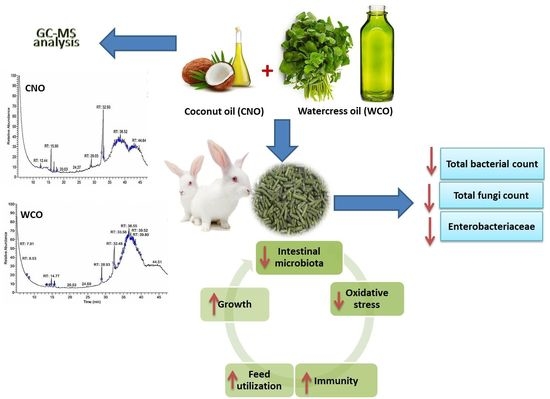
1. Introduction
The use of natural growth promoters in rabbit rations is a widely used strategy to enhance feed efficiency. Phytogenic supplements are products of plant origin that are included in animal feed to amend livestock performance [
1]. The addition of herbs or their extracts and active molecules to animal and poultry feed may have beneficial effects such as the improvement of feed intake, stimulation of digestive enzyme secretion, and activation of immune function as well as antiviral, antibacterial, antioxidant, and anthelminthic effects [
2]. Some of the medical effects of herbal plants are related to their secondary metabolites such as phenolic compounds, saponins, and essential/necessary oils [
3,
4].
Currently, cold pressed oils have become widely and commercially offered. These oils were known to contain higher concentrations of phenolic compounds. Therefore, cold pressed seed oils may be used in diverse diets to provide health benefits. Cold pressed seed oils with high content of natural antioxidants may play a key role in decreasing health disorders and risks of chronic ailments. Phenolic compounds are known as potent bioactive molecules with a remarkable antioxidant effect. This effect is chiefly attributed to the probable redox activity, which can efficiently help in deactivating free radicals, chelating certain metals such as copper and iron, as well as quenching ROS [
5,
6].
Coconut oil (CNO) has been included as a feed additive owing to its beneficial effects, such as antioxidant, anti-inflammatory, and antibacterial activities [
7,
8]. Caproic acid, capric acid, tocotrienols, and lauric acid are natural antioxidants that constitute the major components of CNO. These bioactive components efficiently scavenge reactive oxygen species (ROS), which play a key function in atherosclerosis, aging, diabetes mellitus, and cancer [
9]. Moreover, a substantial reduction in feed efficiency, total cholesterol, serum triglycerides, and low and very low-density lipoproteins as well as glucose levels has been found in diets supplemented with 1% CNO [
10].
Nasturtium officinale R.Br. (watercress) is a perennial herb and belongs to the mustard family, which is native to North America and Eurasia. It is a detoxifying plant that is rich in several vitamins such as vitamin A, C, E, B1, and B2 as well as minerals including iodine, phosphorus, and iron [
11]. Watercress has health benefits that include its effects as a strengthener of immunity, and positive effects have been reported in cancer research [
12]. Watercress contains 95% water and has low concentrations of protein, fiber, carbohydrates, and fat. It is particularly rich in menadione and contains large quantities of riboflavin, vitamin B6, vitamin A, vitamin C, calcium, and manganese. Additionally, 100 g of watercress provides 11 calories [
13]. Saturated oil in CNO makes up approximately 90%, with medium-chain fatty acids (MCFA) representing 60% of this oil with chain length ranging from 6 to 12 carbon atoms [
14], which are incorporated into the hepatic circulation without re-esterification in the digestive tract cells [
15]. MCFA are partially autonomous of the transport mechanism of the carnitine compound into the liver mitochondria and are entirely oxidized for energy release [
16]. On the contrary, generally, long-chain fatty acids present in feeds are assimilated into chylomicrons following absorption in the intestinal cells because these fatty acids are exposed to re-esterification and then proceed to the blood via the lymphatic system [
15]. Rego Costa et al. [
17] stated that most long-chain fatty acids are stored in the body fat (adipose tissue). Despite the palatability of watercress for livestock, there are limited studies on the practical usage of watercress or its derivatives such as watercress oil (WCO) as natural feed additives.
There is a vital need to use effective and economic alternatives or combinations of diverse alternatives to improve the performance, productivity, and health of livestock and poultry. Thus, in the present study, the effect of CNO and WCO as feed additives in growing rabbit diets on growth performance, carcass parameters, antioxidant indices, immunity, and intestinal microbiota was examined.
4. Discussion
In the present study, CNO showed higher antibacterial activity than that of WCO against the tested pathogenic bacteria. Several authors have found that cold pressed oil has antimicrobial activity against Gram-positive and Gram-negative bacteria in vitro, in situ, and in vivo [
29,
30,
31]. Nevin and Rajamohan [
7] demonstrated that CNO has antibacterial action against Gram-positive and Gram-negative bacteria. Additionally, CNO has been used as a feed additive owing to its beneficial applications and health benefits such as antioxidant and anti-inflammatory activities [
7,
8].
The enhancement in LBW, BWG, and FCR with oil mixture (0.5 g CNO +1.5 g WCO and 1 g CNO + 1 g WCO) supplements might be due to providing certain bioactive compounds that improve nutrient digestion and absorption. Similar results were observed by Messens et al. [
32], who stated that the antioxidant activities of coconut can strongly change the digestive system condition via antibacterial and antimicrobial effects, which could be mirrored in the enhancement of digestion. In agreeance with our results, El-Abasy et al. [
10] revealed lower feed conversion ratios in all coconut treated groups than that in the control group (G1). Langhout [
33] reported that these herbs or their derivatives could improve the digestive system of poultry, enhance liver function, and stimulate pancreatic enzymes in the gut. Improvement in nutrient metabolism due to herbal supplementation could improve growth performance [
34]. However, the effects of phytogenic additives or their bioactive components are not always detected in growth indices [
35]. In fact, numerous in vivo and in vitro studies have used the active components (e.g., tannins, saponins, and flavonoids) that were obtained from these plants, showing that coconut, watercress, or their extracts also have antifungal, anti-inflammatory, antimicrobial, and antioxidant properties [
3,
10]. Our findings partially disagree with Van Gerwe et al. [
36] who postulated that 1% MCFA supplementation increased daily weight gain. Furthermore, these results were verified upon investigation of MCFA in broilers [
37] that found that 4% palm oil supplementation as a source of MCFA in diets increased FI.
All carcass parameters were not statistically influenced by dietary treatments in the present study. Our findings are partially in agreeance with Wang et al. [
38] who found no significant effects of dietary CNO on poultry carcass traits including dressing and breast percentages. Skřivanová et al. [
39] found that carcass yield was not significantly affected by MCFA supplementation in rabbit diets. Similarly, Kovitvadhi et al. [
40] mentioned that herbal supplements had no statistical effects on carcass traits or dressing percentage. Furthermore, dressing percentage was not influenced by the addition of herb oils in rabbit diets [
41]. Likewise, Al-Sagheer et al. [
42] stated that there was no beneficial influence of dietary of phytogenic additives on carcass traits of rabbits exposed to a hot climate. Manesh [
43] demonstrated that the addition of watercress extract did not affect breast, liver, and carcass percentages of male broilers. In contrast, rabbits who received 1.5% CNO increased percentages of dressing, trunk, fore parts, hind parts, and total edible parts (
p ≤ 0.05) [
44].
In the present study, the addition of CNO and WCO to the basal diet resulted in a notable elevation of both non-enzymatic (GSH) and enzymatic (SOD) antioxidants. SOD plays a key role in preventing body cells from free radicals and oxidative damage; however, this action requires a supply of definite nutrients in the diet [
1]. The detrimental effects of ROS may be inhibited using antioxidant sources that eliminate detoxifying organisms and radicals [
45]. These bioactive ingredients may delay the oxidation of proteins or lipids and other nutrients by suppressing the propagation of oxidation reactions [
46]. However, there is growing interest in discovering and producing alternative natural antioxidants from medicinal herbs [
21,
47,
48]. Among these plants, coconut and watercress have shown to have higher saturated fatty acids and polyphenolic compounds, as positive correlations have been noted between the content of polyphenolic compounds in herbs and their antioxidant properties [
49]. Similar to our results, Aaby et al. [
50] and Zeb [
51] confirmed that the antioxidant action of WCO may be linked to phenolic molecules such as quercetin and coumaric acid and their derivatives, as well as thymoquinone, caftaric acid, sinapic acid, palmitic acid, oleic acid, and vanillic acid. Dietary supplementation of watercress has shown to reduce the DNA damage and improve the antioxidant activity in the blood [
52].
Phytogenic supplements may have multifaceted mechanisms of action, including modifying feed color and flavor, inducing gastric motility, and improving the secretion of digestive enzymes, endocrine function, immune function, and antioxidant status [
35,
53]. From our results, the addition of natural antioxidants such as herbal additives could be used in the future to enhance the productivity and health status of the rabbit.
Improvement in animal immunity is important to decrease or prevent many infectious ailments via induced immune enhancers and stimulants as the best solutions; herbal oils as feed additives are used in the diets for this purpose. Lysozyme activity is a chief innate immune defense index [
54]. Also, complement component 3 contributes to innate immunity and plays a key function in the complement system, which is the main component of the immune system that attacks the pathogenic microbes in cell membrane and improves the efficacy of phagocytic cells and antibodies to clear microbes and damaged cells from an organism [
55]. Mugnai et al. [
56] has confirmed that serum lysozyme has a synergic action with the serum complement. In the present study, dietary supplementation with WCO, CNO, or their mixtures significantly increased both lysozyme and complement component 3 activities. Our results are in agree with those of El-Abasy et al. [
10] who found that the supplementation of coconut (2%) played a role in enhancing immune parameters and health. In addition, increased lysozyme activity with supplementation of CNO may indicate improved immunity in rabbits. Herbal supplements are rich in flavonoids content and other bioactive components act as antioxidants and can thereby improve immunity [
57]. This may illustrate the influence of cold pressed oils on immune indicators studied in
Table 6. CNO contains lauric acid, myristic acid, palmitic acid, caprylic acid, capric acid, oleic acid, stearic acid, and caproic acid. More than 90% of the fatty acids in CNO are saturated and just less than 10% are unsaturated [
58]. Saturated fatty acids in CNO have been found to significantly enhance the innate immune responses [
59]. Mohiti-Asli and Ghanaatparast-Rashti [
60] observed that broilers fed 300 ppm of essential oil had higher immune parameters than those fed a control diet.
In the present study, dietary CNO, WCO, or their mixtures resulted in a delay in the spoilage emergence. In addition, 1 g CNO + 1 g WCO/kg in the diet inhibited the growth of TBC, lactobacilli, coliform,
Enterobacteriaceae, and
Clostridium spp. in the caecal content of rabbits. This inhibition could be attributed to the antimicrobial effect of CNO and WCO, which is mostly because of its high phenolic content [
7].
Bacteria colonization on mucosal tissues is a vital step in enteric infections. Bacteria must initially attach to the epithelial cells to settle on the mucosal surface. Binding of type 1 fimbriae is one of the main modes for binding to the epithelium surface [
61]. It has been reported that the compressed oil acts as the binding sites for Gram-negative bacteria, inhibiting their enterocytes attachment [
62]. This is the possible way by which CNO or WCO could reduce bacterial populations in the present study. An incremented digestibility by supplemental CNO or WCO is another way that these oils could reduce pathogenic bacterial colonies in the gastrointestinal tract. In addition, it has been documented that inferior digestibility values and inefficiently digested diets lead to an increase in the proliferation of putrefying bacteria in the hindgut, which increases toxic metabolites such as biogenic amines and ammonia [
61]. The antimicrobial activity of natural extracts is closely related to their phenolics content and polyphenols that are considered as potent active compounds with great antimicrobial and antioxidant actions [
63]. The phenolic compound may alter the pH gradient and bacterial cells membrane potential causing disturbance of the intracellular ATP content of
Escherichia coli and
Salmonella via the collapse of the Gram-negative bacteria outer membrane. Various in vivo studies reported that many cold pressed oils might prohibit the Gram-negative growth bacteria [
6,
29,
30,
31]. Conversely, the use of CNO and WCO in the diet led to a decrease in pathogenic bacteria inhabitants. The outcomes from literature revealed that there were no significant effects of cold pressed oils on favorable bacterial population such as lactobacilli in situ [
29,
30].