Diurnal Rhythm of Plasma Melatonin Concentration in the Domestic Turkey and Its Regulation by Light and Endogenous Oscillators
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Materials
2.3. Experiments
2.3.1. Experiment I
2.3.2. Experiment II
2.3.3. Experiment III
2.4. Melatonin Radioimmunoassay
2.5. Statistical Analysis
3. Results
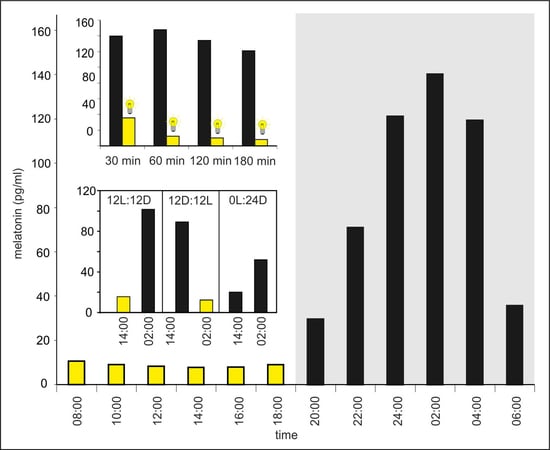
3.1. Experiment I
3.2. Experiment II
3.3. Experiment III
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bermudez, F.F.; Forbes, J.M.; Injidi, M.H. Involvement of melatonin and thyroid hormones in the control of sleep, food intake and energy metabolism in the domestic fowl. J. Physiol. 1983, 337, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siopes, T.D. Pineal gland and ocular influences on turkey breeder hens. 2. Body weight, feed intake, and egg characteristics. Poult. Sci. 1987, 66, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Chabot, C.C.; Menaker, M. Circadian feeding and locomotor rhythms in pigeons and house sparrows. J. Biol. Rhythms 1992, 7, 287–299. [Google Scholar] [CrossRef]
- Kumar, V.; Gwinner, E. Pinealectomy shortens resynchronisation times of house sparrow (Passer domesticus) circadian rhythms. Naturwissenschaften 2005, 92, 419–422. [Google Scholar] [CrossRef]
- Siopes, T.D. Initiation of egg production by turkey breeder hens: Sexual maturation and age at lighting. Poult. Sci. 2010, 89, 1490–1496. [Google Scholar] [CrossRef]
- Siopes, T.D.; Underwood, H.A. Pineal gland and ocular influences on turkey breeder hens. 1. Reproductive performance. Poult. Sci. 1987, 66, 521–527. [Google Scholar] [CrossRef]
- Sharp, P.J. Photoperiodic regulation of seasonal breeding in birds. Ann. N. Y. Acad. Sci. 2005, 1040, 189–199. [Google Scholar] [CrossRef]
- Kumar, V.; Wingfield, J.C.; Dawson, A.; Ramenofsky, M.; Rani, S.; Bartell, P. Biological clocks and regulation of seasonal reproduction and migration in birds. Physiol. Biochem. Zool. 2010, 83, 827–835. [Google Scholar] [CrossRef]
- Moore, C.B.; Siopes, T.D. Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gen. Comp. Endocrinol. 2000, 119, 95–104. [Google Scholar] [CrossRef]
- Moore, C.B.; Siopes, T.D. Effect of melatonin supplementation on the ontogeny of immunity in the Large White turkey poult. Poult. Sci. 2002, 81, 1898–1903. [Google Scholar] [CrossRef]
- Moore, C.B.; Siopes, T.D. Enhancement of cellular and humoral immunity following embryonic exposure to melatonin in turkeys (Meleagris gallopavo). Gen. Comp. Endocrinol. 2005, 143, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Markowska, M.; Majewski, P.M.; Skwarło-Sońta, K. Avian biological clock-immune system relationship. Dev. Comp. 2017, 66, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Harpole, C.E.; Paulose, J.; Cassone, V.M. The role of the pineal gland in the photoperiodic control of bird song frequency and repertoire in the house sparrow, Passer domesticus. Horm. Behav. 2014, 65, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Underwood, H. The circadian rhythm of thermoregulation in Japanese quail: I. Role of the eyes and pineal. J. Comp. Physiol. A 1994, 175, 639–653. [Google Scholar] [CrossRef]
- Underwood, H.; Edmonds, K. The circadian rhythm of thermoregulation in Japanese quail: II. Multioscillator control. J. Biol. Rhythms 1995, 10, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Rozenboim, I.; Miara, L.; Wolfenson, D. The thermoregulatory mechanism of melatonin-induced hypothermia in chicken. Am. J. Physiol. 1998, 274, R232–R236. [Google Scholar] [CrossRef]
- Apeldoorn, E.J.; Schrama, J.W.; Mashaly, M.M.; Parmentier, H.K. Effect of melatonin and lighting schedule on energy metabolism in broiler chickens. Poult. Sci. 1999, 78, 223–229. [Google Scholar] [CrossRef]
- Albarrán, M.T.; Lopez-Burillo, S.; Pablos, M.I.; Reiter, R.J.; Agapito, M.T. Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light. J. Pineal Res. 2001, 30, 227–233. [Google Scholar] [CrossRef]
- Clark, W.D.; Classen, H.L. The effects of continuously or diurnally fed melatonin on broiler performance and health. Poult. Sci. 1995, 74, 1900–1904. [Google Scholar] [CrossRef]
- Nelson, E.; Panksepp, J.; Ikemoto, S. The effects of melatonin on isolation distress in chickens. Pharmacol. Biochem. Behav. 1994, 49, 327–333. [Google Scholar] [CrossRef]
- Saleh, Y.S.; Attia, K.A.; Sawiress, F.A.R. Effect of Melatonin Supplement on Some Fertility Parameters of Turkey Toms. In Proceedings of the 5th International Symposium on Turkey Diseases, Berlin, Germany, 16–19 June 2004; pp. 40–50. [Google Scholar]
- Cassone, V.M.; Menaker, M. Sympathetic regulation of chicken pineal rhythms. Brain Res. 1983, 272, 311–317. [Google Scholar] [CrossRef]
- Underwood, H.; Binkley, S.; Siopes, T.; Mosher, K. Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 1984, 56, 70–81. [Google Scholar] [CrossRef]
- Liou, S.S.; Cogburn, L.A.; Biellier, H.V. Photoperiodic regulation of plasma melatonin levels in the laying chicken (Gallus domesticus). Gen. Comp. Endocrinol. 1987, 67, 221–226. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Rosiak, J.; Vivien-Roels, B.; Skene, D.J.; Pévet, P.; Nowak, J.Z. Daily variation in the concentration of 5-methoxytryptophol and melatonin in the duck pineal gland and plasma. J. Pineal Res. 2002, 32, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Berezińska, M.; Rosiak, J.; Vivien-Roels, B.; Skene, D.J.; Pévet, P.; Nowak, J.Z. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the goose pineal gland, retina, and plasma. Gen. Comp. Endocrinol. 2003, 134, 296–302. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Lorenc, A.; Berezińska, M.; Vivien-Roels, B.; Pévet, P.; Skene, D.J. Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina. Gen. Comp. Endocrinol. 2006, 145, 162–168. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Lorenc, A.; Berezińska, M.; Vivien-Roels, B.; Pévet, P.; Skene, D.J. Photoperiod-dependent changes in melatonin synthesis in the turkey pineal gland and retina. Poult. Sci. 2007, 86, 1397–1405. [Google Scholar] [CrossRef]
- Fraser, S.; Cowen, P.; Franklin, M.; Franey, C.; Arendt, J. Direct radioimmunoassay for melatonin in plasma. Clin. Chem. 1983, 29, 396–397. [Google Scholar] [CrossRef]
- Ravault, J.P.; Arendt, J.; Tobler, I.; Chesneau, D.; Maulin, O. Entrainment of melatonin rhythms in rams by symmetrical light-dark cycles of different period length. Chronobiol. Int. 1989, 6, 329–339. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [Green Version]
- Prusik, M. Mechanisms Regulating Melatonin Secretion in the Turkey Pineal Gland. Ph.D. Thesis, University of Warmia and Mazury, Olsztyn, Poland, 2005. [Google Scholar]
- Prusik, M.; Lewczuk, B. Roles of direct photoreception and the internal circadian oscillator in the regulation of melatonin secretion in the pineal organ of the domestic turkey: A novel in vitro clock and calendar model. Int. J. Mol. Sci. 2019, 20, 4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effect on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Bubenik, G.A.; Pang, S.F.; Cockshut, J.R.; Smith, P.S.; Grovum, L.W.; Friendship, R.M.; Hacker, R.R. Circadian variation of portal, arterial and venous blood levels of melatonin in pigs and its relationship to food intake and sleep. J. Pineal Res. 2000, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Sagar, S.M. Characterization of the day-night variation of retinal melatonin content in the chick. Investig. Ophthalmol. Vis. Sci. 1983, 24, 294–300. [Google Scholar]
- Zieliński, H.; Kozłowska, H.; Lewczuk, B. Melatonin in cereal grains as a potential cancer prevention agent. In Dietary Anticarcinogenes and Antimutagens. Chemical and Biological Aspects; Johnson, I.T., Fenwick, G.R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2000; pp. 266–273. [Google Scholar]
- Zieliński, H.; Kozłowska, H.; Lewczuk, B. Bioactive compunds in the cereal grains before and after hydrotermal processsing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009, 6, 383–410. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Lorenc-Duda, A.; Berezińska, M.; Bothorel, B.; Pévet, P.; Zawilska, J.B. Turkey retina and pineal gland differentially respond to constant environment. J. Comp. Physiol. A 2008, 194, 907–913. [Google Scholar] [CrossRef]
- Cassone, V.M.; Takahashi, J.S.; Blaha, C.D.; Lane, R.F.; Menaker, M. Dynamics of noradrenergic circadian input to the chicken pineal gland. Brain Res. 1986, 384, 334–341. [Google Scholar] [CrossRef]
- Csernus, V.; Ghosh, M.; Mess, B. Development and control of the circadian pacemaker for melatonin release in the chicken pineal gland. Gen. Comp. Endocrinol. 1998, 110, 19–28. [Google Scholar] [CrossRef]
- Murakami, N.; Nakamura, H.; Nishi, R.; Marumoto, N.; Nasu, T. Comparison of circadian oscillation of melatonin release in pineal cells of house sparrow, pigeons and Japanese quail, using cell perfusion system. Brain Res. 1994, 651, 209–214. [Google Scholar] [CrossRef]
- Steele, C.T.; Zivkovic, B.D.; Siopes, T.; Underwood, H. Ocular clocks are tightly coupled and act as pacemakers in the circadian system of Japanese quail. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R208–R218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Liu, Y.; Meng, T.; Xie, C.; Wu, X.; Yin, Y. Circadian calcium feeding regime in laying hens related to zinc concentration, gene expression of circadian clock, calcium transporters and oxidative status. J. Trace Elem. Med. Biol. 2018, 50, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, X.; Zhou, X.; Wan, D.; Wang, Z.; Wu, X.; Yin, Y. Effects of dynamic feeding low and high methionine diets on egg quality traits in laying hens. Poult. Sci. 2017, 96, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, D.; Zhou, X.; Ruan, Z.; Zhang, T.; Wu, X.; Yin, Y. Effects of dynamic feeding low- and high-methionine diets on the variation of glucose and lipid metabolism-related genes in the liver of laying hens. Poult. Sci. 2019, 98, 2231–2240. [Google Scholar] [CrossRef]
- Schwean-Lardner, K.; Vermette, C.; Leis, M.; Classen, H.L. Basing Turkey Lighting Programs on Broiler Research: A Good Idea? A Comparison of 18 Daylength Effects on Broiler and Turkey Welfare. Animals (Basel) 2016, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prusik, M.; Lewczuk, B. Diurnal Rhythm of Plasma Melatonin Concentration in the Domestic Turkey and Its Regulation by Light and Endogenous Oscillators. Animals 2020, 10, 678. https://0-doi-org.brum.beds.ac.uk/10.3390/ani10040678
Prusik M, Lewczuk B. Diurnal Rhythm of Plasma Melatonin Concentration in the Domestic Turkey and Its Regulation by Light and Endogenous Oscillators. Animals. 2020; 10(4):678. https://0-doi-org.brum.beds.ac.uk/10.3390/ani10040678
Chicago/Turabian StylePrusik, Magdalena, and Bogdan Lewczuk. 2020. "Diurnal Rhythm of Plasma Melatonin Concentration in the Domestic Turkey and Its Regulation by Light and Endogenous Oscillators" Animals 10, no. 4: 678. https://0-doi-org.brum.beds.ac.uk/10.3390/ani10040678