Hospital Wastewater—Source of Specific Micropollutants, Antibiotic-Resistant Microorganisms, Viruses, and Their Elimination
Abstract
:1. Introduction
2. Wastewater from Healthcare Facilities
2.1. Presence of Specific Micropollutants
2.2. Presence of Antibiotic-Resistant Microorganisms
2.3. Presence of Viruses in Wastewater from Healthcare Facilities
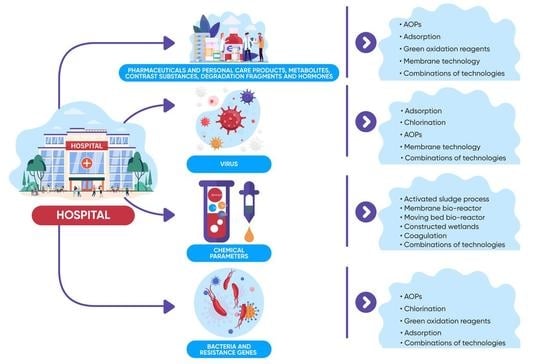
3. Innovative Processes Efficient in the Treatment of Wastewater from Healthcare Facilities
4. Conclusions and Suggestions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, G.; Kaya, Y.; Vergili, I.; Beril Gönder, Z.; Özhan, G.; Ozbek Celik, B.; Altinkum, S.M.; Bagdatli, Y.; Boergers, A.; Tuerk, J. Characterization and toxicity of hospital wastewaters in Turkey. Environ. Monit. Assess. 2017, 189, 55:1–55:19. [Google Scholar] [CrossRef] [PubMed]
- Castillo Meza, L.; Piotrowski, P.; Farnan, J.; Tasker, T.L.; Xiong, B.; Weggler, B.; Murrell, K.; Dorman, F.L.; Vanden Heuvel, J.P.; Burgos, W.D. Detection and removal of biologically active organic micropollutants from hospital wastewater. Sci. Total Environ. 2020, 700, 134469:1–134469:8. [Google Scholar] [CrossRef]
- van Buul, L.W.; van der Steen, J.T.; Doncker, S.M.M.M.; Achterberg, W.P.; Schellevis, F.G.; Veenhuizen, R.B.; Hertogh, C.M.P.M. Factors influencing antibiotic prescribing in long-term care facilities: A qualitative in-depth study. BMC Geriatr. 2014, 14, 136:1–136:11. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef]
- Sengar, A.; Vijayanandan, A. Comprehensive review on iodinated X-ray contrast media: Complete fate, occurrence, and formation of disinfection byproducts. Sci. Total Environ. 2021, 769, 144846:1–144846:23. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021, 41, 101990:1–101990:17. [Google Scholar] [CrossRef]
- Mackuľak, T.; Bodík, I.; Bírošová, L. Drogy a liečivá ako mikropolutanty, 1st ed.; FCHPT STU v Bratislave: Bratislava, Slovakia, 2016; ISBN 978-80-89597-34-5. [Google Scholar]
- Aydin, S.; Aydin, M.E.; Ulvi, A.; Kilic, H. Antibiotics in hospital effluents: Occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ. Sci. Pollut. Res. 2019, 26, 544–558. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Magu, M.M.; Muendo, B.M. Occurrence of antibiotics residues in hospital wastewater, wastewater treatment plant, and in surface water in Nairobi County, Kenya. Environ. Monit. Assess. 2019, 192, 18:1–18:16. [Google Scholar] [CrossRef]
- Heberer, T.; Feldmann, D. Contribution of effluents from hospitals and private households to the total loads of diclofenac and carbamazepine in municipal sewage effluents—Modeling versus measurements. J. Hazard. Mater. 2005, 122, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Saussereau, E.; Lacroix, C.; Guerbet, M.; Cellier, D.; Spiroux, J.; Goullé, J.P. Determination of levels of current drugs in hospital and urban wastewater. Bull. Environ. Contam. Toxicol. 2013, 91, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Jiang, X.; Xia, X.; Zhang, H.; Zheng, S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 2013, 90, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Antimicrobial Resistance and Consumption Remains High in the EU/EEA and the UK, according to New ECDC Data. Available online: https://www.ecdc.europa.eu/en/news-events/antimicrobial-resistance-and-consumption-remains-high-press-release (accessed on 13 July 2021).
- Zhou, C.; Wu, J.; Dong, L.; Liu, B.; Xing, D.; Yang, S.; Wu, X.; Wang, Q.; Fan, J.; Feng, L.; et al. Removal of antibiotic resistant bacteria and antibiotic resistance genes in wastewater effluent by UV-activated persulfate. J. Hazard. Mater. 2020, 388, 122070:1–122070:8. [Google Scholar] [CrossRef]
- Pärnänen, K.M.M.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019, 5, eaau9124:1–eaau9124:10. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.-N.; Chen, H.; Gao, R.-X.; Zhu, Y.-G.; Rensing, C. Organic compounds stimulate horizontal transfer of antibiotic resistance genes in mixed wastewater treatment systems. Chemosphere 2017, 184, 53–61. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Yang, K.; Wang, Z. Correlation between Exogenous Compounds and the Horizontal Transfer of Plasmid-Borne Antibiotic Resistance Genes. Microorganisms 2020, 8, 1211. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Parladé, E.; Villagrasa, M.; Barceló, D.; Rodríguez-Mozaz, S.; Martínez-Alonso, M.; Gaju, N.; Sarrà, M.; Caminal, G. Long-term continuous treatment of non-sterile real hospital wastewater by Trametes versicolor. J. Biol. Eng. 2019, 13, 47:1–47:13. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef]
- Vandael, E.; Latour, K.; Goossens, H.; Magerman, K.; Drapier, N.; Catry, B.; Versporten, A.; Andre, M.; Aouachria, S.; Aoun, M.; et al. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: Results of the Global-PPS and ECDC-PPS 2017. Antimicrob. Resist. Infect. Control 2020, 9, 13:1–13:13. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef]
- Bírošová, L.; Kislíková, K.; Lépesová, K. Antibiotic resistant coliforms: From human gut to wastewater. In Nutrients, Wastewater and Leachate: Testing, Risks and Hazards; Amimul, A., Ed.; Nova Publishers: New York, NY, USA, 2018; ISBN 978-1-53613-949-5. [Google Scholar]
- Lépesová, K.; Olejníková, P.; Mackuľak, T.; Cverenkárová, K.; Krahulcová, M.; Bírošová, L. Hospital Wastewater—Important Source of Multidrug Resistant Coliform Bacteria with ESBL-Production. Int. J. Environ. Res. Public Health 2020, 17, 7827. [Google Scholar] [CrossRef]
- Bírošová, L.; Lépesová, K.; Grabic, R.; Mackuľak, T. Non-antimicrobial pharmaceuticals can affect the development of antibiotic resistance in hospital wastewater. Environ. Sci. Pollut. Res. 2020, 27, 13501–13511. [Google Scholar] [CrossRef] [PubMed]
- Thai-Hoang, L.; Charmaine, N.; Hongjie, C.; Zhu, Y.X.; Hsien, K.T.; Sebastian, B.T.M.; Zhi, Z.; Yew-Hoong, G.K. Occurrences and Characterization of Antibiotic-Resistant Bacteria and Genetic Determinants of Hospital Wastewater in a Tropical Country. Antimicrob. Agents Chemother. 2021, 60, 7449–7456. [Google Scholar] [CrossRef] [Green Version]
- Lépesová, K. Výskyt, Štúdium a Možnosti Redukcie Vybraných Baktérií Rezistentných Voči Antibiotikám v Kaloch a Vodách z Čistiarní Odpadových Vôd. Ph.D. Thesis, Slovak University of Technology, Bratislava, Slovakia, 2018. [Google Scholar]
- Domínguez, J.R.; González, T.; Palo, P.; Cuerda-Correa, E.M. Fenton + Fenton-like Integrated Process for Carbamazepine Degradation: Optimizing the System. Ind. Eng. Chem. Res. 2012, 51, 2531–2538. [Google Scholar] [CrossRef]
- Katouli, M.; Thompson, J.M.; Gündoğdu, A.; Stratton, H.M. Antibiotic Resistant Bacteria in Hospital Wastewaters and Sewage Treatment Plants. In Proceedings of the Science Forum and Stakeholder Engagement: Building Linkages, Collaboration and Science Quality, Brisbane, Australia, 19–20 June 2012; Begbie, D.K., Kenway, S.J., Biermann, S.M., Wakem, S.L., Eds.; Urban Water Security Research Alliance: Brisbane, Australia, 2012. [Google Scholar]
- Lépesová, K.; Olejníková, P.; Mackuľak, T.; Tichý, J.; Birošová, L. Annual changes in the occurrence of antibiotic-resistant coliform bacteria and enterococci in municipal wastewater. Environ. Sci. Pollut. Res. 2019, 26, 18470–18483. [Google Scholar] [CrossRef]
- Maheshwari, M.; Yaser, N.H.; Naz, S.; Fatima, M.; Ahmad, I. Emergence of ciprofloxacin-resistant extended-spectrum β-lactamase-producing enteric bacteria in hospital wastewater and clinical sources. J. Glob. Antimicrob. Resist. 2016, 5, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Lépesová, K.; Mackuľak, T.; Birošová, L. Vplyv odpadovej vody na vznik a šírenie bakteriálnej rezistencie voči antibiotikám. Chem. List. 2017, 111, 374–380. [Google Scholar]
- Prado, T.; Silva, D.M.; Guilayn, W.C.; Rose, T.L.; Gaspar, A.M.C.; Miagostovich, M.P. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011, 45, 1287–1297. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater. In Advances in Applied Microbiology; Sariaslani, S., Geoffrey, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 97, pp. 63–119. ISBN 0065-2164. [Google Scholar]
- Mandal, P.; Gupta, A.K.; Dubey, B.K. A review on presence, survival, disinfection/removal methods of coronavirus in wastewater and progress of wastewater-based epidemiology. J. Environ. Chem. Eng. 2020, 8, 104317:1–104317:10. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Koritnik, T.; Mioč, V.; Trkov, M.; Bolješič, M.; Berginc, N.; Prosenc, K.; Kotar, T.; Paragi, M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2021, 755, 143226:1–143226:7. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef]
- Wang, X.-W.; Li, J.-S.; Guo, T.-K.; Zhen, B.; Kong, Q.-X.; Yi, B.; Li, Z.; Song, N.; Jin, M.; Xiao, W.-J.; et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods 2005, 128, 156–161. [Google Scholar] [CrossRef]
- Corpuz, M.V.A.; Buonerba, A.; Vigliotta, G.; Zarra, T.; Ballesteros, F.J.; Campiglia, P.; Belgiorno, V.; Korshin, G.; Naddeo, V. Viruses in wastewater: Occurrence, abundance and detection methods. Sci. Total Environ. 2020, 745, 140910. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Maresca, M.; Longobardi, C.; Mancon, A.; Romeri, F.; et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Carraturo, F.; Del Giudice, C.; Morelli, M.; Cerullo, V.; Libralato, G.; Galdiero, E.; Guida, M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020, 265, 115010:1–115010:6. [Google Scholar] [CrossRef]
- Kemp, S.A.; Collier, D.A.; Datir, R.P.; Ferreira, I.A.T.M.; Gayed, S.; Jahun, A.; Hosmillo, M.; Rees-Spear, C.; Mlcochova, P.; Lumb, I.U.; et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021, 592, 277–282. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Mak, T.-M.; Cui, L.; Toh, M.P.H.; Lim, Y.D.; Lee, P.H.; Lee, T.H.; Chia, P.Y.; et al. Clinical and Virological Features of SARS-CoV-2 Variants of Concern: A Retrospective Cohort Study Comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665:1–114665:10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ling, H.; Huang, X.; Li, J.; Li, W.; Yi, C.; Zhang, T.; Jiang, Y.; He, Y.; Deng, S.; et al. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020, 741, 140445:1–140445:5. [Google Scholar] [CrossRef] [PubMed]
- Achak, M.; Alaoui Bakri, S.; Chhiti, Y.; M’hamdi Alaoui, F.E.; Barka, N.; Boumya, W. SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: A review on detection, survival and disinfection technologies. Sci. Total Environ. 2021, 761, 143192:1–143192:15. [Google Scholar] [CrossRef]
- Stavbar, S.; Hrnčič, M.K.; Premzl, K.; Kolar, M.; Turk, S.Š. Sub- and super-critical water oxidation of wastewater containing amoxicillin and ciprofloxacin. J. Supercrit. Fluids 2017, 128, 73–78. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Jaén-Gil, A.; Castellet-Rovira, F.; Llorca, M.; Villagrasa, M.; Sarrà, M.; Rodríguez-Mozaz, S.; Barceló, D. Fungal treatment of metoprolol and its recalcitrant metabolite metoprolol acid in hospital wastewater: Biotransformation, sorption and ecotoxicological impact. Water Res. 2019, 152, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Czölderová, M.; Behúl, M.; Filip, J.; Zajíček, P.; Grabic, R.; Vojs-Staňová, A.; Gál, M.; Kerekeš, K.; Híveš, J.; Ryba, J.; et al. 3D printed polyvinyl alcohol ferrate(VI) capsules: Effective means for the removal of pharmaceuticals and illicit drugs from wastewater. Chem. Eng. J. 2018, 349, 269–275. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. What have we learned from worldwide experiences on the management and treatment of hospital effluent?–An overview and a discussion on perspectives. Sci. Total Environ. 2015, 514, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Mackuľak, T.; Grabic, R.; Špalková, V.; Belišová, N.; Škulcová, A.; Slavík, O.; Horký, P.; Gál, M.; Filip, J.; Híveš, J.; et al. Hospital wastewaters treatment: Fenton reaction vs. BDDE vs. ferrate(VI). Environ. Sci. Pollut. Res. 2019, 26, 31812–31821. [Google Scholar] [CrossRef]
- Bimová, P.; Roupcová, P.; Klouda, K.; Matějová, L.; Staňová, A.V.; Grabicová, K.; Grabic, R.; Majová, V.; Híveš, J.; Špalková, V.; et al. Biochar–An efficient sorption material for the removal of pharmaceutically active compounds, DNA and RNA fragments from wastewater. J. Environ. Chem. Eng. 2021, 9, 105746:1–105746:9. [Google Scholar] [CrossRef]
- Tasca, A.L.; Clematis, D.; Stefanelli, E.; Panizza, M.; Puccini, M. Ciprofloxacin removal: BDD anode coupled with solid polymer electrolyte and ultrasound irradiation. J. Water Process Eng. 2020, 33, 101074. [Google Scholar] [CrossRef]
- Tasca, A.L.; Clematis, D.; Panizza, M.; Vitolo, S.; Puccini, M. Chlorpyrifos removal: Nb/boron-doped diamond anode coupled with solid polymer electrolyte and ultrasound irradiation. J. Environ. Heal. Sci. Eng. 2020, 18, 1391–1399. [Google Scholar] [CrossRef]
- Butor Škulcová, A.; Tamášová, K.; Vojs Staňová, A.; Bírošová, L.; Krahulcová, M.; Gál, M.; Konečná, B.; Janíková, M.; Celec, P.; Grabicová, K.; et al. Effervescent ferrate(VI)-based tablets as an effective means for removal SARS-CoV-2 RNA, pharmaceuticals and resistant bacteria from wastewater. J. Water Process Eng. 2021, 43, 102223. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Suntronvipart, N. Evaluation of biodegradability and oxidation degree of hospital wastewater using photo-Fenton process as the pretreatment method. J. Hazard. Mater. 2006, 138, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.; Hastrup, C.; Klausen, M.M.; Pedersen, B.M.; Kristensen, G.H.; Jansen, J.L.C.; Bak, S.N.; Tuerk, J. Removal of APIs and bacteria from hospital wastewater by MBR plus O3, O3 + H2O2, PAC or ClO2. Water Sci. Technol. 2013, 67, 854–862. [Google Scholar] [CrossRef]
- Paulus, G.K.; Hornstra, L.M.; Alygizakis, N.; Slobodnik, J.; Thomaidis, N.; Medema, G. The impact of on-site hospital wastewater treatment on the downstream communal wastewater system in terms of antibiotics and antibiotic resistance genes. Int. J. Hyg. Environ. Health 2019, 222, 635–644. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Noroozi, A. Removal of pharmaceutical compounds from hospital wastewaters using nanomaterials: A review. Anal. Bioanal. Chem. Res. 2016, 3, 1–18. [Google Scholar] [CrossRef]
- Luo, Y.; Feng, L.; Liu, Y.; Zhang, L. Disinfection by-products formation and acute toxicity variation of hospital wastewater under different disinfection processes. Sep. Purif. Technol. 2020, 238, 116405:1–116405:10. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Koottatep, T.; Chapagain, S.K.; Panuvatvanich, A.; Polprasert, C.; Nguyen, T.M.H.; Chaiwong, C.; Nguyen, N.L. Removal and monitoring acetaminophen-contaminated hospital wastewater by vertical flow constructed wetland and peroxidase enzymes. J. Environ. Manag. 2019, 250, 109526:1–109526:9. [Google Scholar] [CrossRef]
- Vo, T.-K.-Q.; Bui, X.-T.; Chen, S.-S.; Nguyen, P.-D.; Cao, N.-D.-T.; Vo, T.-D.-H.; Nguyen, T.-T.; Nguyen, T.-B. Hospital wastewater treatment by sponge membrane bioreactor coupled with ozonation process. Chemosphere 2019, 230, 377–383. [Google Scholar] [CrossRef]
- Shokoohi, R.; Ghobadi, N.; Godini, K.; Hadi, M.; Atashzaban, Z. Antibiotic detection in a hospital wastewater and comparison of their removal rate by activated sludge and earthworm-based vermifilteration: Environmental risk assessment. Process Saf. Environ. Prot. 2020, 134, 169–177. [Google Scholar] [CrossRef]
- Tang, K.; Spiliotopoulou, A.; Chhetri, R.K.; Ooi, G.T.H.; Kaarsholm, K.M.S.; Sundmark, K.; Florian, B.; Kragelund, C.; Bester, K.; Andersen, H.R. Removal of pharmaceuticals, toxicity and natural fluorescence through the ozonation of biologically-treated hospital wastewater, with further polishing via a suspended biofilm. Chem. Eng. J. 2019, 359, 321–330. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of Micropollutants during Post-Treatment of Hospital Wastewater with Powdered Activated Carbon, Ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [Green Version]
- Echevarría, C.; Valderrama, C.; Cortina, J.L.; Martín, I.; Arnaldos, M.; Bernat, X.; De la Cal, A.; Boleda, M.R.; Vega, A.; Teuler, A.; et al. Techno-economic evaluation and comparison of PAC-MBR and ozonation-UV revamping for organic micro-pollutants removal from urban reclaimed wastewater. Sci. Total Environ. 2019, 671, 288–298. [Google Scholar] [CrossRef]
- Moussavi, G.; Fathi, E.; Moradi, M. Advanced disinfecting and post-treating the biologically treated hospital wastewater in the UVC/H2O2 and VUV/H2O2 processes: Performance comparison and detoxification efficiency. Process Saf. Environ. Prot. 2019, 126, 259–268. [Google Scholar] [CrossRef]
- Nardi, G.; Feretti, D.; Bracchi, U.; Tanzi, M.L.; Dore, F.; Francesconi, A.; Grottolo, M.; Bragonzi, G.; Perna, M.C.; Monarca, S. Acque reflue ospedaliere. Valutazione di un trattamento di disinfezione con biossido di cloro. Inquinamento 1995, 7, 77–83. [Google Scholar]
- Suarez, S.; Lema, J.; Omil, F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Kelbert, M.; Daronch, N.A.; Michels, C.; de Oliveira, D.; Soares, H.M. Potential of enzymatic process as an innovative technology to remove anticancer drugs in wastewater. Appl. Microbiol. Biotechnol. 2020, 104, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Le Minh Tri, N.; Kim, J.; Giang, B.L.; Al Tahtamouni, T.M.; Huong, P.T.; Lee, C.; Viet, N.M.; Quang Trung, D. Ag-doped graphitic carbon nitride photocatalyst with remarkably enhanced photocatalytic activity towards antibiotic in hospital wastewater under solar light. J. Ind. Eng. Chem. 2019, 80, 597–605. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Botero-Coy, A.M.; Moncayo-Lasso, A.; Hernández, F.; Torres-Palma, R.A. Effective elimination of fifteen relevant pharmaceuticals in hospital wastewater from Colombia by combination of a biological system with a sonochemical process. Sci. Total Environ. 2019, 670, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, S.; Dolatabadi, M. Removal of acetaminophen from hospital wastewater using electro-Fenton process. Environ. Earth Sci. 2018, 77, 53:1–53:11. [Google Scholar] [CrossRef]
- Arslan, A.; Veli, S.; Bingöl, D. Use of response surface methodology for pretreatment of hospital wastewater by O3/UV and O3/UV/H2O2 processes. Sep. Purif. Technol. 2014, 132, 561–567. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael, I.; García-Fernández, I.; Agüera, A.; Malato, S.; Fernández-Ibáñez, P.; Fatta-Kassinos, D. Reduction of clarithromycin and sulfamethoxazole-resistant Enterococcus by pilot-scale solar-driven Fenton oxidation. Sci. Total Environ. 2014, 468–469, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mackuľak, T.; Vojs, M.; Grabic, R.; Golovko, O.; Staňová, A.V.; Birošová, L.; Medveďová, A.; Híveš, J.; Gál, M.; Kromka, A.; et al. Occurrence of pharmaceuticals, illicit drugs, and resistant types of bacteria in hospital effluent and their effective degradation by boron-doped diamond electrodes. Mon. Chem. 2016, 147, 97–103. [Google Scholar] [CrossRef]
- Ouarda, Y.; Tiwari, B.; Azaïs, A.; Vaudreuil, M.-A.; Ndiaye, S.D.; Drogui, P.; Tyagi, R.D.; Sauvé, S.; Desrosiers, M.; Buelna, G.; et al. Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: Kinetic study and toxicity assessment. Chemosphere 2018, 193, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rajab, M.; Heim, C.; Letzel, T.; Drewes, J.E.; Helmreich, B. Electrochemical disinfection using boron-doped diamond electrode–The synergetic effects of in situ ozone and free chlorine generation. Chemosphere 2015, 121, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.G.; Kümmerer, K.; Henriques, D.M.; Martins, A.F. Ciprofloxacin in hospital effluent: Degradation by ozone and photoprocesses. J. Hazard. Mater. 2009, 169, 1154–1158. [Google Scholar] [CrossRef]
- Munoz, M.; Garcia-Muñoz, P.; Pliego, G.; Pedro, Z.M.D.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. Application of intensified Fenton oxidation to the treatment of hospital wastewater: Kinetics, ecotoxicity and disinfection. J. Environ. Chem. Eng. 2016, 4, 4107–4112. [Google Scholar] [CrossRef] [Green Version]
- Miralles-Cuevas, S.; Oller, I.; Pérez, J.A.S.; Malato, S. Removal of pharmaceuticals from MWTP effluent by nanofiltration and solar photo-Fenton using two different iron complexes at neutral pH. Water Res. 2014, 64, 23–31. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, J.-Q. Reaction kinetics and oxidation products formation in the degradation of ciprofloxacin and ibuprofen by ferrate(VI). Chemosphere 2015, 119, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Mackuľak, T.; Birošová, L.; Bodík, I.; Grabic, R.; Takáčová, A.; Smolinská, M.; Hanusová, A.; Híveš, J.; Gál, M. Zerovalent iron and iron(VI): Effective means for the removal of psychoactive pharmaceuticals and illicit drugs from wastewaters. Sci. Total Environ. 2016, 539, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Yu, T.-H.; Lin, C.-F. Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan. Chemosphere 2008, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Lee, J.-W.; Lee, E.-S.; Shin, S.-K.; Hwang, S.-R.; Oh, J.-E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef]
- Gómez, M.J.; Petrović, M.; Fernández-Alba, A.R.; Barceló, D. Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography–tandem mass spectrometry analysis in hospital effluent wastewaters. J. Chromatogr. A 2006, 1114, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.H.; Thomas, K.V. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ. Int. 2009, 35, 766–770. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital Wastewater Treatment by Membrane Bioreactor: Performance and Efficiency for Organic Micropollutant Elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrodin, Y.; Christine, B.; Sylvie, B.; Alain, D.; Jean-Luc, B.-K.; Cécile, C.-O.; Audrey, R.; Elodie, B. A priori assessment of ecotoxicological risks linked to building a hospital. Chemosphere 2013, 90, 1037–1046. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Wang, X.-H.; Lin, C.-F. Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters. Chemosphere 2010, 81, 562–570. [Google Scholar] [CrossRef]
| Parameters | Range of Values | |
|---|---|---|
| Water quality | pH | 6–9 |
| Redox potential (mV) | 850–950 | |
| Conductivity (μS·cm−1) | 300–1000 | |
| Chlorides (mg·L−1) | 80–400 | |
| Nitrogen (mg N2·L−1) | 60–98 | |
| NH4 (mg NH4·L−1) | 10–68 | |
| Nitrites (mg NO2·L−1) | 0.1–0.58 | |
| Nitrates (mg NO3·L−1) | 1–2 | |
| PO4 (mg P-PO4·L−1) | 6–19 | |
| Soluble compounds (mg·L−1) | 120–400 | |
| Oils (mg·L−1) | 50–210 | |
| COD (mg·L−1) | 1350–2480 | |
| TOC (mg·L−1) | 31–180 | |
| BOC5/CHSK | 0.3–0.4 | |
| AOX (mg·L−1) | 0.55–100 | |
| Microorganisms | E. coli | 103–106 |
| Enterococci | 103–106 | |
| Fecal coliforms | 103–104 | |
| Total coliforms | 105–107 | |
| EC50 (Daphnia), TU | 9.8–117 | |
| Organics | Total disinfective substances (mg·L−1) | 2–200 |
| Total antibiotics (mg·L−1) | 0.03–0.2 | |
| Cytostatics (mg·L−1) | 0.005–0.05 | |
| Lipides regulators (mg·L−1) | 0.001–0.01 | |
| Beta-blocators (mg·L−1) | 0.0004–0.025 |
| Substance | DFNsP | UNB Petržalka | Polyclinic Ružinov |
|---|---|---|---|
| (ng·L−1) | |||
| Caffeine | <LOQ | <LOQ | <LOQ |
| Cotinine | 1100 | 280 | 6700 |
| Codeine | 21 | <LOQ | 10 |
| Amphetamine | <LOQ | <LOQ | 190 |
| Oxycodone | <LOQ | <LOQ | <LOQ |
| Methamphetamine | 28 | 25 | 1100 |
| MDMA | <LOQ | <LOQ | <LOQ |
| Norketamine | <LOQ | <LOQ | <LOQ |
| Mephedrone | <LOQ | <LOQ | <LOQ |
| Ketamine | 18 | 29 | <LOQ |
| Benzoylecgonine | <LOQ | <LOQ | <LOQ |
| Tramadol | 260 | 510 | 2400 |
| Cocaine | <LOQ | <LOQ | <LOQ |
| LSD | <LOQ | <LOQ | <LOQ |
| Venlafaxine | 75 | <LOQ | 600 |
| Oxazepam | 38 | <LOQ | 52 |
| Citalopram | 173 | 47 | 250 |
| Midazolam | 680 | 18 | <LOQ |
| Buprenorphine | <LOQ | <LOQ | <LOQ |
| EDDP | <LOQ | <LOQ | <LOQ |
| Methadone | <LOQ | <LOQ | <LOQ |
| THC-COOH | 52 | <LOQ | <LOQ |
| Terbutaline | 15 | 240 | 20 |
| Atenolol | <LOQ | 160 | <LOQ |
| Bisoprolol | 42 | 320 | 5200 |
| Ampicillin | <LOQ | <LOQ | <LOQ |
| Penicillin V | <LOQ | <LOQ | <LOQ |
| Clonazepam | <LOQ | <LOQ | <LOQ |
| Atorvastatin | 12 | 40 | 294 |
| Flumequine | <LOQ | <LOQ | <LOQ |
| Metoprolol | 96 | 310 | 2600 |
| Ranitidine | 31 | 1400 | 32 |
| Furosemide | 450 | 340 | 560 |
| Substance (ng·L−1) | Effluent Psych. Hospital A | Secondary Effluent from WWTP | Effluent Psych. Hospital B | Secondary Effluent from WWTP |
|---|---|---|---|---|
| Clozapine | 5600 | 300 | 5000 | 1200 |
| Oxazepam | 940 | 750 | 290 | 190 |
| Sulpiride | 2800 | 430 | 9800 | 11,000 |
| Quetiapine | 2000 | <LOQ | 5000 | 1200 |
| Citalopram | 67 | 19 | 260 | 160 |
| Carbamazepine | 88 | <LOQ | 160 | 180 |
| Treatment Process | Aim |
|---|---|
| Ozonation | Disinfection/degradation |
| Chlorination | Disinfection |
| Photo-Fenton reaction | Disinfection/degradation |
| Fenton reaction and modifications | Disinfection/degradation |
| Coagulation—filtration—disinfection | Disinfection/degradation |
| Ozonation/UV radiation | Disinfection/degradation |
| Ozonation/UV radiation/H2O2 | Disinfection/degradation |
| Ozonation/UV radiation/H2O2/biological degree | Disinfection/degradation |
| Septic/anaerobic filter | Degradation |
| Septic/Fenton reaction | Disinfection/degradation |
| Flocculation/activated sludge | Degradation |
| Anaerobic and aerobic reactor with stabilized biofilm | Degradation |
| Aerobic reactor with stabilized biofilm/ozonation | Disinfection/degradation |
| Activated sludge | Degradation |
| Activated sludge/chlorination | Disinfection/degradation |
| Bioreactor—filamentous fungi | Degradation |
| Membrane bioreactor (MBR) | Degradation |
| MBR in combination with sorbents, AOPs, chlorination, catalysis | Disinfection/degradation |
| BDD—boron-doped diamond electrode | Disinfection/degradation |
| Ferrates (Fe6+) Anodic Oxidation with solid polymer electrolyte Ultrasound irradiation | Disinfection/degradation Disinfection/Degradation Disinfection/Degradation |
| Compound | Effluent Concentration (µg·L−1) | Study |
|---|---|---|
| Caffeine | 12.3–42 | [87] |
| 15.6 | [88] | |
| 12.1–182 | [89] | |
| <7.2 | [53] | |
| Carbamazepine | 0.03–0.07 | [90] |
| <0.017–1.7 | [87] | |
| LOD–0.24 | [14] | |
| 0.7–2.7 | [91] | |
| 0.64–1.2 | [92] | |
| 0.222 | [93] | |
| 0.018–6.08 | [89] | |
| 0.003–0.036 | [94] | |
| 0.163 | [88] | |
| Citalopram | 0.019–0.322 | [14] |
| 47–490 | [53] | |
| Cocaine | 0.05 | [95] |
| <19 | [53] | |
| Benzoylecognine (metabolite cocaine) | 0.029 | [95] |
| <7 | [53] | |
| Codeine | 0.378 | [95] |
| 0.01–5.7 | [90] | |
| 0.26–3.2 | [92] | |
| <2.3–58 | [53] | |
| 6-acetylcodeine | <0.002 | [95] |
| Diazepam | <0.001–0.038 | [92] |
| 0.069 | [93] | |
| Ketamine | 0.206 | [95] |
| <4.2–29 | [53] | |
| Lorazepam | 0.17–0.79 | [92] |
| LOD–0.353 | [14] | |
| Lidocaine | 9.133 | [93] |
| Methamphetamine | 0.26 | [95] |
| <4.2–1100 | [53] | |
| Morphine | 1.24 | [95] |
| 3.679 | [93] | |
| 6-acetylmorphine | <0.0005–0.039 | [95] |
| Oxazepam | 0.186–0.942 | [14] |
| 1.123 | [93] | |
| <24–52 | [53] | |
| Tramadol | 0.958 | [14] |
| 260–2400 | [53] | |
| Venlafaxine | 0.811 | [14] |
| <24–600 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackuľak, T.; Cverenkárová, K.; Vojs Staňová, A.; Fehér, M.; Tamáš, M.; Škulcová, A.B.; Gál, M.; Naumowicz, M.; Špalková, V.; Bírošová, L. Hospital Wastewater—Source of Specific Micropollutants, Antibiotic-Resistant Microorganisms, Viruses, and Their Elimination. Antibiotics 2021, 10, 1070. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10091070
Mackuľak T, Cverenkárová K, Vojs Staňová A, Fehér M, Tamáš M, Škulcová AB, Gál M, Naumowicz M, Špalková V, Bírošová L. Hospital Wastewater—Source of Specific Micropollutants, Antibiotic-Resistant Microorganisms, Viruses, and Their Elimination. Antibiotics. 2021; 10(9):1070. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10091070
Chicago/Turabian StyleMackuľak, Tomáš, Klára Cverenkárová, Andrea Vojs Staňová, Miroslav Fehér, Michal Tamáš, Andrea Bútor Škulcová, Miroslav Gál, Monika Naumowicz, Viera Špalková, and Lucia Bírošová. 2021. "Hospital Wastewater—Source of Specific Micropollutants, Antibiotic-Resistant Microorganisms, Viruses, and Their Elimination" Antibiotics 10, no. 9: 1070. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10091070