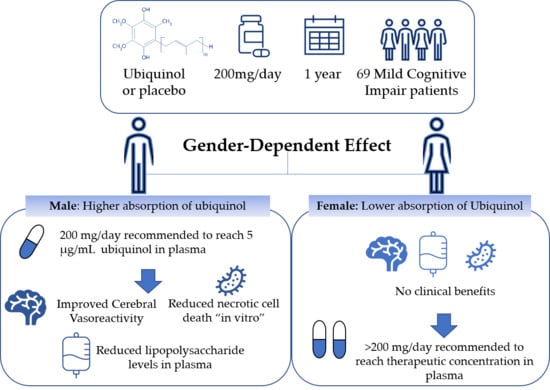
Ubiquinol Supplementation Improves Gender-Dependent Cerebral Vasoreactivity and Ameliorates Chronic Inflammation and Endothelial Dysfunction in Patients with Mild Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Evaluation
2.3. Transcranial Doppler Studies
2.4. CoQ10 Quantification
2.5. Lipopolysaccharide (Endotoxin-LPS) Concentration in Plasma
2.6. Cell Cultures
2.7. Statistical Analysis
3. Results
3.1. Participants’ Cognitive Evaluation
3.2. Clinical Effects of Ubiquinol
3.3. Oral Supplementation with Ub Increased CoQ Concentration in Plasma
3.4. Gender-Dependent Effects in CVR
3.5. Gender-Dependent Effects in Inflammation
3.6. Gender-Dependent Effects on Necrotic Death Within in Vitro Brain Microvascular Endothelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayden, K.M.; Zandi, P.P.; Lyketsos, C.G.; Khachaturian, A.S.; Bastian, L.A.; Charoonruk, G.; Tschanz, J.T.; Norton, M.C.; Pieper, C.F.; Munger, R.G.; et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: The Cache County study. Alzheimer Dis. Assoc. Disord. 2006, 20, 93–100. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Mora-Simón, S.; García-García, R.; Perea-Bartolomé, M.V.; Ladera-Fernández, V.; Unzueta-Arce, J.; Patino-Alonso, M.C.; Rodríguez-Sánchez, E. Deterioro cognitivo leve: Detección temprana y nuevas perspectivas. Rev. Neurol. 2012, 54, 0303–0310. [Google Scholar] [CrossRef] [Green Version]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; Pasqualetti, P.; Baruffaldi, R.; Bartolini, M.; Handouk, Y.; Matteis, M.; Moffa, F.; Provinciali, L.; Vernieri, F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 2006, 37, 1010–1015. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.K.; Elias, M.F.; Robbins, M.A.; Budge, M.M. Blood pressure-related cognitive decline: Does age make a difference? Hypertension 2004, 44, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Kasagi, F.; Sasaki, H.; Masunari, N.; Mimori, Y.; Suzuki, G. Association between dementia and midlife risk factors: The radiation effects research foundation adult health study. J. Am. Geriatr. Soc. 2003, 51, 410–414. [Google Scholar] [CrossRef]
- Van Sloten, T.T.; Henry, R.M.; Dekker, J.M.; Nijpels, G.; Unger, T.; Schram, M.T.; Stehouwer, C.D. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: The Hoorn study. Hypertension 2014, 64, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Tsuneki, H.; Sekizaki, N.; Suzuki, T.; Kobayashi, S.; Wada, T.; Okamoto, T.; Kimura, I.; Sasaoka, T. Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2007, 566, 1–10. [Google Scholar] [CrossRef]
- Basuroy, S.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C422–C432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuneki, H.; Tokai, E.; Suzuki, T.; Seki, T.; Okubo, K.; Wada, T.; Okamoto, T.; Koya, S.; Kimura, I.; Sasaoka, T. Protective effects of coenzyme Q10 against angiotensin II-induced oxidative stress in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2013, 701, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Anrather, J.; Zhou, P.; Frys, K.; Pitstick, R.; Younkin, S.; Carlson, G.A.; Iadecola, C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J. Neurosci. 2005, 25, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Duran-Prado, M.; Frontinan, J.; Santiago-Mora, R.; Peinado, J.R.; Parrado-Fernandez, C.; Gomez-Almagro, M.V.; Moreno, M.; Lopez-Dominguez, J.A.; Villalba, J.M.; Alcain, F.J. Coenzyme Q10 protects human endothelial cells from beta-amyloid uptake and oxidative stress-induced injury. PLoS ONE 2014, 9, e109223. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Anrather, J.; Forster, C.; Kazama, K.; Carlson, G.A.; Iadecola, C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J. Cereb. Blood Flow Metab. 2004, 24, 334–342. [Google Scholar] [CrossRef]
- Dai, W.; Lopez, O.L.; Carmichael, O.T.; Becker, J.T.; Kuller, L.H.; Gach, H.M. Mild cognitive impairment and alzheimer disease: Patterns of altered cerebral blood flow at MR imaging. Radiology 2009, 250, 856–866. [Google Scholar] [CrossRef]
- Lacalle-Aurioles, M.; Mateos-Perez, J.M.; Guzman-De-Villoria, J.A.; Olazaran, J.; Cruz-Orduna, I.; Aleman-Gomez, Y.; Martino, M.E.; Desco, M. Cerebral blood flow is an earlier indicator of perfusion abnormalities than cerebral blood volume in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2014, 34, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Akkawi, M.N.; Borroni, B.; Agosti, C.; Magoni, M.; Broli, M.; Pezzini, A.; Padovani, A. Volume cerebral blood flow reduction in pre-clinical stage of Alzheimer disease: Evidence from an ultrasonographic study. J. Neurol. 2005, 252, 559–563. [Google Scholar] [CrossRef]
- Mattsson, N.; Tosun, D.; Insel, P.S.; Simonson, A.; Jack, C.R., Jr.; Beckett, L.A.; Donohue, M.; Jagust, W.; Schuff, N.; Weiner, M.W. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain 2014, 137, 1550–1561. [Google Scholar] [CrossRef]
- Wang, Z.; Das, S.R.; Xie, S.X.; Arnold, S.E.; Detre, J.A.; Wolk, D.A. Arterial spin labeled MRI in prodromal Alzheimer’s disease: A multi-site study. Neuroimage Clin. 2013, 2, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Glodzik, L.; Randall, C.; Rusinek, H.; de Leon, M.J. Cerebrovascular reactivity to carbon dioxide in Alzheimer’s disease. J. Alzheimers Dis. 2013, 35, 427–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, C.C.; Zlatar, Z.Z.; Wierenga, C.E. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell. Mol. Neurobiol. 2016, 36, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, A.; Lindegaard, K.F.; Russell, D.; Nyberg-Hansen, R.; Rootwelt, K.; Sorteberg, W.; Nornes, H. A comparison of transcranial Doppler and cerebral blood flow studies to assess cerebral vasoreactivity. Stroke 1992, 23, 15–19. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, M.N.; Berry, N.M.; Cutting, M.A.; Keage, H.A.; Buckley, J.D.; Howe, P.R. Transcranial Doppler ultrasound to assess cerebrovascular reactivity: Reliability, reproducibility and effect of posture. Peer J. 2013, 1, e65. [Google Scholar] [CrossRef]
- Dai, Y.L.; Luk, T.H.; Yiu, K.H.; Wang, M.; Yip, P.M.; Lee, S.W.; Li, S.W.; Tam, S.; Fong, B.; Lau, C.P.; et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis 2011, 216, 395–401. [Google Scholar] [CrossRef]
- Lee, B.J.; Huang, Y.C.; Chen, S.J.; Lin, P.T. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition 2012, 28, 767–772. [Google Scholar] [CrossRef]
- Olivieri, F.; Lazzarini, R.; Babini, L.; Prattichizzo, F.; Rippo, M.R.; Tiano, L.; di Nuzzo, S.; Graciotti, L.; Festa, R.; Bruge, F.; et al. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via miR-146a modulation. Free Radic. Biol. Med. 2013, 63, 410–420. [Google Scholar] [CrossRef]
- Yang, Y.K.; Wang, L.P.; Chen, L.; Yao, X.P.; Yang, K.Q.; Gao, L.G.; Zhou, X.L. Coenzyme Q10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction. Clin. Chim. Acta 2015, 450, 83–89. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Whitworth, J.A. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens. 2003, 21, 1983–1992. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Reitan, R.M. Trail Making Test: Manual for Administration and Scoring; Reitan Neuropsychology Laboratory: Length Tucson, AZ, USA, 1992. [Google Scholar]
- Benedet, M.J.; Alejandre, M.Á. TAVEC: Test de Aprendizaje Verbal España-Complutense; Ed Tea: Madrid, Spain, 1998. [Google Scholar]
- Wechsler, D.; de la Guía, E.; Vallar, F. WAIS-IV: Escala de Inteligencia de Wechsler para Adultos-IV; Ed Pearson: Madrid, Spain, 2012. [Google Scholar]
- Alosco, M.L.; Brickman, A.M.; Spitznagel, M.B.; Garcia, S.L.; Narkhede, A.; Griffith, E.Y.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Heart Fail. 2013, 19, E29–E34. [Google Scholar] [CrossRef] [Green Version]
- Parks, R.W.; Loewenstein, D.A.; Dodrill, K.L.; Barker, W.W.; Yoshii, F.; Chang, J.Y.; Emran, A.; Apicella, A.; Sheramata, W.A.; Duara, R. Cerebral metabolic effects of a verbal fluency test: A PET scan study. J. Clin. Exp. Neuropsychol. 1988, 10, 565–575. [Google Scholar] [CrossRef]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- De Renzi, A.; Vignolo, L.A. Token test: A sensitive test to detect receptive disturbances in aphasics. Brain 1962, 85, 665–678. [Google Scholar] [CrossRef]
- Hamilton, S.J.; Chew, G.T.; Watts, G.F. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care 2009, 32, 810–812. [Google Scholar] [CrossRef] [Green Version]
- Peña-Casanova, J.; Blesa, R.; Aguilar, M.; Gramunt-Fombuena, N.; Gómez-Ansón, B.; Oliva, R.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Antúnez, C.; et al. Spanish multicenter normative studies (NEURONORMA Project): Methods and sample characteristics. Arch. Clin. Neuropsychol. 2009, 24, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Peña-Casanova, J.; Gramunt-Fombuena, N.; Quiñones-Úbeda, S.; Sánchez-Benavides, G.; Aguilar, M.; Badenes, D.; Molinuevo, J.L.; Robles, A.; Barquero, M.S.; Payno, M.; et al. Spanish multicenter normative studies (NEURONORMA Project): Norms for the Rey–Osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch. Clin. Neuropsychol. 2009, 24, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Peña-Casanova, J.; Quiñones-Úbeda, S.; Gramunt-Fombuena, N.; Aguilar, M.; Casas, L.; Molinuevo, J.L.; Robles, A.; Rodríguez, D.; Barquero, M.S.; Antúnez, C.; et al. Spanish multicenter normative studies (NEURONORMA Project): Norms for Boston naming test and token test. Arch. Clin. Neuropsychol. 2009, 24, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Peña-Casanova, J.; Quiñones-Úbeda, S.; Gramunt-Fombuena, N.; Quintana-Aparicio, M.; Aguilar, M.; Badenes, D.; Cerulla, N.; Molinuevo, J.L.; Ruiz, E.; Robles, A.; et al. Spanish multicenter normative studies (NEURONORMA Project): Norms for verbal fluency tests. Arch. Clin. Neuropsychol. 2009, 24, 395–411. [Google Scholar] [CrossRef] [Green Version]
- Peña-Casanova, J.; Quiñones-Úbeda, S.; Quintana-Aparicio, M.; Aguilar, M.; Badenes, D.; Molinuevo, J.L.; Torner, L.; Robles, A.; Barquero, M.S.; Villanueva, C.; et al. Spanish multicenter normative studies (NEURONORMA Project): Norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch. Clin. Neuropsychol. 2009, 24, 321–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; Harrison, M.J. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke 1992, 23, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontinan-Rubio, J.; Sancho-Bielsa, F.J.; Peinado, J.R.; LaFerla, F.M.; Gimenez-Llort, L.; Duran-Prado, M.; Alcain, F.J. Sex-dependent co-occurrence of hypoxia and beta-amyloid plaques in hippocampus and entorhinal cortex is reversed by long-term treatment with ubiquinol and ascorbic acid in the 3xTg-AD mouse model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 92, 67–81. [Google Scholar] [CrossRef]
- Kaikkonen, J.; Tuomainen, T.P.; Nyyssonen, K.; Salonen, J.T. Coenzyme Q10: Absorption, antioxidative properties, determinants, and plasma levels. Free Radic. Res. 2002, 36, 389–397. [Google Scholar] [CrossRef]
- Niklowitz, P.; Onur, S.; Fischer, A.; Laudes, M.; Palussen, M.; Menke, T.; Doring, F. Coenzyme Q10 serum concentration and redox status in European adults: Influence of age, sex, and lipoprotein concentration. J. Clin. Biochem. Nutr. 2016, 58, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, S.L.; Florkowski, C.M.; Lever, M.; George, P.M. Biological variation of coenzyme Q10. Clin. Chem. 2005, 51, 455–457. [Google Scholar] [CrossRef] [Green Version]
- Hughes, K.; Lee, B.L.; Feng, X.; Lee, J.; Ong, C.N. Coenzyme Q10 and differences in coronary heart disease risk in Asian Indians and Chinese. Free Radic. Biol. Med. 2002, 32, 132–138. [Google Scholar] [CrossRef]
- Miles, M.V.; Horn, P.S.; Morrison, J.A.; Tang, P.H.; DeGrauw, T.; Pesce, A.J. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin. Chim. Acta 2003, 332, 123–132. [Google Scholar] [CrossRef]
- Hosoe, K.; Kitano, M.; Kishida, H.; Kubo, H.; Fujii, K.; Kitahara, M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul. Toxicol. Pharmacol. 2007, 47, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Langsjoen, P.H.; Langsjoen, A.M. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin. Pharmacol. Drug. Dev. 2014, 3, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of coenzyme Q10: An overview of the absorption process and subsequent metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex differences in lipid and lipoprotein metabolism: It’s not just about sex hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharm. 2003, 42, 107–121. [Google Scholar] [CrossRef]
- Ochiai, A.; Itagaki, S.; Kurokawa, T.; Kobayashi, M.; Hirano, T.; Iseki, K. Improvement in intestinal coenzyme q10 absorption by food intake. Yakugaku Zasshi 2007, 127, 1251–1254. [Google Scholar] [CrossRef] [Green Version]
- Palan, P.R.; Connell, K.; Ramirez, E.; Inegbenijie, C.; Gavara, R.Y.; Ouseph, J.A.; Mikhail, M.S. Effects of menopause and hormone replacement therapy on serum levels of coenzyme Q10 and other lipid-soluble antioxidants. Biofactors 2005, 25, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.H.; Kandiah, N. Early identification of Alzheimer’s disease: What have we learned from mild cognitive impairment? CNS Spectr. 2008, 13, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Bidzan, L.; Pachalska, M.; Bidzan, M. Predictors of clinical outcome in MCI. Med. Sci. Monit. 2007, 13, CR398–CR405. [Google Scholar] [PubMed]
- Sachdev, P.S.; Lipnicki, D.M.; Crawford, J.; Reppermund, S.; Kochan, N.A.; Trollor, J.N.; Wen, W.; Draper, B.; Slavin, M.J.; Kang, K.; et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: A population-based study. PLoS ONE 2013, 8, e59649. [Google Scholar] [CrossRef] [Green Version]
- Langsjoen, P.H. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J. Am. Coll. Cardiol. 2000, 35, 816–817. [Google Scholar] [CrossRef] [Green Version]
- Lavi, S.; Gaitini, D.; Milloul, V.; Jacob, G. Impaired cerebral CO2 vasoreactivity: Association with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1856–H1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavi, S.; Egbarya, R.; Lavi, R.; Jacob, G. Role of nitric oxide in the regulation of cerebral blood flow in humans: Chemoregulation versus mechanoregulation. Circulation 2003, 107, 1901–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Shim, Y.; Yoon, B.; Shim, D.S.; Kim, W.; An, J.Y.; Yang, D.W. Cognitive correlates of cerebral vasoreactivity on transcranial Doppler in older adults. J. Stroke Cerebrovasc. Dis. 2015, 24, 1262–1269. [Google Scholar] [CrossRef]
- Wolters, F.J.; de Bruijn, R.F.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A.; Heart brain connection collaborative research, G. cerebral vasoreactivity, apolipoprotein E, and the risk of dementia: A population-based study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, X.; Stamova, B.; Jin, L.W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [Green Version]
- Schmelzer, C.; Lorenz, G.; Rimbach, G.; Doring, F. In vitro effects of the reduced form of coenzyme Q (10) on secretion levels of TNF-alpha and chemokines in response to LPS in the human monocytic cell line THP-1. J. Clin. Biochem. Nutr. 2009, 44, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Simon, F.; Fernandez, R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J. Hypertens. 2009, 27, 1202–1216. [Google Scholar] [CrossRef]
- Tsai, K.L.; Chen, L.H.; Chiou, S.H.; Chiou, G.Y.; Chen, Y.C.; Chou, H.Y.; Chen, L.K.; Chen, H.Y.; Chiu, T.H.; Tsai, C.S.; et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011, 55, S227–S240. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Tan, H.H.; Goh, S.K.; Subramaniam, T.; Sum, C.F.; Tan, I.K.; Lee, B.L.; Ong, C.N. Oxidative burden in prediabetic and diabetic individuals: Evidence from plasma coenzyme Q (10). Diabet. Med. 2006, 23, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Overvad, K.; Diamant, B.; Holm, L.; Holmer, G.; Mortensen, S.A.; Stender, S. Coenzyme Q10 in health and disease. Eur. J. Clin. Nutr. 1999, 53, 764–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.J.; Lin, Y.C.; Huang, Y.C.; Ko, Y.W.; Hsia, S.; Lin, P.T. The relationship between coenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronary artery disease. Sci. World J. 2012, 2012, 792756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Pierce, J.D. Supplementation of coenzyme Q10 among patients with type 2 diabetes mellitus. Healthcare 2015, 3, 296–309. [Google Scholar] [CrossRef]
- Kure, C.E.; Rosenfeldt, F.L.; Scholey, A.B.; Pipingas, A.; Kaye, D.M.; Bergin, P.J.; Croft, K.D.; Wesnes, K.A.; Myers, S.P.; Stough, C. Relationships among cognitive function and cerebral blood flow, oxidative stress, and inflammation in older heart failure patients. J. Card. Fail. 2016, 22, 548–559. [Google Scholar] [CrossRef]
- Watts, G.F.; Playford, D.A.; Croft, K.D.; Ward, N.C.; Mori, T.A.; Burke, V. Coenzyme Q (10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia 2002, 45, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, R.; Akbari, M.; Sharifi, N.; Lankarani, K.; Moosazadeh, M.; Kolahdooz, F.; Taghizadeh, M.; Asemi, Z. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: A Systematic review and meta-analysis of randomized controlled trials. High Blood Press. Cardiovasc. Prev. 2018, 25. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Mxinwa, V.; Mokgalaboni, K.; Nkambule, B.B.; Louw, J.; Muller, C.J.F.; Tiano, L. The impact of coenzyme Q10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2020, 3, e00118. [Google Scholar] [CrossRef] [Green Version]
- Farkas, E.; Luiten, P.G. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 2001, 64, 575–611. [Google Scholar] [CrossRef] [Green Version]




| Group Characteristics | Study Population (n = 69) | Ubiquinol (n = 33) | Placebo (n = 36) | p Value Ub vs. Placebo |
|---|---|---|---|---|
| Age (years) (Mean ± SEM) | 72.2 (±5.7) | 72.3 (±5.8) | 72.08(±5.6) | 0.858 |
| Female | 39 (56.5%) | 17 (51.5%) | 22 (61.1%) | χ2 = 0.645; p = 0.422 |
| Hypertension | 49 (71.0%) | 23 (69.7%) | 26 (72.2%) | χ2 = 0.530; p = 0.817 |
| Type 2 diabetes mellitus | 20 (29.0%) | 7 (21.2%) | 13 (36.1%) | χ2 = 1.857; p = 0.173 |
| Dyslipidemia | 35 (43.8%) | 15 (45.5%) | 20 (55.6%) | χ2 = 0.703; p = 0.473 |
| Smoking | 40 (57.5%) | 17 (51.51%) | 23 (64%) | χ2 = 0.809; p = 0.565 |
| BMI (kg/m2) (Mean ± SEM) | 24.58 (±5.1) | 24.87 (±5.1) | 24.32 (±5.1) | 0.654 |
| CRP (Mean ± SEM) | 1.10 (±1.33) | 0.92 (±0.93) | 1.25 (±1.62) | 0.308 |
| Barthel scale [46] | 99.71 (±1.69) | 100 (±0) | 99.44 (±2.32) | 0.174 |
| Lawton & Brody scale [47] | 7.68 (±0.67) | 7.6 (±0.7) | 7.75 (±0.65) | 0.380 |
| TEST | Study Population T0 (n = 69) | Ubiquinol T0 (n = 33) | Placebo T0 (n = 36) | p Value Ub vs. Placebo T0 |
| Part A, Trail Making Test [33] | 123.97 ± 73.46 | 119.25 ± 55.84 | 128.52 ± 88.09 | 0.664 |
| Digit span (forward) [35] Visuospatial span (forward) [36] | 4.46 ± 0.85 4.37 ± 0.76 | 4.48 ± 0.90 4.48 ± 0.87 | 4.44 ± 0.80 4.27 ± 0.65 | 0.845 0.267 |
| Part B, Trail Making Test [33] Verbal abstract reasoning (WAIS-III Similarities) [35] | 273.25 ± 129.12 11.5 ± 4.01 | 286.91 ± 116.65 11.90 ± 3.81 | 257.55 ± 143.57 11.13 ± 4.20 | 0.464 0.435 |
| Digit span (backward) [35] Visuospatial span (backward) [36] | 3.20 ± 0.87 3.69 ± 0.82 | 3.18 ± 0.98 3.78 ± 0.92 | 3.22 ± 2.13 3.61 ± 0.72 | 0.839 0.880 |
| TAVEC [34] Trial 1 and 5 free recall Delayed free and cued recall (short term) Delayed free and cued recall (long term) Recognition corrects False positives Rey–Osterrieth Complex Figure. Immediate recall [40] Rey–Osterrieth Complex Figure. Delayed recall [40] | 7.60 ± 2.14 5.03 ± 2.36 6.57 ± 2.94 13.96 ± 2.55 5.52 ± 4.26 10.39 ± 5.46 9.76 ± 5.35 | 7.54 ± 2.20 5.03 ± 2.44 6.50 ± 2.76 14.25 ± 2.47 5.31 ± 4.14 9.00 ± 5.43 8.79 ± 5.35 | 7.64 ± 143.57 5.03 ± 2.33 6.64 ± 3.13 13.70 ± 2.63 5.70 ± 4.41 11.45 ± 5.30 10.54 ± 5.34 | 0.855 0.996 0.844 0.388 0.717 0.177 0.322 |
| Animal list generation [37] Boston Naming Test [38] Token Test [39] | 11.50 ± 4.01 39.18 ± 7.79 8.25 ± 4.18 | 11.77 ± 3.80 39.75 ± 8.34 8.61 ± 3.05 | 11.13 ± 4.20 38.66 ± 7.33 7.90 ± 4.98 | 0.521 0.565 0.601 |
| Rey–Osterrieth Complex Figure. Copy [40] | 25.89 ± 8.89 | 25.03 ± 9.11 | 25.80 ± 8.43 | 0.934 |
| TEST | Study Population T1 (n = 69) | Ubiquinol T1 (n = 33) | Placebo T1 (n = 36) | p Value Ub vs. Placebo T1 |
| Part A, Trail Making Test [33] | 133.42 ± 97.23 | 128.12 ± 71.59 | 138.30 ± 117.31 | 0.715 |
| Digit span (forward) [35] Visuospatial span (forward) [36] | 4.42 ± 0.97 4.37 ± 0.86 | 4.42 ± 1.03 4.50 ± 0.75 | 4.41 ± 0.93 4.30 ± 0.92 | 0.975 0.467 |
| Part B, Trail Making Test [33] Verbal abstract reasoning (WAIS-III Similarities) [35] | 267.83 ± 118.65 10.59 ± 5.03 | 290.69 ± 115.76 10.93 ± 4.89 | 241.55 ± 119.34 10.28 ± 5.20 | 0.179 0.600 |
| Digit span (backward) [35] Visuospatial span (backward) [36] | 3.20 ± 0.87 3.49 ± 1.00 | 3.30 ± 0.80 3.72 ± 0.87 | 3.11 ± 0.82 3.27 ± 0.82 | 0.332 0.064 |
| TAVEC [34] Trial 1 and 5 free recall Delayed free and cued recall (short term) Delayed free and cued recall (long term) Recognition correct False positives Rey–Osterrieth Complex Figure. Immediate recall [40] Rey–Osterrieth Complex Figure. Delayed recall [40] | 7.98 ± 3.12 5.74 ± 2.95 6.78 ± 4.01 13.13 ± 2.63 5.03 ± 4.65 10.65 ± 6.84 10.60 ± 7.20 | 7.64 ± 2.13 5.66 ± 3.02 7.26 ± 3.77 13.21 ± 2.76 4.41 ± 3.45 9.23 ± 6.83 9.41 ± 7.35 | 7.82 ± 3.07 5.82 ± 2.94 6.35 ± 4.22 13.05 ± 2.53 5.62 ± 5.56 11.80 ± 6.79 11.57 ± 7.20 | 0.669 0.837 0.368 0.807 0.308 0.255 0.370 |
| Animal list generation [37] Boston Naming Test [38] Token Test [39] | 10.59 ± 5.03 38.50 ± 8.88 7.62 ± 4.21 | 10.61 ± 4.60 39.15 ± 9.12 6.80 ± 3.48 | 10.28 ± 5.20 37.91 ± 8.73 8.28 ± 4.68 | 0.789 0.568 0.247 |
| Rey–Osterrieth Complex Figure. Copy [40] | 25.03 ± 8.42 | 25.50 ± 8.88 | 24.90 ± 8.30 | 0.899 |
| Group | Ubiquinol (n = 33) | Placebo (n = 36) | ||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Evolution (T1) | Dementia (n = 7) | Stability/MCI (n = 25) | Normal Cognition (n = 1) | Statistics | Dementia (n = 10) | Stability/MCI (n = 22) | Normal Cognition (n = 4) | Statistics |
| Barthel scale | 95 ± 2.98 | 99.09 ± 4.26 | 100 ± 0 | 0.125 | 95.71 ± 7.31 | 99.40 ± 2.19 | 100 ± 0 | 0.090 |
| Lawton & Brody scale | 4.30 ± 8.16a | 7.68 ± 0.89 b | 8 ± 0 b | ≥0.001 * | 4.14 ± 2.11 a | 7.28 ± 1.17 b | 8 ± 0 b | ≥0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Carpintero, S.; Domínguez-Bértalo, J.; Pedrero-Prieto, C.; Frontiñán-Rubio, J.; Amo-Salas, M.; Durán-Prado, M.; García-Pérez, E.; Vaamonde, J.; Alcain, F.J. Ubiquinol Supplementation Improves Gender-Dependent Cerebral Vasoreactivity and Ameliorates Chronic Inflammation and Endothelial Dysfunction in Patients with Mild Cognitive Impairment. Antioxidants 2021, 10, 143. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020143
García-Carpintero S, Domínguez-Bértalo J, Pedrero-Prieto C, Frontiñán-Rubio J, Amo-Salas M, Durán-Prado M, García-Pérez E, Vaamonde J, Alcain FJ. Ubiquinol Supplementation Improves Gender-Dependent Cerebral Vasoreactivity and Ameliorates Chronic Inflammation and Endothelial Dysfunction in Patients with Mild Cognitive Impairment. Antioxidants. 2021; 10(2):143. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020143
Chicago/Turabian StyleGarcía-Carpintero, Sonia, Javier Domínguez-Bértalo, Cristina Pedrero-Prieto, Javier Frontiñán-Rubio, Mariano Amo-Salas, Mario Durán-Prado, Eloy García-Pérez, Julia Vaamonde, and Francisco J. Alcain. 2021. "Ubiquinol Supplementation Improves Gender-Dependent Cerebral Vasoreactivity and Ameliorates Chronic Inflammation and Endothelial Dysfunction in Patients with Mild Cognitive Impairment" Antioxidants 10, no. 2: 143. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020143