Haplopine Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and TNF-α/IFN-γ-Induced Inflammation in Human Keratinocyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Quantitative Real-Time PCR
2.4. ELISA Assay
2.5. Western Blot Analysis
2.6. Experimental Animals
2.7. AD Induction and Treatment
2.8. Clinical Dermatitis Score
2.9. Measurement of Serum IgE Levels
2.10. Spleen and Body Weight
2.11. Histopathological Analysis
2.12. Statistical Analysis
3. Results
3.1. Anti-Inflammatory Effects of Dictamine, Fraxinellone, Haplopine, and Obacunone on IL-6 mRNA Expression in HaCaT Cells
3.2. Inhibitory Effects of Haplopine on TSLP, GM-CSF, G-CSF, and IL-6 Expressions in HaCaT Cells
3.3. Inhibitory Effects of Haplopine on the Expressions of IL-4, IL-13, and COX-2 in Jurkat T Cells
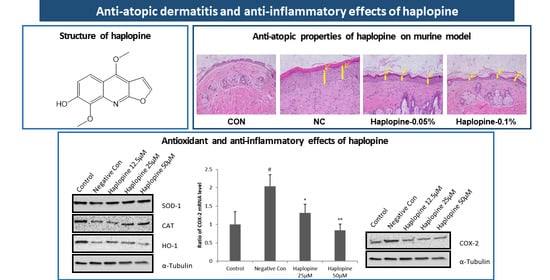
3.4. Inhibitory Effects of Haplopine on SOD, CAT, and HO-1 Activities in Jurkat T Cells
3.5. Effect of Haplopine on AD-Like Skin Lesions in the DNCB-Induced Animal Model
3.6. Effects of Haplopine on Total Serum IgE and Splenic Cellularity
3.7. Effects of Haplopine on Histologic Manifestations in DNCB Treat Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pezzolo, E.; Naldi, L. Epidemiology of major chronic inflammatory immune-related skin diseases in 2019. Expert Rev. Clin. Immunol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cork, M.J.; Robinson, D.A.; Vasilopoulos, Y.; Ferguson, A.; Moustafa, M.; MacGowan, A.; Duff, G.W.; Ward, S.J.; Tazi-Ahnini, R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene-environment interactions. J. Allergy Clin. Immunol. 2006, 118, 3–21. [Google Scholar] [CrossRef]
- Bin, L.; Leung, D.Y.M. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin. Immunol. 2016, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Fuchs, J.; Zollner, T.M.; Kaufmann, R.; Podda, M. Redox-modulated pathways in inflammatory skin diseases. Free Radic. Biol. Med. 2001, 30, 337–353. [Google Scholar] [CrossRef]
- Tsukahara, H.; Shibata, R.; Ohshima, Y.; Todoroki, Y.; Sato, S.; Ohta, N.; Hiraoka, M.; Yoshida, A.; Nishima, S.; Mayumi, M. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003, 72, 2509–2516. [Google Scholar] [CrossRef]
- Oh, S.Y.; Chung, J.; Kim, M.K.; Kwon, S.O.; Cho, B.H. Antioxidant nutrient intakes and corresponding biomarkers associated with the risk of atopic dermatitis in young children. Eur. J. Clin. Nutr. 2010, 64, 245–252. [Google Scholar] [CrossRef]
- Choi, M.; Yi, J.K.; Kim, S.-Y.; Ryu, J.H.; Lee, J.; Kwon, W.; Jang, S.; Kim, D.; Kim, M.; Kim, H.; et al. Anti-inflammatory effects of a methanol extract of Dictamnus dasycarpus Turcz. root bark on imiquimod-induced psoriasis. BMC Complement. Altern. Med. 2019, 19, 347. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Nakano, Y.; Rahman, M.A.; Yatsuzuka, R.; Kamei, C. Effects of a Dictamnus dasycarpus T. extract on allergic models in mice. Biosci. Biotechnol. Biochem. 2008, 72, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.M.; Yang, T.Y.; Niu, Y.L.; Huang, H.C. The Extract of D. dasycarpus Ameliorates Oxazolone-Induced Skin Damage in Mice by Anti-Inflammatory and Antioxidant Mechanisms. Antioxidants 2018, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qin, Y.; Gong, F.-Y.; Wu, X.-F.; Hua, Z.-C.; Chen, T.; Xu, Q. Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice. Biochem. Pharmacol. 2009, 77, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, R.; Liu, F.; Liu, H.; Fei, Q.; Han, Y.; Cai, R.; Peng, C.; Qi, Y. Obacunone causes sustained expression of MKP-1 thus inactivating p38 MAPK to suppress pro-inflammatory mediators through intracellular MIF. J. Cell. Biochem. 2018, 119, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, F.; Ruan, J.; Yan, J.; Huang, P.; Wang, J.; Han, L.; Zhang, Y.; Wang, T. Anti-inflammatory constituents from Cortex Dictamni. Fitoterapia 2019, 134, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Y.; Lin, X.; Geng, Y.; Wang, X.; Zhang, J.; Qiu, J. Extraction and isolation of dictamnine, obacunone and fraxinellone from Dictamnus dasycarpus Turcz. by supercritical fluid extraction and high-speed counter-current chromatography. Química Nova 2012, 35, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Jeong, E.J.; Yang, H.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Inhibitory alkaloids from Dictamnus dasycarpus root barks on lipopolysaccharide-induced nitric oxide production in BV2 cells. J. Enzyme Inhib. Med. Chem. 2012, 27, 490–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Wolfender, J.L.; Hostettmann, K.; Xu, R.; Qin, G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry 1998, 47, 7–11. [Google Scholar] [CrossRef]
- Abramovits, W. A clinician’s paradigm in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2005, 53, S70–S77. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- Novak, N.; Simon, D. Atopic dermatitis—From new pathophysiologic insights to individualized therapy. Allergy 2011, 66, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of new oxidative stress markers in chronic spontaneous urticaria. Postepy Derm. Alergol. 2017, 34, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Li, S.P.; Chang, H.T.; Wang, Y.T.; Tu, P.F. Pressurized liquid extraction followed by high-performance liquid chromatography for determination of seven active compounds in Cortex Dictamni. J. Chromatogr. A 2006, 1108, 268–272. [Google Scholar] [CrossRef]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Ansel, J.; Perry, P.; Brown, J.; Damm, D.; Phan, T.; Hart, C.; Luger, T.; Hefeneider, S. Cytokine Modulation of Keratinocyte Cytokines. J. Investig. Dermatol. 1990, 94, s101–s107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Saredy, J.; Zhang, R.; Shao, Y.; Sun, Y.; Yang, W.Y.; Wang, J.; Liu, L.; Drummer, C., IV; Johnson, C.; et al. Approaching Inflammation Paradoxes-Proinflammatory Cytokine Blockages Induce Inflammatory Regulators. Front. Immunol. 2020, 11, 554301. [Google Scholar] [CrossRef]
- Mueller, M.M.; Fusenig, N.E. Constitutive expression of G-CSF and GM-CSF in human skin carcinoma cells with functional consequence for tumor progression. Int. J. Cancer 1999, 83, 780–789. [Google Scholar] [CrossRef]
- Jang, S.; Morris, S.; Lukacs, N.W. TSLP promotes induction of Th2 differentiation but is not necessary during established allergen-induced pulmonary disease. PLoS ONE 2013, 8, e56433. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, M.J.; Bak, D.-H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.C.; Scheerens, H. Targeting IgE production in mice and humans. Curr. Opin. Immunol. 2014, 31, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manohar, M.; Nadeau, K.C. The Potential of Anti-IgE in Food Allergy Therapy. Curr. Treat. Options Allergy 2014, 1, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.-T.; Goodarzi, H.; Chen, H.-Y. IgE, Mast Cells, and Eosinophils in Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Yu-Lee, L.Y. Prolactin modulation of immune and inflammatory responses. Recent Prog. Horm. Res. 2002, 57, 435–455. [Google Scholar] [CrossRef]
- Yang, C.; Ling, H.; Zhang, M.; Yang, Z.; Wang, X.; Zeng, F.; Wang, C.; Feng, J. Oxidative stress mediates chemical hypoxia-induced injury and inflammation by activating NF-κb-COX-2 pathway in HaCaT cells. Mol. Cells 2011, 31, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.; Sanchez, T.; Moreno, J.J. Regulation of prostaglandin E2 production by the superoxide radical and nitric oxide in mouse peritoneal macrophages. Free Radic. Res. 2000, 32, 303–311. [Google Scholar] [CrossRef]
- Sanchez, T.; Moreno, J.J. Induction by interleukin-1β peptide of prostaglandin E2 formation via enhanced prostaglandin H synthase-2 expression in 3T6 fibroblasts. Biochem. Pharmacol. 1998, 56, 759–761. [Google Scholar] [CrossRef]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell. Immunol. 2011, 2. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Araujo, J.; Zhang, M.; Yin, F. Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Diniyah, N.; Alam, M.B.; Choi, H.-J.; Lee, S.-H. Lablab purpureus Protects HaCaT Cells from Oxidative Stress-Induced Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression via the Activation of p38 and ERK1/2. Int. J. Mol. Sci. 2020, 21, 8583. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Kim, Y.J.; Jegal, J.; Jo, B.-G.; Choi, H.-S.; Yang, M.H. Haplopine Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and TNF-α/IFN-γ-Induced Inflammation in Human Keratinocyte. Antioxidants 2021, 10, 806. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050806
Kim T-Y, Kim YJ, Jegal J, Jo B-G, Choi H-S, Yang MH. Haplopine Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and TNF-α/IFN-γ-Induced Inflammation in Human Keratinocyte. Antioxidants. 2021; 10(5):806. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050806
Chicago/Turabian StyleKim, Tae-Young, Ye Jin Kim, Jonghwan Jegal, Beom-Geun Jo, Han-Seok Choi, and Min Hye Yang. 2021. "Haplopine Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and TNF-α/IFN-γ-Induced Inflammation in Human Keratinocyte" Antioxidants 10, no. 5: 806. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050806