Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Small Mammal Trapping
2.3. Data Analysis
3. Results
3.1. Small Mammal Diversity and Abundance in Relation to Crop Type
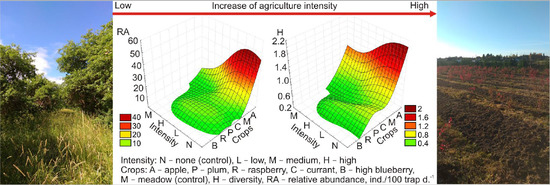
3.2. Small Mammal Diversity and Abundance in Relation to Intensity of Agricultural Practices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A





Appendix B
| Data Source 1 | Habitat 2 | S. ara 3c | S. min | N. fod | N. ano | A. agr | A. fla | A. ura | A. syl | M. min | M. mus | S. bet | M. arv | M. agr | M. oec | A. amp | M. gla | S | N | D | H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 10 | 1 | 0 | 0 | 7 | 159 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 404 | 6 | 585 | 0.55 | 0.78 |
| M | 38 | 4 | 0 | 0 | 17 | 3 | 0 | 0 | 0 | 1 | 0 | 252 | 70 | 14 | 0 | 2 | 9 | 401 | 0.44 | 1.20 | |
| C | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 1 | 0 | 81 | 6 | 0 | 0 | 0 | 4 | 94 | 0.75 | 0.53 | |
| 2 | M | 82 | 21 | 1 | 0 | 58 | 45 | 0 | 0 | 5 | 3 | 0 | 99 | 64 | 25 | 0 | 83 | 11 | 486 | 0.14 | 2.05 |
| YF | 78 | 32 | 4 | 0 | 36 | 44 | 0 | 0 | 1 | 0 | 0 | 82 | 73 | 1 | 0 | 193 | 10 | 544 | 0.20 | 1.81 | |
| F | 62 | 19 | 4 | 0 | 68 | 11 | 0 | 0 | 5 | 0 | 0 | 16 | 25 | 1 | 0 | 287 | 10 | 498 | 0.37 | 1.42 | |
| 3 | FLM | 207 | 51 | 1 | 1 | 432 | 4 | 0 | 0 | 137 | 1 | 0 | 27 | 1 | 408 | 1 | 19 | 13 | 1290 | 0.25 | 1.58 |
| NFM | 96 | 16 | 0 | 1 | 204 | 1 | 0 | 0 | 4 | 1 | 0 | 14 | 0 | 222 | 0 | 12 | 10 | 571 | 0.31 | 1.38 | |
| FF | 22 | 4 | 0 | 0 | 39 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 6 | 41 | 1 | 9 | 9 | 126 | 0.24 | 1.64 | |
| 4 | NM | 18 | 2 | 0 | 0 | 56 | 43 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 35 | 0 | 10 | 9 | 170 | 0.23 | 1.65 |
| SM | 23 | 2 | 1 | 0 | 8 | 39 | 7 | 0 | 2 | 1 | 0 | 1 | 2 | 17 | 0 | 19 | 12 | 122 | 0.19 | 1.91 | |
| FM | 31 | 20 | 1 | 0 | 3 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 22 | 0 | 46 | 8 | 150 | 0.19 | 1.78 | |
| YF | 3 | 0 | 1 | 0 | 2 | 10 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 23 | 8 | 45 | 0.32 | 1.48 | |
| RC | 28 | 20 | 1 | 0 | 10 | 22 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 18 | 8 | 121 | 0.16 | 1.90 | |
| FW | 30 | 26 | 6 | 0 | 2 | 23 | 0 | 0 | 0 | 1 | 0 | 0 | 19 | 3 | 0 | 86 | 9 | 196 | 0.26 | 1.64 | |
| MF | 8 | 1 | 6 | 0 | 1 | 96 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 178 | 9 | 295 | 0.47 | 0.97 | |
| AF | 2 | 0 | 0 | 0 | 7 | 106 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 36 | 7 | 157 | 0.51 | 0.96 | |
| 5 | CF | 93 | 3 | 1 | 0 | 7 | 98 | 0 | 2 | 2 | 1 | 1 | 4 | 4 | 2 | 0 | 840 | 13 | 1058 | 0.65 | 0.76 |
| W | 211 | 24 | 2 | 0 | 60 | 69 | 0 | 1 | 19 | 1 | 1 | 16 | 29 | 3 | 3 | 1084 | 14 | 1523 | 0.53 | 1.08 | |
| M | 105 | 3 | 0 | 0 | 37 | 28 | 0 | 0 | 22 | 4 | 0 | 158 | 45 | 1 | 0 | 120 | 10 | 523 | 0.20 | 1.79 | |
| C | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 7 | 0 | 2 | 2 | 0 | 0 | 0 | 5 | 15 | 0.30 | 1.40 | |
| DF | 11 | 0 | 0 | 0 | 1 | 66 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 1 | 0 | 467 | 8 | 551 | 0.73 | 0.55 |
References
- Official Statistics Portal. Available online: https://osp.stat.gov.lt/statistiniu-rodikliu-analize?indicator=S9R017#/ (accessed on 3 April 2019).
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntke, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2018, 55, 548–558. [Google Scholar] [CrossRef]
- Fischer, C.; Schröder, B. Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Hansen, S.C.; Stolter, C.; Jacob, J.J. Effect of plant secondary metabolites on feeding behavior of microtine and arvicoline rodent species. J. Pest Sci. 2016, 89, 955–963. [Google Scholar] [CrossRef]
- Heroldová, M.; Tkadlec, E. Harvesting behaviour of three central European rodents: Identifying the rodent pest in cereals. Crop Prot. 2011, 30, 82–84. [Google Scholar] [CrossRef]
- Luque-Larena, J.J.; Mougeot, F.; Roig, D.V.; Lambin, X.; Rodríguez-Pastor, R.; Rodríguez-Valín, E.; Anda, P.; Escudero, R. Tularemia outbreaks and common vole (Microtus arvalis) irruptive population dynamics in northwestern Spain, 1997–2014. Vector Borne Zoonotic Dis. 2015, 15, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Buckle, A. Use of Anticoagulant Rodenticides in Different Applications around the World. In Anticoagulant Rodenticides and Wildlife. Emerging Topics in Ecotoxicology (Principles, Approaches and Perspectives); Van den Brink, N., Elliott, J., Shore, R., Rattner, B., Eds.; Springer: Cham, Switzerland, 2018; pp. 11–44. [Google Scholar]
- Broughton, R.K.; Shore, R.F.; Heard, M.S.; Amy, S.R.; Meek, W.R.; Redhead, J.W.; Turk, A.; Pywell, R.F. Agri-environment scheme enhances small mammal diversity and abundance at the farm-scale. Agric. Ecosyst. Environ. 2014, 192, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Butet, A.; Paillat, G.; Delettre, Y. Seasonal changes in small mammal assemblages from field boundaries in an agricultural landscape of western France. Agric. Ecosyst. Environ. 2006, 113, 364–369. [Google Scholar] [CrossRef]
- Fischer, C.; Thies, C.; Tscharntke, T. Small mammals in agricultural landscapes: Opposing responses to farming practices and landscape complexity. Biol. Conserv. 2011, 144, 1130–1136. [Google Scholar] [CrossRef]
- Janova, E.; Heroldova, M. Response of small mammals to variable agricultural landscapes in central Europe. Mamm. Biol. 2016, 81, 488–493. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Hogue, E.J.; Lautenschlager, R.A.; Wagner, R.G. Population dynamics of small mammals in relation to vegetation management in orchard agroecosystems: Compensatory responses in abundance and biomass. Crop Prot. 1998, 17, 1–11. [Google Scholar] [CrossRef]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Heroldová, M.; Bryja, J.; Zejda, J.; Tkadlec, E. Structure and diversity of small mammal communities in agriculture landscape. Agric. Ecosyst. Environ. 2007, 120, 206–210. [Google Scholar] [CrossRef]
- Lund, M. Rodent problems in Europe. In Rodent Pest Management; Prakash, I., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2018; pp. 39–44. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S.; Granatstein, D.M. Influence of living mulches on vole populations and feeding damage to apple trees. Crop Prot. 2018, 108, 78–86. [Google Scholar] [CrossRef]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Benedek, A.M.; Sîrbu, I. Responses of small mammal communities to environment and agriculture in a rural mosaic landscape. Mamm. Biol. 2018, 90, 55–65. [Google Scholar] [CrossRef]
- Golet, G.H.; Hunt, J.W.; Koenig, D. Decline and recovery of small mammals after flooding: Implications for pest management and floodplain community dynamics. River Res. Appl. 2013, 29, 183–194. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Wang, Y.; Guo, C.; Li, B.; Huang, H. Recovery of a rodent community in an agro-ecosystem after flooding. J. Zool. 2007, 272, 138–147. [Google Scholar] [CrossRef]
- Riojas-López, M.E.; Mellink, E.; Luévano, J. A semiarid fruit agroecosystem as a conservation-friendly option for small mammals in an anthropized landscape in Mexico. Ecol. Appl. 2018, 28, 495–507. [Google Scholar] [CrossRef]
- Panzacchi, M.; Linnell, J.D.; Melis, C.; Odden, M.; Odden, J.; Gorini, L.; Andersen, R. Effect of land-use on small mammal abundance and diversity in a forest–farmland mosaic landscape in south-eastern Norway. For. Ecol. Manag. 2010, 259, 1536–1545. [Google Scholar] [CrossRef]
- Men, X.; Guo, X.; Dong, W.; Ding, N.; Qian, T. Influence of Human Disturbance to the Small Mammal Communities in the Forests. Open J. For. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995; 436p. [Google Scholar]
- Bonnet, T.; Crespin, L.; Pinot, A.; Bruneteau, L.; Bretagnolle, V.; Gauffre, B. How the common vole copes with modern farming: Insights from a capture–mark–recapture experiment. Agric. Ecosyst. Environ. 2013, 177, 21–27. [Google Scholar] [CrossRef]
- Quinn, N.; Baldwin, R.A. Managing Roof Rats and Deer Mice in Nut and Fruit Orchards. ANR Publ. 2014, 8513, 1–7. [Google Scholar] [CrossRef]
- Witmer, G.; Snow, N.; Humberg, L.; Salmon, T. Vole problems, management options, and research needs in the United States. In Proceedings of the 13th WDM Conference; Boulanger, J.R., Ed.; DigitalCommons@University of Nebraska—Lincoln: Lincoln, NE, USA, 2009; pp. 235–249. [Google Scholar]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Murano, C.; Kasahara, S.; Kudo, S.; Inada, A.; Sato, S.; Watanabe, K.; Azuma, N. Effectiveness of vole control by owls in apple orchards. J. Appl. Ecol. 2018. [Google Scholar] [CrossRef]
- Jacob, J. Response of small rodents to manipulations of vegetation height in agro-ecosystems. Integr. Zool. 2008, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sellers, L.; Long, R.; Baldwin, R.A.; Jay-Russell, M.; Li, X.; Atwill, E.R.; Engeman, R.M. Impact of Field Border Plantings on Rodents and Food Safety Concerns. Proc. Vertebr. Pest Conf. 2016, 27, 264–267. [Google Scholar] [CrossRef]
- Somoano, A.; Ventura, J.; Miñarro, M. Continuous breeding of fossorial water voles in northwestern Spain: Potential impact on apple orchards. Folia Zool. 2017, 66, 29–36. [Google Scholar] [CrossRef]
- Baldwin, R.A.; Meinerz, R.; Witmer, G.W.; Werner, S.J. The elusive search for an effective repellent against voles: An assessment of anthraquinone for citrus crops. J. Pest. Sci. 2018, 91, 1107–1113. [Google Scholar] [CrossRef]
- Elmeros, M.; Christensen, T.K.; Lassen, P. Concentrations of anticoagulant rodenticides in stoats Mustela erminea and weasels Mustela nivalis from Denmark. Sci. Total Environ. 2011, 409, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Geduhn, A.; Esther, A.; Schenke, D.; Mattes, H.; Jacob, J. Spatial and temporal exposure patterns in non-target small mammals during brodifacoum rat control. Sci. Total Environ. 2014, 496, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Sellers, L.A.; Long, R.F.; Jay-Russell, M.T.; Li, X.; Atwill, E.R.; Engeman, R.M.; Baldwin, R.A. Impact of field-edge habitat on mammalian wildlife abundance, distribution, and vectored foodborne pathogens in adjacent crops. Crop Prot. 2018, 108, 1–11. [Google Scholar] [CrossRef]
- Balčiauskas, L. Sausumos Ekosistemų Tyrimo Metodai. I dalis. Gyvūnų Apskaitos [Methods of Investigation of Terrestrial Ecosystems. Part I. Animal Surveys]; VU leidykla: Vilnius, Lithuania, 2004; 183p. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S.; Ransome, D.B.; Lindgren, P.M.F. Impact of removal trapping on abundance and diversity attributes in small mammal communities. Wildl. Soc. Bull. 2003, 31, 464–474. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S. Influence of removal sampling of small mammals on abundance and diversity attributes: Scientific implications. Hum.–Wildl. Interact. 2013, 7, 85–98. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Juškaitis, R. Diversity of small mammal communities in Lithuania (1. A review). Acta Zool. Litu. Biodivers. 1997, 7, 29–45. [Google Scholar] [CrossRef]
- Balčiauskas, L. Results of the long-term monitoring of small mammal communities in the Ignalina Nuclear Power Plant Region (Drūkšiai LTER site). Acta Zool. Litu. 2005, 15, 79–84. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small mammal community response to early meadow–forest succession. For. Ecosyst. 2017, 4, 11. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Balčiauskienė, L.; Jasiulionis, M. Resource partitioning confirmed by isotopic signatures allows small mammals to share seasonally flooded meadows. Ecol. Evol. 2019, 9, 5479–5489. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Janonytė, A. Reproduction of the root vole (Microtus oeconomus) at the edge of its distribution range. Turk. J. Zool. 2012, 36, 668–675. [Google Scholar]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Addison-Wesley Educational Publishers, Inc.: Menlo Park, CA, USA, 1999; 620p. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST–palaeontological statistics, ver. 1.89. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Balčiauskas, L.; Alejūnas, P. Small mammal species diversity and abundance in Žagarė Regional Park. Acta Zool. Litu. 2011, 21, 163–172. [Google Scholar] [CrossRef]
- Mažeikytė, R.; Balčiauskas, L.; Baranauskas, K.; Ulevičius, A. Smulkiųjų žinduolių rūšių įvairovė ir gausumas integruoto monitoringo teritorijose ir agrostacionaruose. [Species richness and abundance of small mammals in the territories of integrated monitoring and agro-stationars]. Aplinkos monitoringas 1993–1995; Lietuvos Respublikos Aplinkos Apsaugos Ministerija: Vilnius, Lithuania, 1996; pp. 73–79. Available online: https://www.researchgate.net/publication/329775865_Smulkiuju_zinduoliu_rusiu_ivairove_ir_gausumas_integruoto_monitoringo_teritorijose_ir_agrostacionaruose_1993-1995_m (accessed on 11 October 2018).
- Past 3.x—The Past of the Future. Available online: http://folk.uio.no/ohammer/past (accessed on 10 October 2018).
- EstimateS. Available online: http://viceroy.eeb.uconn.edu/estimates/EstimateSPages/EstimateS.php (accessed on 10 October 2018).
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. 2013. Available online: purl.ococ.org/estimates (accessed on 5 September 2018).
- Ott, R.L.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis, 6th ed.; Cengage Learning Brooks/Cole: Belmont, CA, USA, 2010; pp. 878–949. [Google Scholar]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Aschwanden, J.; Holzgang, O.; Jenni, L. Importance of ecological compensation areas for small mammals in intensively farmed areas. Wildl. Biol. 2007, 13, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Krijger, I.M.; Belmain, S.R.; Singleton, G.R.; Groot Koerkamp, P.W.; Meerburg, B.G. The need to implement the landscape of fear within rodent pest management strategies. Pest Manag. Sci. 2017, 73, 2397–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolino, S.; Asteggiano, L.; Saladini, M.A.; Giordani, L.; Vittone, G.; Alma, A. Environmental factors and agronomic practices associated with Savi’s pine vole abundance in Italian apple orchards. J. Pest. Sci. 2015, 88, 135–142. [Google Scholar] [CrossRef]
- Garratt, C.M.; Minderman, J.; Whittingham, M.J. Should we stay or should we go now? What happens to small mammals when grass is mown, and the implications for birds of prey. Ann. Zool. Fennici 2012, 49, 113–122. [Google Scholar] [CrossRef]
- Jacob, J.; Brown, J.S. Microhabitat use, giving-up densities and temporal activity as short- and long-term anti-predator behaviors in common voles. Oikos 2000, 91, 131–138. [Google Scholar] [CrossRef]
- Larsen, A.L.; Homyack, J.A.; Wigley, T.B.; Miller, D.A.; Kalcounis-Rueppell, M.C. Altered understory characteristics affect rodent spatial and foraging behaviors and reproduction patterns. For. Ecol. Manag. 2018, 409, 119–128. [Google Scholar] [CrossRef]
- Jacob, J.; Tkadlec, E. Rodent outbreaks in Europe: Dynamics and damage. In Rodent Outbreaks: Ecology and Impacts; Singleton, G., Belmain, S., Brown, P., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 207–223. [Google Scholar]
- Tulis, F.; Ambros, M.; Baláz, I.; Ziak, D.; Sládkovicová, V.H.; Miklós, P.; Dudich, A.; Stollmann, A.; Klimant, P.; Somogyi, B.; et al. Expansion of the Striped field mouse (Apodemus agrarius) in the south-western Slovakia during 2010–2015. Folia Oecol. 2016, 43, 64–73. [Google Scholar]



| Site No. | Crops | Age 1 | Appendix Figure | Intensity 2 | Agricultural Practices 3 | Control Habitat 4 | Appendix Figure | Mowing Practices 5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | S | PPA | RD | T | GR | |||||||
| 1 | Apple | O | A2a | M | + | − | + | + | MM | A4a | S | R |
| 2 | Apple | O | H | + | − | + | + | MM | LA | R/M | ||
| 3 | Currant | MD | A3a | L | + | − | − | − | MM | A4b | S | R |
| 4 | Currant | MD | L | + | − | − | − | MM | S | R | ||
| 5 | Plum | Y | A2d | M | + | − | + | − | NM | A4c | ||
| 6 | Apple | O | A1a | H | + | − | + | + | MM | A4d | LA | R |
| 7 | Apple | O | A3d | L | +/− | − | − | − | NM | |||
| 8 | Raspberry | MD | A3b | L | + | − | − | − | NM | A4e | ||
| 9 | Apple | O | A2b | M | + | − | + | − | MM | A4f | S | R |
| 10 | Raspberry | Y | A1c | H | + | + | + | − | MM | LA | R | |
| 11 | Raspberry | MD | H/M | + | + | + | − | FE | ||||
| Plum | MD | A3c | L | +/− | − | − | − | MM | S | NR | ||
| 12 | Apple | O | H | + | − | + | + | MM | LA | R | ||
| 13 | Highbush blueberry | MD | A1b | H | + | +/− | + | + | MM | LA | R | |
| 14 | Currant | MD | A2c | M | + | − | + | − | MM | S | R | |
| 15 | Apple | MD | A1d | H | + | + | + | + | MM | LA | R/M | |
| Season | Orchards and Plantations 1 | Control Habitats | Total | |||
|---|---|---|---|---|---|---|
| TE | N | TE | N | TE | N | |
| Summer | 2970 | 46 | 1440 | 46 | 4410 | 92 |
| Autumn | 2925 | 257 | 1545 | 163 | 4470 | 420 |
| Total | 5895 | 303 | 2985 | 209 | 8880 | 512 |
| Species | A 1 | AC | P | PC | C | CC | R | RC | HB | HBC |
|---|---|---|---|---|---|---|---|---|---|---|
| Sorex araneus | 3 | 6 | 0 | 0 | 1 | 0 | 0 | 3 | 2 | |
| Sorex minutus | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Apodemus agrarius | 47 | 61 | 5 | 6 | 15 | 1 | 15 | 9 | ||
| Apodemus flavicollis | 50 | 27 | 2 | 1 | 1 | 0 | 6 | 1 | ||
| Micromys minutus | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Mus musculus | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| Microtus agrestis | 8 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Microtus arvalis | 44 | 13 | 6 | 7 | 38 | 7 | 7 | 11 | ||
| Microtus oeconomus | 10 | 7 | 0 | 0 | 0 | 2 | 1 | 0 | ||
| Myodes glareolus | 38 | 21 | 0 | 0 | 1 | 0 | 0 | 8 | ||
| Total, N 2 | 203 | 149 | 13 | 14 | 56 | 11 | 31 | 33 | 2 | |
| S | 9 | 10 | 3 | 3 | 5 | 4 | 6 | 6 | 0 | 1 |
| TD | 3495 | 1665 | 300 | 225 | 1200 | 570 | 600 | 375 | 300 | 150 |
| D (95% CI) | 0.20 (0.18–0.23) | 0.24 (0.18–0.24) | 0.38 (0.35–0.62) | 0.44 (0.35–0.59) | 0.53 (0.41–0.64) | 0.45 (0.32–0.83) | 0.33 (0.20–0.38) | 0.25 (0.20–0.37) | 2 | |
| H (95% CI) | 1.74 (1.64–1.86) | 1.74 (1.62–1.86) | 1.01 (0.54–1.07) | 0.90 (0.60–1.08) | 0.83 (0.64–1.17) | 1.03 (0.30–1.24) | 1.34 (1.17–1.76) | 1.49 (1.19–1.76) | ||
| RAs ± SE | 2.63 ± 1.65 | 4.95 ± 1.94 | 2.00 | 0.67 ± 0.67 | 0.56 ± 0.40 | 0 | 1.11 ± 0.80 | 6.00 ± 2.00 | 0 | 0 |
| RAa ± SE | 11.03 ± 4.82 | 14.00 ± 4.07 | 6.67 | 17.33 | 6.67 ± 4.44 | 4.44 ± 2.47 | 12.44 ± 8.23 | 10.67 ± 5.05 | 0 | 2.67 |
| Species | Intensity of Agricultural Practices and Controls 1 | |||||
|---|---|---|---|---|---|---|
| L | LC | M | MC | H | HC | |
| S. araneus | 4 | 12 | 2 | 7 | ||
| S. minutus | 1 | 7 | 1 | 2 | ||
| A. agrarius | 46 | 6 | 19 | 30 | 46 | |
| A. flavicollis | 14 | 1 | 6 | 14 | 39 | 8 |
| M. minutus | 11 | 2 | 2 | |||
| M. musculus | 1 | 1 | 1 | |||
| M. agrestis | 8 | 8 | 1 | |||
| M. arvalis | 46 | 9 | 12 | 12 | 37 | 15 |
| M. oeconomus | 2 | 2 | 8 | 3 | 1 | 6 |
| M. glareolus | 36 | 1 | 8 | 2 | 12 | |
| Total, N 2 | 149 | 43 | 42 | 68 | 112 | 98 |
| S | 7 | 7 | 7 | 8 | 7 | 9 |
| TD | 1500 | 825 | 1650 | 870 | 2745 | 1290 |
| D (95% CI) | 0.26 (0.17–0.23) | 0.22 (0.16–0.28) | 0.20 (0.16–0.28) | 0.18 (0.16–0.26) | 0.30 (0.17–0.24) | 0.27 (0.17–0.24) |
| H (95% CI) | 1.48 (1.69–1.95) | 1.65 (1.48–1.99) | 1.72 (1.47–1.97) | 1.84 (1.58–1.98) | 1.31 (1.66–1.95) | 1.64 (1.64–1.96) |
| RAs ± SE | 4.07 ± 2.08 | 4.00 ± 2.53 | 1.00 ± 1.00 | 3.17 ± 2.74 | 0.44 ± 0.22 | 2.56 ± 1.07 |
| RAa ± SE | 15.60 ± 5.46 | 9.00 ± 7.23 | 2.58 ± 2.14 | 11.67 ± 4.01 | 8.51 ± 2.71 | 11.52 ± 2.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals 2019, 9, 334. https://0-doi-org.brum.beds.ac.uk/10.3390/ani9060334
Balčiauskas L, Balčiauskienė L, Stirkė V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals. 2019; 9(6):334. https://0-doi-org.brum.beds.ac.uk/10.3390/ani9060334
Chicago/Turabian StyleBalčiauskas, Linas, Laima Balčiauskienė, and Vitalijus Stirkė. 2019. "Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms" Animals 9, no. 6: 334. https://0-doi-org.brum.beds.ac.uk/10.3390/ani9060334