 4.2 ± 0.05 a | 3.2 ± 0.10 b | 5.26 ± 0.33 a |
| ES-R | 16.7 ± 0.4 a | 4.0 ± 0.11 a | 4.0 ± 0.30 a | 4.27 ± 0.33 b | |
| NoES-NoR | - | - | - | - | |
| ES-NoR | - | - | - | - | |
| t-test | n.s. | n.s. | * | * | |
| 24 | NoES-R | 21.3 ± 1.1 a | 4.3 ± 0.06 a | 2.9 ± 0.26 b | 7.40 ± 6.4 a |
| ES-R | 23.3 ± 2.4 a | 4.1 ± 0.04 b | 3.7 ± 0.22 a | 6.49 ± 8.6 a | |
| NoES-NoR | - | - | - | - | |
| ES-NoR | - | - | - | - | |
| t-test | n.s. | * | * | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Salinas, R.; Acosta-Motos, J.R.; Núñez-Delicado, E.; Gabaldón, J.A.; López-Miranda, S. Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures. Agronomy 2022, 12, 616. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12030616
Alonso-Salinas R, Acosta-Motos JR, Núñez-Delicado E, Gabaldón JA, López-Miranda S. Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures. Agronomy. 2022; 12(3):616. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12030616
Chicago/Turabian StyleAlonso-Salinas, Ramiro, José Ramón Acosta-Motos, Estrella Núñez-Delicado, José Antonio Gabaldón, and Santiago López-Miranda. 2022. "Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures" Agronomy 12, no. 3: 616. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12030616