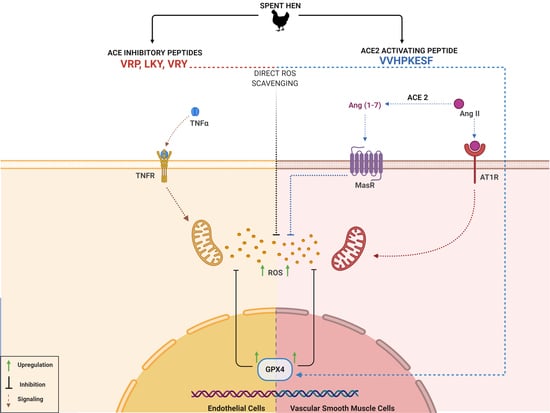
Spent Hen Muscle Protein-Derived RAS Regulating Peptides Show Antioxidant Activity in Vascular Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cytotoxicity of VRP, LKY, VRY, and V-F in Vascular Cells
2.3. Cell Culture of VSMCs and ECs
2.4. Superoxide Detection
2.5. Lipid Peroxidation Assay
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Cytotoxicity of VRP, LKY, VRY, and V-F in ECs and VSMCs
3.2. Antioxidant Effect of VRP, LKY, VRY, and V-F in ECs and VSMCs
3.3. Effect of VRP, LKY, VRY, and V-F on the Expression of TNF-R1/R2 in ECs
3.4. Effects of VRP, LKY, VRY, and V-F on the Expression of AT1R and MasR Receptors in VSMCs
3.5. Involvement of AT1R or MasR in Antioxidant Effect of VRP, LKY, VRY, and V-F in VSMCs
3.6. Effect of VRP, LKY, VRY, and V-F on Expressions of Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e138. [Google Scholar]
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Tedla, Y.G.; Bautista, L.E. Drug side effect symptoms and adherence to antihypertensive medication. Am. J. Hypertens. 2016, 29, 772–779. [Google Scholar] [CrossRef]
- Khanna, A.; Lefkowitz, L.; White, W.B. Evaluation of recent fixed-dose combination therapies in the management of hypertension. Curr. Opin. Nephrol. Hypertens. 2008, 17, 477–483. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoia, C.; Sada, L.; Zezza, L.; Pucci, L.; Lauri, F.M.; Befani, A.; Alonzo, A.; Volpe, M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int. J. Hypertens. 2011, 2011, 281240. [Google Scholar] [CrossRef]
- Unger, T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am. J. Cardiol. 2002, 89, 3–9. [Google Scholar] [CrossRef]
- Touyz, R.M. Oxidative stress and vascular damage in hypertension. Curr. Hypertens. Rep. 2000, 2, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.; Schiffrin, E. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem. Cell Biol. 2004, 122, 339–352. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Cau, S.B.; Carneiro, F.S.; Tostes, R.C. Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Front. Physiol. 2012, 3, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.R.; Visioli, F.; Frei, B.; Hagen, T.M. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: Evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 2006, 5, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Juurlink, B.H. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension 2002, 39, 809–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalba, G.; Beaumont, F.J.; José, G.S.; Fortuno, A.; Fortuño, M.A.A.; Etayo, J.C.; Diíez, J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 2000, 35, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleenor, B.S.; Seals, D.R.; Zigler, M.L.; Sindler, A.L. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 2012, 11, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, G.; ud Din, J.; Zhao, F.; Liu, X. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Tsai, B.C.-K.; Hsieh, D.J.-Y.; Lin, W.-T.; Tamilselvi, S.; Day, C.H.; Ho, T.-J.; Chang, R.-L.; Viswanadha, V.P.; Kuo, C.-H.; Huang, C.-Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2020, 129, 108862. [Google Scholar] [CrossRef]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.; Chan, C.B.; Wu, J. Egg white ovotransferrin-derived ACE inhibitory peptide ameliorates angiotensin II-stimulated insulin resistance in skeletal muscle cells. Mol. Nutr. Food Res. 2018, 62, 1700602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory effects of a pea-derived peptide Leu-Arg-Trp (LRW) on dysfunction of rat aortic vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liang, Y.; Bai, J.; Wu, W.; Lin, Q.; Wu, J. Spent hen-derived ACE inhibitory peptide IWHHT shows antioxidative and anti-inflammatory activities in endothelial cells. J. Funct. Foods 2019, 53, 85–92. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. Poultry and Egg Market Information: Report 108–Chicks/Poults Destroyed. Available online: https://www.agr.gc.ca/eng/animal-industry/poultry-and-egg-market-information/hatcheries/?id=1384971854401 (accessed on 30 December 2020).
- Shahbandeh, M. Total Number of Laying Hens in the U.S. from 2000 to 2019. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/195823/total-number-of-laying-hens-in-the-us-since-2000/ (accessed on 30 December 2020).
- Fan, H.; Yu, W.; Liao, W.; Wu, J. Spent hen protein hydrolysate with good gastrointestinal stability and permeability in caco-2 cells shows antihypertensive activity in SHR. Foods 2020, 9, 1384. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J. Purification and identification of novel ACE inhibitory and ACE2 upregulating peptides from spent hen muscle proteins. Food Chem. 2020, 345, 128867. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Fan, H.; Liao, W.; Wu, J. Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J. Food Biochem. 2019, 43, e12572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Xu, Q.; Hong, H.; Wu, J. Stability and transport of spent hen-derived ACE-inhibitory peptides IWHHT, IWH, and IW in human intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2018, 66, 11347–11354. [Google Scholar] [CrossRef]
- Liao, W. Food Protein-Derived Peptides Targeting Angiotensin Converting Enzyme 2. Ph.D. Thesis, University of Alberta, Ecmonton, AB, Canada, 2019. [Google Scholar]
- Liao, W.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Modulatory effects of egg white ovotransferrin-derived tripeptide IRW (Ile-Arg-Trp) on vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2016, 64, 7342–7347. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative Stress and Endothelial Dysfunction in Hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Vaziri, N.D. Up-regulation of kidney NAD (P) H oxidase and calcineurin in SHR: Reversal by lifelong antioxidant supplementation. Kidney Int. 2004, 65, 219–227. [Google Scholar]
- Wang, X.; Hong, H.; Wu, J. Hen collagen hydrolysate alleviates UVA-induced damage in human dermal fibroblasts. J. Funct. Foods 2019, 63, 103574. [Google Scholar] [CrossRef]
- Yu, W.; Field, C.J.; Wu, J. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018, 253, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.-H.; Zhang, W.-G. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Antioxidant peptides identified from ovotransferrin by the ORAC method did not show anti-inflammatory and antioxidant activities in endothelial cells. J. Agri. Food Chem. 2015, 64, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agri. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K.K. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Yang, Y.; Wang, Z.; Xing, C.; Yuan, J.; Wang, L.; Udenigwe, C.; Ju, X. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin–angiotensin system pathway in spontaneously hypertensive rat. NPJ Sci. Food 2019, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Wang, J.; Pan, H.; Wu, H.; Ren, D.; Lu, J. Effects of IQP, VEP and Spirulina platensis hydrolysates on the local kidney renin angiotensin system in spontaneously hypertensive rats. Mol. Med. Rep. 2017, 16, 8485–8492. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Davidge, S.T.; Wu, J. Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) reduces blood pressure in spontaneously hypertensive rats via the ACE2/Ang (1-7)/Mas receptor axis. Mol. Nut. Food Res. 2019, 63, 1900063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabelo, L.A.; Alenina, N.; Bader, M. ACE2–angiotensin-(1–7)–Mas axis and oxidative stress in cardiovascular disease. Hypertens. Res. 2011, 34, 154–160. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Sáez, G.T.; Están-Capell, N. Antioxidant Enzymes. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 288–294. [Google Scholar]
- Liu, Z.; Gou, Y.; Zhang, H.; Zuo, H.; Zhang, H.; Liu, Z.; Yao, D. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biol. 2014, 3, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by Gpx4 and Ferroptosis. In Apoptotic and Non-Apoptotic Cell Death; Springer: Berlin/Heidelberg, Germany, 2016; pp. 143–170. [Google Scholar]
- Tsubouchi, K.; Araya, J.; Yoshida, M.; Sakamoto, T.; Koumura, T.; Minagawa, S.; Hara, H.; Hosaka, Y.; Ichikawa, A.; Saito, N. Involvement of GPx4-regulated lipid peroxidation in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. 2019, 203, 2076–2087. [Google Scholar] [CrossRef]
- Du, Y.; Esfandi, R.; Willmore, W.G.; Tsopmo, A. Antioxidant activity of oat proteins derived peptides in stressed hepatic HepG2 cells. Antioxidants 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Bhullar, K.S.; Wu, J. Spent Hen Muscle Protein-Derived RAS Regulating Peptides Show Antioxidant Activity in Vascular Cells. Antioxidants 2021, 10, 290. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020290
Fan H, Bhullar KS, Wu J. Spent Hen Muscle Protein-Derived RAS Regulating Peptides Show Antioxidant Activity in Vascular Cells. Antioxidants. 2021; 10(2):290. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020290
Chicago/Turabian StyleFan, Hongbing, Khushwant S. Bhullar, and Jianping Wu. 2021. "Spent Hen Muscle Protein-Derived RAS Regulating Peptides Show Antioxidant Activity in Vascular Cells" Antioxidants 10, no. 2: 290. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020290