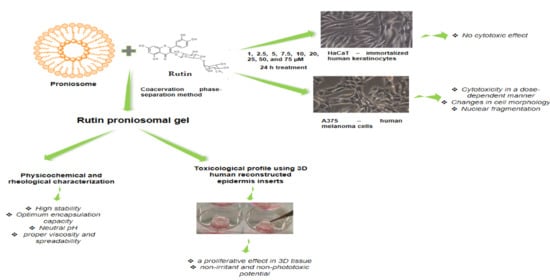
Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Lines
2.2. Preparation and Characterization of Proniosomal Gel Formulations
2.3. EpiDerm Testing
2.3.1. Reconstructed Human EpiDermal Model (EPI-200-SIT)—Skin Irritation Assay
2.3.2. EpiDermTM Skin Model (EPI 200)—Phototoxicity Test
2.4. Cytotoxicity on 2D Cell Culture
2.5. Nuclear Staining
2.6. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Medicated Proniosomal Gel
3.2. Toxicological Profile Evaluation of Rutin Proniosomal Gel in 3D Human EpiDerm Reconstructed Tissue
3.2.1. Proniosomal Rutin Gel Showed a Proliferative Effect in 3D Human EpiDerm Reconstructed Tissue
3.2.2. Proniosomal Rutin Gel Classifies as Non-Irritant to Skin
3.2.3. Proniosomal Rutin Gel Showed a Non-Phototoxic Potential in 3D Human EpiDerm Reconstructed Tissue
3.3. Cytotoxicity Assessment in 2D Cells
3.3.1. Rutin Showed a Non-Cytotoxic Effect in Human Immortalized Keratinocytes—HaCaT
3.3.2. Rutin Induced a Dose-Dependent Cytotoxic Effect in A375 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, Y.-W.; Liu, Q.; Liu, L.-P.; Luo, F.-L.; Zhou, H.-C.; Isoda, H.; Ohkohchi, N.; Li, Y.-M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of increased ROS and redox imbalance in skin carcinogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 8127362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejera-Vaquerizo, A.; Descalzo-Gallego, M.A.; Otero-Rivas, M.M.; Posada-García, C.; Rodríguez-Pazos, L.; Pastushenko, I.; Marcos-Gragera, R.; García-Doval, I. Skin cancer incidence and mortality in Spain: A systematic review and meta-analysis. Actas Dermo-Sifiliogr. 2016, 107, 318–328. [Google Scholar] [CrossRef]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 2 January 2021).
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global incidence and mortality of skin cancer by histological subtype and its relationship with the human development index (HDI); An ecology study in 2018. WCRJ 2019, 6, e1265. [Google Scholar]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; VanBeek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. Contribution of health care factors to the burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 1151–1160.e21. [Google Scholar] [CrossRef] [Green Version]
- Venza, M.; Visalli, M.; Beninati, C.; De Gaetano, G.V.; Teti, D.; Venza, I. Cellular mechanisms of oxidative stress and action in melanoma. Oxidative Med. Cell. Longev. 2015, 2015, 481782. [Google Scholar] [CrossRef] [Green Version]
- PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rutin (accessed on 10 November 2020).
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019, 109, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin ageing: Pathophysiology and current market treatment approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Vertuani, S.; Bino, A.; De Lucia, D.; Lampronti, I.; Milani, R.; Gambari, R.; Manfredini, S. Design, synthesis and biological activity of a novel Rutin analogue with improved lipid soluble properties. Bioorg. Med. Chem. 2015, 23, 264–271. [Google Scholar] [CrossRef]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Peres, D.A.; de Oliveira, C.A.; da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.; Baby, A.R. Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Skin Res. Technol. 2016, 22, 325–333. [Google Scholar] [CrossRef]
- Pivec, T.; Kargl, R.; Maver, U.; Bračič, M.; Elschner, T.; Žagar, E.; Gradišnik, L.; Kleinschek, K.S. Chemical structure-antioxidant activity relationship of water-based enzymatic polymerized rutin and its wound healing potential. Polymers 2019, 11, 1566. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Bin Kwon, S.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural substances for prevention of skin photoaging: Screening systems in the development of sunscreen and rejuvenation cosmetics. Rejuvenation Res. 2018, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Vaidya, V.; Sakpal, G. Formulation and development of rutin and gallic acid loaded herbal gel for the treatment of psoriasis and skin disease. J. Sci. Technol. 2020, 5, 192–203. [Google Scholar] [CrossRef]
- Choi, J.K.; Kim, S.H. Rutin suppresses atopic dermatitis and allergic contact dermatitis. Exp. Biol. Med. 2013, 238, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Basha, M.; Abd El-Alim, S.H. Development of a novel vesicular system using a binary mixture of sorbitan monostearate and polyethylene glycol fatty acid esters for rectal delivery of rutin. J. Liposome Res. 2013, 23, 28–36. [Google Scholar] [CrossRef]
- Pyo, S.M.; Meinke, M.; Keck, C.M.; Muller, R.H. Rutin—Increased antioxidant activity and skin penetration by nanocrystal technology (smartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Kalita, B.; Das, M.K. Rutin-phospholipid complex in polymer matrix for long-term delivery of rutin via skin for the treatment of inflammatory diseases. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 41–56. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Formulation and stability characterization of rutin-loaded oil-in-water emulsions. Food Bioprocess Technol. 2017, 10, 926–939. [Google Scholar] [CrossRef]
- Cândido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and antioxidant efficacy profiles of rutin-loaded ethosomes for topical application. AAPS PharmSciTech 2018, 19, 1773–1780. [Google Scholar] [CrossRef]
- Khatoon, M.; Shah, K.U.; Din, F.U.; Shah, S.U.; Rehman, A.U.; Dilawar, N.; Khan, A.N. Proniosomes derived niosomes: Recent advancements in drug delivery and targeting. Drug Deliv. 2017, 24, 56–69. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- European Pharmacopeia. Directorate for the Quality of Medicines & Healthcare of the Council of Europe, 10th ed.; European Pharmacopeia: Strasburg, France, 2020; Volume 1, p. 337. [Google Scholar]
- Popa, E.A.; Popovici, I.; Braha, S.L. Forme farmaceutice bioadezive, cap. XXIX. In Tehnologie Farmaceutică; Popovici, I., Lupuleasa, D., Eds.; Polirom: Iaşi, Romania, 2017; Volume 2, p. 782. [Google Scholar]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef] [PubMed]
- MatTek Corporation Protocol—MTT Effective Time 50 (ET-50). Available online: https://www.mattek.com/wp-content/uploads/EPI-200-MTT-ET-50-Protocol-MK-24-007-0001.pdf (accessed on 24 November 2020).
- MatTek Corporation Protocol—In Vitro EpiDerm Skin Irritation Test (EPI-200-SIT). Available online: https://www.mattek.com/wp-content/uploads/EPI-200-SIT-Skin-Irritation_MK-24-007-0023_10_02_19.pdf (accessed on 24 November 2020).
- Kandarova, H.; Willoughby, J.A.; De Jong, W.H.; Letasiova, S.; Milasova, T.; Bachelor, M.A.; Breyfogle, B.; Handa, Y.; De la Fonteyne, L.; Coleman, K.P. Pre-validation of an in vitro skin irritation test for medical devices using the reconstructed human tissue model EpiDerm™. Toxicol. In Vitro 2018, 50, 407–417. [Google Scholar] [CrossRef]
- MatTek Corporation Protocol—Phototoxicity Protocol for Use with EpiDermTM Model (EPI-200). Available online: https://www.mattek.com/wp-content/uploads/EPI-200-Phototoxicity-Protocol-ZEBET.pdf (accessed on 24 November 2020).
- Líšková, A.; Letašiová, S.; Jantová, S.; Brezová, V.; Kandarova, H. Evaluation of phototoxic and cytotoxic potential of TiO2 nanosheets in a 3D reconstructed human skin model. ALTEX 2020. [Google Scholar] [CrossRef] [Green Version]
- Kandarova, H.; Liebsch, M. The EpiDermTM Phototoxicity Test (EpiDermTM H3D-PT). In Alternatives for Dermal Toxicity Testing; Eskes, C., van Vliet, E., Maibach, H., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Anderson, A.; Bowman, A.; Boulton, S.J.; Manning, P.; Birch-Machin, M.A. A role for human mitochondrial complex II in the production of reactive oxygen species in human skin. Redox Biol. 2014, 2, 1016–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Cytoprotective effect of ascorbic acid and rutin against oxidative changes in the proteome of skin fibroblasts cultured in a three-dimensional system. Nutrients 2020, 12, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, C.A.; Peres, D.D.A.; Graziola, F.; Chacra, N.A.B.; de Araújo, G.L.B.; Flórido, A.C. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017, 12, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Viswanad, V. Formulation and optimization of clotrimazole-loaded proniosomal gel using 3(2) factorial design. Sci. Pharm. 2012, 80, 731–748. [Google Scholar] [CrossRef] [Green Version]
- Soliman, S.M.; Abdelmalak, N.S.; El-Gazayerly, O.N.; Abdelaziz, N. Novel non-ionic surfactant proniosomes for transdermal delivery of lacidipine: Optimization using 23 factorial design and in vivo evaluation in rabbits. Drug Deliv. 2016, 23, 1608–1622. [Google Scholar] [CrossRef] [Green Version]
- Ramkanth, S.; Madhusudhana Chetty, C.; Sudhakar, Y.; Thiruvengadarajan, V.S.; Anitha, P.; Gopinath, C. Development, characterization & in vivo evaluation of proniosomal based transdermal delivery system of Atenolol. Future J. Pharm. Sci. 2018, 4, 80–87. [Google Scholar]
- Sammour, R.M.F.; Taher, M.; Chatterjee, B.; Shahiwala, A.; Mahmood, S. Optimization of aceclofenac proniosomes by using different carriers, Part 1: Development and characterization. Pharmaceutics 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Carraway, J.W.; Liu, C.; Fan, C.; Liu, J.; Turley, A.P.; Rollins, T.S.; Coleman, K.P. The suitability of reconstructed human epidermis models for medical device irritation assessment: A comparison of in vitro and in vivo testing results. Toxicol. In Vitro 2020, 69, 104995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, E.; Vatanara, A.; Najafabadi, A.R.; Rouini, M.R.; Gilani, K.; Darabi, M. Preparation, characterization and optimization of sildenafil citrate loaded PLGA nanoparticles by statistical factorial design. DARU J. Pharm. Sci. 2013, 21, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive betulinic acid-loaded magnetoliposomes: A promising antitumor potential for highly aggressive human breast adenocarcinoma cells under hyperthermic conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Pavel, I.; Brittle, S.; Marti, D.; Calina, D.; et al. Stable PEG-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Andhale, V.A.; Patil, P.R.; Dhas, A.U.; Chauhan, P.D.; Desai, S.V. Liposome: An emerging tool in drug carrier system. J. Pharm. Technol. 2016, 8, 10982–11011. [Google Scholar]
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B.W. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004, 276, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, N.S.; Camponogara, C.; Gehrcke, M.; Giuliani, L.M.; da Silva, D.T.; Maurer, L.H.; Dias, P.; Emanuelli, T.; Cruz, L.; Oliveira, S.M. Oleic acid-containing semisolid dosage forms exhibit in vivo anti-inflammatory effect via glucocorticoid receptor in a UVB radiation-induced skin inflammation model. Inflammopharmacology 2020, 28, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Bachelor, M.; Ayehunie, S.; Letasiova, S.; Kaluzhny, Y.; Klausner, M.; Kandarova, H. Application of MatTek in vitro reconstructed human skin models for safety, efficacy screening, and basic preclinical research. Appl. In Vitro Toxicol. 2015, 1, 226–233. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Section 4 Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Publisher: Paris, France, 2020.
- European Medicine Agency. Available online: www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/12/WC500136404.pdf (accessed on 2 December 2020).
- Ceridono, M.; Tellner, P.; Bauer, D.; Barroso, J.; Alépée, N.; Corvi, R.; De Smedt, A.; Fellows, M.D.; Gibbs, N.K.; Heisler, E.; et al. The 3T3 neutral red uptake phototoxicity test: Practical experience and implications for phototoxicity testing—The report of an ECVAMEFPIA workshop. Regul. Toxicol. Pharmacol. 2012, 63, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.X.; Yin, S.J.; Oh, S.; Wang, Z.J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An integrated study of tyrosinase inhibition by rutin: Progress using a computational simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Natural exogenous antioxidant defense against changes in human skin fibroblast proteome disturbed by UVA radiation. Oxidative Med. Cell. Longev. 2020, 2020, 3216415. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Chamani, E. Molecular interaction and cellular studies on combination photodynamic therapy with rutoside for melanoma A375 cancer cells: An in vitro study. Cancer Cell Int. 2020, 20, 525. [Google Scholar] [CrossRef]













| Components | Composition |
|---|---|
| Span 60 (mg) | 180 |
| Cholesterol (mg) | 30 |
| Soy lecithin (mg) | 90 |
| Absolute ethanol (mL) | 0.3 |
| Distilled water (mL) | 0.1 |
| Bioactive compound (rutin) (%) | 0.3 |
| Gel Code | Viscosity (Pas) | Thixotropy (Pa/s) | Penetration Value (mm) | pH Value | Size (nm) | ζ Potential (mV) | EE (%) |
|---|---|---|---|---|---|---|---|
| PNS GEL | 0.502 ± 0.24 | 2576 | 233.00 ± 1.15 | 7.105 ± 0.09 | 116.2 ± 1.13 | 25.53 ± 0.2 | - |
| RUT_PNS GEL | 0.488 ± 0.62 | 2930 | 249.00 ± 0.82 | 7.002 ± 0.18 | 140.5 ± 2.56 | 27.33 ± 0.09 | 59.6 ± 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells. Antioxidants 2021, 10, 85. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10010085
Pinzaru I, Tanase A, Enatescu V, Coricovac D, Bociort F, Marcovici I, Watz C, Vlaia L, Soica C, Dehelean C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells. Antioxidants. 2021; 10(1):85. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10010085
Chicago/Turabian StylePinzaru, Iulia, Alina Tanase, Virgil Enatescu, Dorina Coricovac, Flavia Bociort, Iasmina Marcovici, Claudia Watz, Lavinia Vlaia, Codruta Soica, and Cristina Dehelean. 2021. "Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells" Antioxidants 10, no. 1: 85. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10010085