Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine
Abstract
:1. Introduction
2. Methods of Review
3. Herbal Diversity of Monofloral Honeys
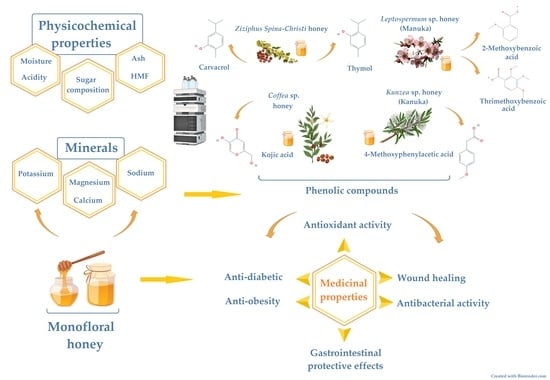
4. Phenolic Compounds
5. Physicochemical Properties of Monofloral Honeys
6. Major Mineral Composition of Some Monofloral Honey
7. Antioxidant Activity of Monofloral Honeys
8. Health Effects of Monofloral Honeys
8.1. Wound Healing
8.2. Antioxidant Activity
8.3. Anti-Obesity
8.4. Gastrointestinal Protective Effects
8.5. Anti-Fatigue and Antidepressant Effects
8.6. Antibacterial and Antimicrobial Activity
8.7. Antidiabetic Effects
8.8. In Vitro Studies Correlated to Polyphenols
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Requier, F. Bee Colony Health Indicators: Synthesis and Future Directions. CAB Rev. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A National Survey of Managed Honey Bee 2015–2016 Annual Colony Losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, B.K. Do More Bees Imply Higher Fees? Honey Bee Colony Strength as a Determinant of Almond Pollination Fees. Food Policy 2019, 83, 150–160. [Google Scholar] [CrossRef]
- Topal, E.; Yücel, B.; Altunoğlu, E.; Acar, A.A.; Kösoğlu, M.; Tekintaş, F.E. Bal ve Bombus Arısı Tozlaşmasının ve Doğal Tozlayıcıların Kirazda Meyve Tutumu ve Kalitesi Üzerine Etkisi. J. Aari. 2018, 28, 61–74. [Google Scholar]
- Mărgăoan, R.; Aradăvoaicei, Ș.; Cornea-Cipcigan, M.; Sisea, C.R. The Role of Pollinators in Maintaining the Biodiversity of Some Exotic Cultures. Int. J. Envrion. Res. Technol. 2019, 2, 17–23. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, A.J.; Genersch, E. Honey Bee Colony Losses and Associated Viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are Bee Diseases Linked to Pesticides?—A Brief Review. Environ. Int. 2016, 89, 7–11. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honey Bee (Apis mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef] [Green Version]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.; Vejnović, B.; Stevanović, J. Looking for The Causes of and Solutions to The Issue of Honey Bee Colony Losses. Acta Vet. Beogr. 2019, 69, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, A.E.; El-Ansari, M.K.; Zahra, A.A. Effect of the Honeybee Hybrid and Geographic Region on the Honey Bee Venom Production. J. Plant Prot. Pathol. 2019, 10, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Bărnuţiu, L.I.; Mărghitaş, L.; Dezmirean, D.; Bobiş, O.; Mihai, C.; Pavel, C. Physicochemical Composition of Apilarnil (Bee Drone Larvae). Lucr. Ştiinţifice Ser. Zooteh. 2013, 59, 199–202. [Google Scholar]
- Estevinho, M.L.M.F.; Rodrigues, S.S.Q.; Pereira, A.P.; Feás, X. Portuguese Bee Pollen: Palynological Study, Nutritional and Microbiological Evaluation. Int. J. Food Sci. Technol. 2012, 47, 429–435. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 8367846. [Google Scholar] [CrossRef] [Green Version]
- Gürbüz, S.; Güngör Çelikel, A.; Toprak, A. Sağlık ve Beslenme Açısından Bal. Anadolu, I. In Uluslararası Multidisipliner Çalışmalar Kongresi; Sayfa: Diyarbakır, Turkey, 2018; pp. 692–695. [Google Scholar]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. High 5-Hydroxymethylfurfural Concentrations are Found in Malaysian Honey Samples Stored for More Than One Year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Buckwheat Honey Increases Serum Antioxidant Capacity in Humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Çakıcı, N.; Yassıhüyük, N. Balın Antioksidan Aktivitesi ve Antibakteriyel Etkisi. Arıcılık Araştırma Dergisi 2013, 9, 12–13. [Google Scholar]
- Malkoç, M.; Kara, Y.; Özkök, A.; Ertürk, Ö.; Kolaylı, S. Karaçalı (Paliurus spina-christi Mill.) Balının Karakteristik Özellikleri. Uludag Bee J. 2019, 19, 69–81. [Google Scholar]
- Özenirler, Ç. Dandelion Honey: A New Monofloral Honey Record for Turkey. Uludag Bee J. 2018, 18, 87–93. [Google Scholar]
- Gül, A.; Pehlivan, T. Antioxidant Activities of Some Monofloral Honey Types Produced Across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Can, Z. Biyoaktiviteleri Yönünden Türkiye Florasına Ait Baskın Ballar Ile Manuka Ballarının Karşılaştırılması. Ph.D. Thesis, Karadeniz Teknik Üniversitesi/Fen Bilimleri Enstitüsü, Trabzon, Turkey, 2014. [Google Scholar]
- Ekmekci, N. Investigation of Antimutagenic Effects of Some Local Honey Kinds in Turkey by Salmonella/Microsome (Ames) Test System. Ph.D. Thesis, Selçuk Üniversitesi Fen Bilimleri Enstitüsü, Konya, Turkey, 2010. [Google Scholar]
- Ölmez, Ç. Türkiye’de Üretilen Farklı Çiçek ve Salgı Bal Çeşitlerinin Bazı Kalitatif ve Besinsel Özellikleri. Ph.D. Thesis, Selçuk Üniversitesi Fen Bilimleri Enstitüsü, Konya, Turkey, 2009. [Google Scholar]
- Haroun, M.I. Türkiye’de Üretilen Bazı Çiçek ve Salgı Ballarının Fenplik Asit ve Flavonoid Profilinin Belirlenmesi. Ph.D. Thesis, Ankara Üniversitesi Fen Bilimleri Enstitüsü Gıda Mühendisliği Anabilim Dalı, Ankara, Turkey, 2006. [Google Scholar]
- Silici, S.; Tolon, B. Further Chemical and Palynological Properties of Some Unifloral Turkish Honeys. In Proceedings of the First German Congress for Bee Products and Apitherapy, Passau, Germany, 23–24 March 2002. [Google Scholar]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Balkanska, R.; Stefanova, K.; Stoikova–Grigorova, R.; Manolova, V. Preliminary Study of DNA Extractıon from Bulgarian Honeys and Its Amplification by PCR for Botanıcal Identıfıcatıon. Food Health 2018, 3, 194–201. [Google Scholar] [CrossRef]
- Nikolova, K.; Pisanova, E.; Ivanova, I. Use of Cluster and Factor Analysis for Grouping Bulgarians Honeys according to Botanical Origin. J. Commun. Comput. 2017, 14, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, K.; Gentscheva, G.; Ivanova, E. Survey of the Mineral Content and Some Physico-Chemical Parameters of Bulgarian Bee Honeys. Bulg. Chem. Commun. 2013, 45, 244–249. [Google Scholar]
- Atanassova, J.; Lazarova, M.; Yurukova, L. Significant Parameters of Bulgarian Honeydew Honey. J. Cent. Eur. Agric. 2016, 17, 640–651. [Google Scholar] [CrossRef] [Green Version]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Berhilevych, O.; Kasianchuk, V.; Kukhtyn, M.; Dimitrijevich, L.; Marenkova, T. The Study Correlation between Physicochemical Properties, Botanical Origin and Microbial Contamination of Honey from the South of Ukraine. Potravin. Slovak. J. Food Sci. 2019, 13, 863–869. [Google Scholar] [CrossRef] [Green Version]
- Kotenko, P.; Miyaura, R. Regional Typology of Beekeeping and Consumption of Honeybee Products in Ukraine. J. Agric. Sci. Tokyo Univ. Agric. 2019, 64, 11–19. [Google Scholar]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Carloni, P. Chemometric Approach to the Analysis of Antioxidant Properties and Colour of Typical Italian Monofloral Honeys. Int. J. Food Sci. Technol. 2017, 52, 1138–1146. [Google Scholar] [CrossRef]
- Di Marco, G.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, A.; Canini, A. Geographical, Botanical and Chemical Profile of Monofloral Italian Honeys as Food Quality Guarantee and Territory Brand. Plant Biosyst. 2017, 153, 450–463. [Google Scholar] [CrossRef]
- Mattonai, M.; Parri, E.; Querci, D.; Degano, I.; Ribechini, E. Development and Validation of an HPLC-DAD and HPLC/ESI-MS2 Method for the Determination of Polyphenols in Monofloral Honeys from Tuscany (Italy). Microchem. J. 2016, 126, 220–229. [Google Scholar] [CrossRef]
- Parri, E.; Santinami, G.; Domenici, V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy). Appl. Sci. 2020, 10, 1776. [Google Scholar] [CrossRef] [Green Version]
- Tuberoso, C.I.; Bifulco, E.; Jerkovic, I.; Caboni, P.; Cabras, P.; Floris, I. Methyl Syringate: A Chemical Marker of Asphodel (Asphodelus Microcarpus Salzm. Et Viv.) Monofloral Honey. J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Ribeiro, I.; Marçalo, J.; Rijo, P.; Faustino, C.; Pinheiro, L. Physicochemical, Antioxidant and Antimicrobial Properties of Selected Portuguese Commercial Monofloral Honeys. J. Food Nutr. Res. 2018, 6, 645–654. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant Activity, Quality Parameters and Mineral Content of Portuguese Monofloral Honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Silva, L.R.; Videira, R.; Monteiro, A.P.; Valentão, P.; Andrade, P.B. Honey from Luso Region (Portugal): Physicochemical Characteristics and Mineral Contents. Microchem. J. 2009, 93, 73–77. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, Antioxidant Activity and Multielement Analysis of Two Types of Early Spring Honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Czipa, N.; Alexa, L.; Phillips, C.J.; Kovács, B. Macro-Element Ratios Provide Improved Identification of the Botanical Origin of Mono-Floral Honeys. Eur. Food Res. Technol. 2018, 244, 1439–1445. [Google Scholar] [CrossRef]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of Essential and Toxic Elements in Hungarian Honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; El Midaoui, A.; Bourkhis, B.; Ennassir, J.; Alem, C.; Filali-Zegzouti, Y. Physicochemical Properties of Eleven Monofloral Honey Samples Produced in Morocco. Arab J. Basic Appl. Sci. 2019, 26, 476–487. [Google Scholar] [CrossRef] [Green Version]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G. Physicochemical Characterization and Antioxidant Activity of 17 Commercial Moroccan Honeys. Int. J. Food Sci. Nutr. 2014, 65, 449–457. [Google Scholar] [CrossRef]
- Chakir, A.; Romane, A.; Marcazzan, G.L.; Ferrazzi, P. Physicochemical Properties of Some Honeys Produced from Different Plants in Morocco. Arab J. Ochem. 2016, 9, S946–S954. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.; Sirajudeen, K.N.S.; Swamy, M.; Yaacob, M.; Sulaiman, S. Studies on the Antioxidant Properties of Tualang Honey of Malaysia. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Yaacob, N.S.; Nengsih, A.; Norazmi, M. Tualang Honey Promotes Apoptotic Cell Death Induced by Tamoxifen in Breast Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2013, 2013, 989841. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a Novel Glycoside, Leptosin, as a Chemical Marker of Manuka Honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Semprini, A.; Singer, J.; Braithwaite, I.; Shortt, N.; Thayabaran, D.; McConnell, M.; Weatherall, M.; Beasley, R. Kanuka Honey Versus Aciclovir for the Topical Treatment of Herpes Simplex Labialis: A Randomised Controlled Trial. BMJ Open 2019, 9, e026201. [Google Scholar] [CrossRef] [Green Version]
- Vanhanen, L.P.; Emmertz, A.; Savage, G.P. Mineral Analysis of Mono-floral New Zealand Honey. Food Chem. 2011, 128, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Bobis, O.; Mãrghitas, L.A.; Dezmirean, D.S.; Bãrnutiu, L.I.; Mãrgãoan, R.; Bogdan Gherman, B.; Bonta, V. The Importance of Melissopalynology in Addition to Physical-chemical Analysis on Botanical Authenticity Testing of Monofloral Honey. Bull. UASVM Anim. Sci. Biotechnol. 2013, 70, 24–30. [Google Scholar]
- Bong, J.; Loomes, K.M.; Lin, B.; Stephens, J.M. New Approach: Chemical and Fluorescence Profiling of NZ Honeys. Food Chem. 2018, 267, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Durán, S.S.; Sánchez-Sánchez, J.; Quintanai, S.C.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus honey: Quality parameters, chemical composition and health-promoting properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of Honey Phenolic Acids by HPLC, Its Application to Honey Botanical Characterization. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2281–2288. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomas-Barberan, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, Phenolic Acids and Abscisic Acid in Australian and New Zealand Leptospermum Honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Das, A.; Datta, S.; Mukherjee, S.; Bose, S.; Ghosh, S.; Dhar, P. Evaluation of Antioxidative, Antibacterial and Probiotic Growth Stimulatory Activities of Sesamum Indicum Honey Containing Phenolic Compounds and Lignans. LWT Food Sci. Technol. 2015, 61, 244–250. [Google Scholar] [CrossRef]
- Gašić, U.M.; Natić, M.M.; Mišić, D.M.; Lušić, D.V.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Lušić, D. Chemical Markers for the Authentication of Unifloral Salvia Officinalis, L. Honey. J. Food Compos. Anal. 2015, 44, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Buelga, C.S.; Battino, M. Phenolics from Monofloral Honeys Protect Human Erythrocyte Membranes Against Oxidative Damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Abd Hamid, H.; Khazaai, H. Malaysian Stingless Bee and Tualang Honeys: A Comparative Characterization of Total Antioxidant Capacity and Phenolic Profile Using Liquid Chromatography-Mass Spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A Comparative Study on Phenolic Profile, Vitamin C Content and Antioxidant Activity of Italian Honeys of Different Botanical Origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Arráez-Román, D.; Gómez-Caravaca, A.M.; Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of Phenolic Compounds in Rosemary Honey Using Solid-Phase Extraction by Capillary Electrophoresis–Electrospray Ionization-Mass Spectrometry. J. Pharm. Biomed. Anal. 2006, 41, 1648–1656. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Di Lorenzo, A.; Vista, S.; Tenore, G.C.; Daglia, M. Chemical Composition of Different Botanical Origin Honeys Produced by Sicilian Black Honeybees (Apis mellifera ssp. sicula). J. Agric. Food Chem. 2015, 63, 5864–5874. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical Properties, Antibacterial and Cellular Antioxidant Activities of Buckwheat Honey in Comparison to Manuka Honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef]
- Kečkeš, S.; Gašić, U.; Veličković, T.Ć.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. The Determination of Phenolic Profiles of Serbian Unifloral Honeys Using Ultra-High-Performance Liquid Chromatography/High Resolution Accurate Mass Spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Biesaga, M. Analysis of Phenolic Acids and Flavonoids in Honey. Trac. Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Zhao, J.; Du, X.; Cheng, N.; Chen, L.; Xue, X.; Wu, L.; Cao, W. Identification of Monofloral Honeys Using HPLC–ECD and Chemometrics. Food Chem. 2016, 194, 167–174. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Florez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the Phenolic and Flavonoid Fractions and Antioxidant Power of Italian Honeys of Different Botanical Origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Sun, C.; Tan, H.; Zhang, Y.; Zhang, H. Phenolics and Abscisic Acid Identified in Acacia Honey Comparing Different SPE Cartridges Coupled with HPLC-PDA. J. Food Compos. Anal. 2016, 53, 91–101. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Férez-Melgarejo, G.; Hernández-Córdoba, M. Dispersive Liquid–Liquid Microextraction for the Determination of Flavonoid Aglycone Compounds in Honey Using Liquid Chromatography with Diode Array Detection and Time-of-Flight Mass Spectrometry. Talanta 2015, 131, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Bioactive Components, Antioxidant and DNA Damage Inhibitory Activities of Honeys from Arid Regions. Food Chem. 2014, 153, 28–34. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of Phenolic Compounds in Honey Using Dispersive Liquid–Liquid Microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef]
- Petrus, K.; Schwartz, H.; Sontag, G. Analysis of Flavonoids in Honey by HPLC Coupled with Coulometric Electrode Array Detection and Electrospray Ionization Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 2555–2563. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, W.; Chen, W.J.; Xiao, X.H.; Zheng, J.B. Simultaneous determination of four phenolic components in citrus honey by high performance liquid chromatography using electrochemical detection. Food Chem. 2009, 114, 1537–1541. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Ahmed, Y.B.H.; Alothman, Z.A.; Obbed, M.S.; AL-Harbi, N.M.; AL-Turki, T.M. Ultra High Performance Liquid Chromatography with Mass Spectrometry Method for the Simultaneous Determination of Phenolic Constituents in Honey from Various Floral Sources Using Multiwalled Carbon Nanotubes as Extraction Sorbents. J. Sep. Sci. 2015, 38, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Wu, H.L.; Wang, J.Y.; Tu, D.Z.; Kang, C.; Zhao, J.; Chen, Y.; Miu, X.X.; Yu, R.Q. Fast HPLC-DAD Quantification of Nine Polyphenols in Honey by Using Second-Order Calibration Method Based on Trilinear Decomposition Algorithm. Food Chem. 2013, 138, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant Activity, Color Characteristics, Total Phenol Content and General HPLC Fingerprints of Six Polish Unifloral Honey Types. LWT Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Kuś, P.M.; Jerković, I.; Tuberoso, C.I.G.; Marijanović, Z.; Congiu, F. Cornflower (Centaurea cyanus L.) Honey Quality Parameters: Chromatographic Fingerprints, Chemical Biomarkers, Antioxidant Capacity and Others. Food Chem. 2014, 142, 12–18. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of Phenolic Acids in Honeys of Different Floral Origin by Solid-Pase Extraction and High-Performance Liquid Chromatography. Phytochem. Anal. 2007, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bobiș, O.; Mărghitaş, L.; Bonta, V.; Dezmirean, D.; Maghear, O. Free Phenolic Acids, Flavonoids and Abscisic Acid Related to HPLC Sugar Profile in Acacia Honey. Bul. USAMV CN 2007, 64, 179–185. [Google Scholar]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic Compounds and Methylglyoxal in Some New Zealand Manuka and Kanuka Honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Badjah Hadj Ahmed, A.Y.; Obbed, M.S.; Wabaidur, S.M.; AlOthman, Z.A.; Al-Shaalan, N.H. High-Performance Liquid Chromatography Analysis of Phenolic Acid, Flavonoid, and Phenol Contents in Various Natural Yemeni Honeys Using Multi-Walled Carbon Nanotubes as a Solid-Phase Extraction Adsorbent. J. Agric. Food Chem. 2014, 62, 5443–5450. [Google Scholar] [CrossRef]
- Oelschlaegel, S.; Pieper, L.; Staufenbiel, R.; Gruner, M.; Zeippert, L.; Pieper, B.; Koelling-Speer, I.; Speer, K. Floral markers of cornflower (Centaurea cyanus) honey and its peroxide antibacterial activity for an alternative treatment of digital dermatitis. J. Agric. Food Chem. 2012, 60, 11811–11820. [Google Scholar] [CrossRef]
- Jerković, I.; Tuberoso, C.; Kuś, P.M.; Marijanović, Z.; Kranjac, M. Screening of Coffea spp. honey by different methodologies: Theobromine and caffeine as chemical markers. RSC Adv. 2014, 4, 60557–60562. [Google Scholar] [CrossRef]
- Jerković, I.; Kuś, P.M.; Tuberoso, C.I.G.; Šarolić, M. Phytochemical and Physical–Chemical Analysis of Polish Willow (Salix spp.) Honey: Identification of the Marker Compounds. Food Chem. 2014, 145, 8–14. [Google Scholar] [CrossRef]
- Gambacorta, E.; Simonetti, A.; Garrisi, N.; Intaglietta, I.; Perna, A. Antioxidant Properties and Phenolic Content of Sulla (H edysarum spp.) Honeys from Southern Italy. Int. J. Food Sci. Technol. 2014, 49, 2260–2268. [Google Scholar] [CrossRef]
- Weston, R.J.; Mitchell, K.R.; Allen, K.L. Antibacterial Phenolic Components of New Zealand Manuka Honey. Food Chem. 1999, 64, 295–301. [Google Scholar] [CrossRef]
- Chan, C.W.; Deadman, B.J.; Manley-Harris, M.; Wilkins, A.L.; Alber, D.G.; Harry, E. Analysis of the Flavonoid Component of Bioactive New Zealand Mānuka (Leptospermum scoparium) Honey and the Isolation, Characterisation and Synthesis of an Unusual Pyrrole. Food Chem. 2013, 141, 1772–1781. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, S.; Devarajan, S. Estimation of Total Flavonoids, Phenols and Antioxidant Activity of Local and New Zealand Manuka Honey. J. Pharm. Res. 2011, 4, 464–466. [Google Scholar]
- Czipa, N. Különböző Eredetű Mézek Összehasonlító Vizsgálata És A Gyártmánykialakítás Hatása A Minőségre. [Comparative Study of Honeys with Different Origin, the Effect of Production-Forming on the Quality. Ph.D. Thesis, University of Debrecen, Debrecen, Hungary, 2010. [Google Scholar]
- National Hungarian Beekeeping Association. Mézvizsgálat II. Méhészújság 2020, 7, 20–23. [Google Scholar]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G. Physicochemical Characterization and Antioxidant Activity of Commercial Portuguese Honeys. J. Food Sci. 2013, 78, C1159–C1165. [Google Scholar] [CrossRef]
- Zhelyazkova, I.; Lazarov, S. Comparative Study of Rapeseed, Monofloral Types and Multifloral Honey by Some Physicochemical Parameters. Agric. Sci. Technol. 2017, 9, 277–281. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, Microbiological and Antimicrobial Properties of Commercial Honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkanska, R.; Ignatova, M. Physicochemical Parameters of Bulgarian Rape Honey (Brassica Sp.) and Coriander Honey (Coriandrum sativum L.). In Proceedings of the 10th International Symposium Modern Trends in Livestock Production, Belgrade, Serbia, 2–4 October 2013; pp. 618–626. [Google Scholar]
- Pires, J.; Estevinho, M.L.; Feás, X.; Cantalapiedra, J.; Iglesias, A. Pollen Spectrum and Physico-Chemical Attributes of Heather (Erica sp.) Honeys of North Portugal. J. Sci. Food Agric. 2009, 89, 1862–1870. [Google Scholar] [CrossRef] [Green Version]
- Anjos, O.; Iglesias, C.; Peres, F.; Martínez, J.; García, Á.; Taboada, J. Neural Networks Applied to Discriminate Botanical Origin of Honeys. Food Chem. 2015, 175, 128–136. [Google Scholar] [CrossRef]
- Pauliuc, D.; Oroian, M. Organic Acids and Physico-Chemical Parameters of Romanian Sunflower Honey. Food Environ. Saf. J. 2020, 19, 148–155. [Google Scholar]
- Eremia, N.; Neicovcena, I.; Grițunic, I. Physical and Chemical Indicators, Content of Micro and Macroelements and Heavy Metals in Sunflower Honey. Sci. Pap. Anim. Sci. Ser. 2019, 71, 105–108. [Google Scholar]
- Chua, L.S.; Adnan, N.A. Biochemical and Nutritional Components of Selected Honey Samples. Acta Sci. Pol. Technol. Aliment. 2014, 13, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Wanjai, C.; Sringarm, K.; Santasup, C.; Pak-Uthai, S.; Chantawannakul, P. Physicochemical and Microbiological Properties of Longan, Bitter Bush, Sunflower and Litchi Honeys Produced by Apis mellifera in Northern Thailand. J. Apic. Res. 2012, 51, 36–44. [Google Scholar] [CrossRef]
- Alpat, U. Comparison of Functional and Bioactive Properties of Pine Honey with Other Important Honeys Produced in Our Country. Ph.D. Thesis, Yıldız Technical University, İstanbul, Turkey, 2018. [Google Scholar]
- Çinar, S.B.; Ekşi, A.; Coşkun, İ. Carbon Isotope Ratio (13C/12C) of Pine Honey and Detection of HFCS Adulteration. Food Chem. 2014, 157, 10–13. [Google Scholar] [CrossRef]
- Özkök, A. Muğla Bölgesinde Üretilen Çam Balı ve Propolisin Mikroskobik, Organoleptik ve Kimyasal Analizi. Ph.D. Thesis, Hacettepe Üniversitesi, Ankara, Turkey, 2009. [Google Scholar]
- Tolon, B. Muğla ve Yöresi Çam Ballarının Biyokimyasal Özellikleri Üzerine Bir Araştırma. Ph.D Thesis, Ege Üniversitesi Fen Bilimleri Enstitüsü Doktora Tezi İzmir, Bornova, İzmir, 1999. [Google Scholar]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V. Bioactivity of Greek Honey Extracts on Breast Cancer (MCF-7), Prostate Cancer (PC-3) and Endometrial Cancer (Ishikawa) Cells: Profile Analysis of Extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Dinkov, D.H. Quality Parameters of Bulgarian Kinds of Bee Honey. Maced. Vet. Rev. 2014, 37, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Akgün, N. Ordu İlinde Üretilen Kestane Balı, Akasya Balı, Orman Gülü Balı ve Yayla Ballarının Fiziksel ve Kimyasal Aktiviteleri ile Antioksidan Özelliklerinin İncelenmesi; Yüksek Lisans; Fen Bilimleri Enstitüsü, Ordu Üniversitesi: Ordu, Turkey, 2017. [Google Scholar]
- Bobiş, O.; Mărghitaş, L.A.; Dezmirean, D.; Bonta, V.; Mihai, C.M. Beehive Products: Source of Nutrients and Natural Biologically Active Compounds. J. Agroalim. Proc. Technol. 2010, 16, 104–109. [Google Scholar]
- Zerrouk, S.; Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Characterization Of Ziziphus Lotus (Jujube) Honey Produced in Algeria. J. Apic. Res. 2017, 57, 166–174. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.K.; Kim, M.S.; Lee, S.H. Antioxidant and Antibacterial Properties of Hovenia (Hovenia Dulcis) Monofloral Honey Produced in South Korea. Food Sci. Anim. Resour. 2020, 40, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Maieves, H.A.; Züge, L.C.B.; Teixeira, G.L.; Cámara, M.; Ribani, R.H.; Sánchez-Mata, M.C. Chemical Properties, Rheological Behavior, and Melissopalynological Analysis of Selected Brazilian Honeys from Hovenia dulcis Flowering. Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- EU. Council Directive 2001/110/CE Concerning Honey. Off. J. Eur. Communities 2002, L10, 47–52. [Google Scholar]
- Codex Alimentarius Commission Standards. Revised Codex Standards for Honey; Codex Standard 12–1981, Rev. 2; Joint FAO/WHO Food Standards Programme. 24th Session; FAO Headquarters: Rome, Italy, 2001. [Google Scholar]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of pH and Electrical Conductivity Upon Honey Dilution as a Complementary Tool for Discriminating Geographical Origin of Honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez, E.R.; Romero, C.D. Physicochemical characteristics of minor monofloral honeys from Tenerife, Spain. LWT Food Sci. Technol. 2014, 55, 572–578. [Google Scholar] [CrossRef]
- Kabbani, D.; Sepulcre, F.; Wedekind, J. Ultrasound-Assisted Liquefaction of Rosemary Honey: Influence on Rheology and Crystal Content. J. Food Eng. 2011, 107, 173–178. [Google Scholar] [CrossRef]
- Önür, İ.; Misra, N.N.; Barba, F.J.; Putnik, P.; Lorenzo, J.M.; Gökmen, V.; Alpas, H. Effects of Ultrasound and High Pressure on Physicochemical Properties and HMF Formation in Turkish Honey Types. J. Food Eng. 2018, 219, 129–136. [Google Scholar] [CrossRef]
- Bartáková, K.; Dračková, M.; Borkovcová, I.; Vorlová, L. Impact of Microwave Heating on Hydroxymethylfurfural Content in Czech Honeys. Czech J. Food Sci. 2011, 29, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.; Diunase, K.N. Comparing the Antibacterial and Functional Properties of Cameroonian and Manuka Honeys for Potential Wound Healing—Have We Come Full Cycle in Dealing with Antibiotic Resistance? Molecules 2015, 20, 16068–16084. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial Activity of Manuka Honey and its Components: An Overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of Physicochemical and Antioxidant Properties of Sourwood and Other Malaysian Honeys: A Comparison with Manuka Honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beitlich, N.; Koelling-Speer, I.; Oelschlaegel, S.; Speer, K. Differentiation of Manuka Honey from Kanuka Honey and from Jelly Bush Honey Using HS-SPME-GC/MS and UHPLC-PDA-MS/MS. J. Agric. Food Chem. 2014, 62, 6435–6444. [Google Scholar] [CrossRef]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka Honey Dressings on the Surface pH of Chronic Wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.D.; Sancho, M.T.; Pascual-Maté, A. Composition and Properties of Apis mellifera Honey: A Review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- De Almeida-Muradian, L.B. Tetragonisca Angustula Pot-Honey Compared to Apis mellifera Honey from Brazil. Pot Honey 2013, 375–382. [Google Scholar] [CrossRef]
- Sabatini, A.G. Il Miele: Origine, Composizione E Proprieta; Sabatini, A.G., Botolotti, L., Marcazzan, G.L., Eds.; Conscere Il Miele; Avenue Media: Bologna, Italy; Milano, Italy, 2007; pp. 3–37. [Google Scholar]
- International Honey Commission. Harmonised Methods of the International Honey Comm. 2009. Available online: http://www.bee-hexagon.net/en/network.htm. (accessed on 1 May 2021).
- Doner, L.W. Honey. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P.M., Trugo, L.C., Eds.; Academic Press: London, UK, 2003; pp. 3125–3130. [Google Scholar]
- Alda-Garcilope, C.; Gallego-Pico, A.; Bravo-Yague, J.C.; Garcinuno-Martınez, R.M.; Fernandez-Hernando, P. Characterization of Spanish Honeys with Protected Designation of Origin “Miel de Granada” According to Their Mineral Content. Food Chem. 2012, 135, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, C.; Chang, Q.; Pang, G.; Hu, X.; Lu, M.; Wang, W. Chemometric Determination of the Botanical Origin for Chinese Honeys on the Basis of Mineral Elements Determined by ICP-MS. J. Agric. Food Chem. 2014, 62, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torres, R.; Perez-Bernal, J.L.; Bello-Lopez, M.A.; Callejon-Mochon, M.; Jimenez-Sanchez, J.C.; Guiraúm-Pérez, A. Mineral Content and Botanical Origin of Spanish Honeys. Talanta 2005, 65, 686–691. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and Geographical Discrimination of Greek Pine and Thyme Honeys Based on Their Mineral Content, Using Chemometrics. Eur. Food Res. Technol. 2017, 243, 101–113. [Google Scholar] [CrossRef]
- Louppis, A.P.; Karabagias, I.K.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C. Botanical Discrimination of Greek Unifloral Honeys Based on Mineral Content in Combination with Physicochemical Parameter Analysis, Using a Validated Chemometric Approach. Microchem. J. 2017, 135, 180–189. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Chowdhury, M.A.Z.; Rahman, M.A.; Sulaiman, S.A.; Gan, S.H. Determination of Mineral, Trace Element, and Pesticide Levels in Honey Samples Originating from Different Regions of Malaysia Compared to Manuka Honey. BioMed Res. Int. 2014, 2014, 359890. [Google Scholar] [CrossRef]
- Tudoreanu, L.; Codreanu, M.D.; Crivineanu, V.; Goran, G.V. The Quality of Romanian Honey Varieties-Mineral Content and Textural Properties. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Vet. Med. 2012, 69, 1–2. [Google Scholar]
- Atanassova, J.; Yurukova, L.; Lazarova, M. Pollen and inorganic characteristics of Bulgarian unifloral honeys. Czech J. Food Sci. 2012, 30, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Nalda, M.N.; Yagüe, J.B.; Calva, J.D.; Gómez, M.M. Classifying Honeys from the Soria Province of Spain Via Multivariate Analysis. Anal. Bioanal. Chem. 2005, 382, 311–319. [Google Scholar] [CrossRef]
- Di Bella, G.; Turco, V.L.; Potorti, A.G.; Bua, G.D.; Fede, M.R.; Dugo, G. Geographical Discrimination of Italian Honey by Multi-Element Analysis with a Chemometric Approach. J. Food Compos. Anal. 2015, 44, 25–35. [Google Scholar] [CrossRef]
- Atanassova, J.; Yurukova, L.; Lazarova, M. Palynological, Physical, and Chemical Data on Honey from the Kazanlak Region (Central Bulgaria). Phytol. Balc. 2009, 15, 107–114. [Google Scholar]
- Marghitas, L.A.; Dezmirean, D.S.; Pocol, C.B.; Marioara, I.L.E.A.; Bobis, O.; Gergen, I. The Development of a Biochemical Profile of Acacia Honey by Identifying Biochemical Determinants of its Quality. Not. Bot. Horti Agrobot. Cluj Napoca 2010, 38, 84–90. [Google Scholar]
- Popa, M.; Bostan, R.; Popa, D. Honey—Marker of Environmental Pollution. Case study—The Transylvania Region, Romania. J. Environ. Prot. Ecol. 2013, 14, 273–280. [Google Scholar]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Physicochemical Characterization and Antioxidant Activity of Palestinian Honey Samples. Food Sci. Nutr. 2018, 6, 2056–2065. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 15, 219–233. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Aliyu, M.; Odunola, O.A.; Farooq, A.D.; Mesaik, A.M.; Choudhary, M.I.; Fatima, B.; Qureshi, T.A.; Erukainure, O.L. Acacia Honey Modulates Cell Cycle Progression, Pro-Inflammatory Cytokines and Calcium Ions Secretion in PC-3 Cell Line. Cancer Sci. Ther. 2012, 4, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Aliyu, M.; Ibrahim, S.; Inuwa, H.M.; Sallau, A.B.; Abbas, O.; Aimola, I.A.; Habila, N.; Uche, N.S. Ameliorative Effects of Acacia Honey Against Sodium Arsenite-Induced Oxidative Stress in Some Viscera of Male Wistar Albino Rats. Biochem. Res. Int. 2013, 2013, 502438. [Google Scholar] [CrossRef] [Green Version]
- Salleh, M.A.M.; Eshak, Z.; Ismail, W.I.W. Acacia Honey Induces Apoptosis in Human Breast Adenocarcinoma Cell Lines (MCF-7). J. Teknologi. 2017, 79. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi-Aliabadi, H.; Hamzeh, J.; Mirian, M. Investigation of Astragalus honey and propolis extract’s cytotoxic effect on two human cancer cell lines and their oncogen and proapoptotic gene expression profiles. Adv. Biomed. Res. 2015, 4, 42. [Google Scholar] [CrossRef]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache Rugosa and its Correlation with Colour and Poly-Phenol Content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef] [Green Version]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Muzi, M.G.; Canini, A. Acacia Honey and Chrysin Reduce Proliferation of Melanoma Cells Through Alterations in Cell Cycle Progression. Int. J. Oncol. 2010, 37, 973–981. [Google Scholar] [PubMed] [Green Version]
- Van den Berg, A.J.J.; Van den Worm, E.; Quarles van Ufford, H.C.; Halkes, S.B.A.; Hoekstra, M.J.; Beukelman, C.J. An In Vitro Examination of the Antioxidant and Anti-Inflammatory Properties of Buckwheat Honey. J. Wound Care 2008, 17, 172–178. [Google Scholar] [CrossRef] [Green Version]
- García-Tenesaca, M.; Navarrete, E.S.; Iturralde, G.A.; Villacrés Granda, I.M.; Tejera, E.; Beltrán-Ayala, P.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Influence of Botanical Origin and Chemical Composition on the Protective Effect Against Oxidative Damage and the Capacity to Reduce in Vitro Bacterial Biofilms of Monofloral Honeys from the Andean Region of Ecuador. Int. J. Mol. Sci. 2018, 19, 45. [Google Scholar] [CrossRef] [Green Version]
- Dobre, I.; Escuredo, O.; Rodriguez-Flores, S.; Seijo, M.C. Evaluation of Several Romanian Honeys Based on Their Palynological and Biochemical Profiles. Int. J. Food Prop. 2014, 17, 1850–1860. [Google Scholar] [CrossRef]
- Sipahi, H.; Aydoğan, G.; Helvacioğlu, S.; Charehsaz, M.; Guzelmeric, E.; Aydin, A. Antioxidant, Antiinflammatory and Antimutagenic Activities of Various Kinds of Turkish Honey. FABAD J. Pharm. Sci. 2017, 42, 7–13. [Google Scholar]
- Seyhan, M.F.; Yılmaz, E.; Timirci-Kahraman, Ö.; Saygılı, N.; Kısakesen, H.İ.; Eronat, A.P.; Ceviz, A.B.; Gazioğlu, S.B.; Aydoğan, H.Y.; Öztürk, O. Anatolian Honey is not only Sweet But Can Also Protect from Breast Cancer: Elixir for Women from Artemis to Present. IUBMB Life 2017, 69, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Sahin, K. Honey Shows Potent Inhibitory Activity Against the Bovine Testes Hyaluronidase. J. Enzym. Inhib. Med. Chem. 2016, 31, 599–602. [Google Scholar] [CrossRef]
- Badria, F.; Fathy, H.; Fatehe, A.; Elimam, D.; Ghazy, M. Evaluate the Cytotoxic Activity of Honey, Propolis, and Bee Venom from Different Localities in Egypt Against Liver, Breast, and Colorectal Cancer. J Apither. 2017, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ciappini, M.C.; Stoppani, F.S. Determination of Antioxidant Capacity, Flavonoids, and Total Phenolic Content in Eucalyptus and Clover Honeys. J. Apic. Sci. 2014, 58, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Radonić, A.; Kranjac, M.; Zekić, M.; Marijanović, Z.; Gudićc, S.; Kliškić, M. Red clover (Trifolium pratense L.) Honey: Volatiles Chemical-Profiling and Unlocking Antioxidant and Anticorrosion Capacity. Chem. Pap. 2016, 70, 726–736. [Google Scholar] [CrossRef]
- Akbari, E.; Baigbabaei, A.; Shahidi, M. Determination of the Floral Origin of Honey Based on its Phenolic Profile and Physicochemical Properties Coupled with Chemometrics. Int. J. Food Prop. 2020, 23, 506–519. [Google Scholar] [CrossRef] [Green Version]
- Kıvrak, Ş.; Kıvrak, İ. Assessment of Phenolic Profile of Turkish Honeys. Int. J. Food Prop. 2017, 20, 864–876. [Google Scholar] [CrossRef] [Green Version]
- Aumeeruddy, M.Z.; Aumeeruddy-Elalfi, Z.; Neetoo, H.; Zengin, G.; van Staden, A.B.; Fibrich, B.; Lambrechts, I.A.; Rademan, S.; Szuman, K.M.; Lall, N.; et al. Pharmacological Activities, Chemical Profile, and Physicochemical Properties of Raw and Commercial Honey. Biocatal. Agric. Biotechnol. 2019, 18, 101005. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic Fingerprint, Antioxidant Activity, and Colour Characteristic of Polish Goldenrod (Solidago virgaurea L.) Honey and Flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Chen, X.; Liu, T.; Zhuo, Q.; Zhang, S.Q. Evaluation of Cellular Antioxidant Components of Honeys Using UPLC-MS/MS and HPLC-FLD Based on the Quantitative Composition-Activity Relationship. Food Chem. 2019, 293, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.K.; Halim, A.S.; Syazana, M.N.; Sirajudeen, K.N.S. Tualang Honey has Higher Phenolic Content and Greater Radical Scavenging Activity Compared with Other Honey Sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef]
- Batumalaie, K.; Qvist, R.; Yusof, K.M.; Ismail, I.S.; Sekaran, S.D. The Antioxidant Effect of the Malaysian Gelam Honey on Pancreatic Hamster Cells Cultured Under Hyperglycemic Conditions. Clin. Exp. Med. 2014, 14, 185–195. [Google Scholar] [CrossRef]
- Morales, P.; Haza, A.I. Antiproliferative and Apoptotic Effects of Spanish Honeys. Pharmacogn. Mag. 2013, 9, 231–237. [Google Scholar] [PubMed] [Green Version]
- Haza, A.I.; Morales, P. Spanish Honeys Protect Against Food Mutagen-Induced DNA Damage. J. Sci. Food Agric. 2013, 93, 2995–3000. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the Phenolic Content, Antioxidant Activity and Colour of Slovenian Honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Sharma, R.; Martins, N.; Chaudhary, A.; Garg, N.; Sharma, V.; Kuca, K.; Nepovimova, E.; Tuli, H.S.; Bishayee, A.; Chaudhary, A.; et al. Adjunct Use of Honey in Diabetes Mellitus: A Consensus or Conundrum? Trends Food Sci. Technol. 2020, 106, 254–274. [Google Scholar] [CrossRef]
- Fernandez-Cabezudo, M.J.; El-Kharrag, R.; Torab, F.; Bashir, G.; George, J.A.; El-Taji, H.; Al-Ramadi, B.K. Intravenous Administration of Manuka Honey Inhibits Tumor Growth and Improves Host Survival When Used in Combination with Chemotherapy in a Melanoma Mouse Model. PLoS ONE 2013, 8, e55993. [Google Scholar] [CrossRef] [Green Version]
- Kocyigit, A.; Aydogdu, G.; Balkan, E.; Yenigun, V.B.; Guler, E.M.; Bulut, H.; Koktasoğlu, F.; Gören, A.C.; Atayoglu, A.T. Quercus Pyrenaica Honeydew Honey with High Phenolic Contents Cause DNA Damage, Apoptosis, and Cell Death Through Generation of Reactive Oxygen Species in Gastric Adenocarcinoma Cells. Integr. Cancer Ther. 2019, 18, 1534735419876334. [Google Scholar] [CrossRef] [Green Version]
- Samat, S.; Salleh, M.A.M.; Adam, Z.; Ismail, W.W. Pineapple Honey Inhibits Adipocytes Proliferation and Reduces Lipid Droplet Accumulation in 3T3-L1 Adipocytes. Malays. Appl. Biol. 2019, 48, 21–26. [Google Scholar]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2018, 67, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Staver, M.M.; Ratkaj, I.; Broznić, D.; Jerković, I.; Marijanović, Z.; Željezić, D.; Pavelić, S.K. Bioactivity of Satureja Montana L. Honey Extracts and Their Profile Screening. RSC Adv. 2014, 4, 47329–47340. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: A comparison with Manuka honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Pistollato, F.; Zhang, J.; Pacetti, M.; Amici, A.; Reboredo-Rodriguez, P.; Simal-Gandara, J.; Bompadre, S.; et al. Strawberry Tree Honey as a New Potential Functional Food. Part 2: Strawberry Tree Honey Increases ROS Generation by Suppressing Nrf2-ARE and NF-κB Signaling Pathways and Decreases Metabolic Phenotypes and Metastatic Activity in Colon Cancer Cells. J. Funct. Foods 2019, 57, 477–487. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Maia, M.; Brigas, A.F. Physicochemical Parameters and Bioactive Compounds of Strawberry Tree (Arbutus unedo L.) Honey. J. Chem. 2015, 2015, 602792. [Google Scholar] [CrossRef] [Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.M.; Gurtu, S. Antioxidant Protection of Malaysian Tualang Honey in Pancreas of Normal and Streptozotocin-Induced Diabetic Rats. Ann. D Endocrinol. 2010, 71, 291–296. [Google Scholar] [CrossRef]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang Honey Induces Apoptosis and Disrupts the Mitochondrial Membrane Potential of Human Breast and Cervical Cancer Cell Lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative Effect of Tualang Honey on Oral Squamous Cell Carcinoma and Osteosarcoma Cell Lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Idris, Y.M.A.; Mariod, A.A.; Hamad, S.I. Physicochemical Properties, Phenolic Contents and Antioxidant Activity of Sudanese Honey. Int. J. Food Prop. 2011, 14, 450–458. [Google Scholar] [CrossRef]
- Suleiman, M.H.; ALaerjani, W.M.A.; Mohammed, M.E.A. Influence of Altitudinal Variation on the Total Phenolic and Flavonoid Content of Acacia and Ziziphus Honey. Int. J. Food Prop. 2020, 23, 2077–2086. [Google Scholar] [CrossRef]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dessì, M.A. Antioxidant Profile of Strawberry Tree Honey and its Marker Homogentisic Acid in Several Models of Oxidative Stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; He, J.; Chen, L.; Zhang, J.; Jin, Y.; Zhou, J.; Zhang, Y. A Green Triple-Locked Strategy Based on Volatile-Compound Imaging, Chemometrics, and Markers to Discriminate Winter Honey and Sapium Honey Using Headspace Gas Chromatography-Ion Mobility Spectrometry. Food Res. Int. 2019, 119, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic Profile and Antioxidant Properties of Polish Honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Rodriguez, B.A.; Mendoza, S.; Iturriga, M.H.; Castaño-Tostado, E. Quality Parameters and Antioxidant and Antibacterial Properties of Some Mexican Honeys. J. Food Sci. 2012, 77, C121–C127. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Battino, M. Antioxidant Characterization of Native Monofloral Cuban Honeys. J. Agric. Food Chem. 2010, 58, 9817–9824. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and Antimicrobial Capacity of Several Monofloral Cuban Honeys and Their Correlation with Color, Polyphenol Content and Other Chemical Compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Demirhan, A. Balın Halk Tedavilerindeki Yeri ve Modern Tıp Açısından Önemi. Dirim 1981, 96, 364–367. [Google Scholar]
- Üçer, M. İbni Sina’nın Kanun fi’t-tıbb’ında Bal ve Kına ile Yapılan Ilaçlar Üzerine Etkileri; Bildiriler Kitapçığı, Uluslararası İbni Sina Sempozyumu: Ankara, Turkey, 1984; pp. 323–331. [Google Scholar]
- Yücel, B.; Akçiçek, E. Balın Modern Tıpta Kullanımı. Hasad Gıda 2005, 244, 22–25. [Google Scholar]
- Šedík, P.; Horská, E.; Adam, Š.; Miškeje, M. Mineral Content as an Aspect of Nutrition Marketing: Case Study of Honey Market in Slovakia. J. Food Nutr. Res. 2020, 59, 185–192. [Google Scholar]
- Omotayo, E.O.; Gurtu, S.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, M.S.M. Hypoglycemic and Antioxidant Effects of Honey Supplementation in Streptozotocin-Induced Diabetic Rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74–82. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, S.M.; Gurtu, S. Antioxidant Protective Effect of Glibenclamide and Metformin in Combination with Honey in Pancreas of Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2010, 11, 2056–2066. [Google Scholar] [CrossRef] [Green Version]
- Kadir, E.A.; Sulaiman, S.A.; Yahya, N.K.; Othman, N.H. Inhibitory Effects of Tualang Honey on Experimental Breast Cancer in Rats: A Preliminary Study. Asian Pac. J. Cancer Prev. 2013, 14, 2249–2254. [Google Scholar] [CrossRef] [Green Version]
- Sibel, S.; Enis, Y.M.; Hüseyin, S.; Timucin, A.A.; Duran, O. Analysis of Grayanatoxin in Rhododendron Honey and Effect on Antioxidant Parameters in Rats. J. Ethnopharmacol. 2014, 156, 155–161. [Google Scholar] [CrossRef]
- Eraslan, G.; Kanbur, M.; Silici, S.; Karabacak, M. Beneficial Effect of Pine Honey on Trichlorfon Induced Some Biochemical Alterations in Mice. Ecotoxicol. Environ. Saf. 2010, 73, 1084–1091. [Google Scholar] [CrossRef]
- Bezerra, M.L.R.; De Souza, E.L.; de Sousa, J.M.B.; Lima, M.D.S.; Alves, A.F.; Almeida, M.D.G.; Alves, R.C.; de Araújo, E.V.; Soares, N.L.; Da Silva, G.A.; et al. Effects of Honey from Mimosa quadrivalvisl (malícia) Produced by the Melipona Subnitidad. (jandaíra) Stingless Bee on Dyslipidaemic Rats. Food Funct. 2018, 9, 4480–4492. [Google Scholar] [CrossRef] [PubMed]
- Mooty, M.A.; Nisr, N.A.E.; Wahba, N.M.; Complement, I. Influence of Intravenous Egyptian Fennel Honey Infusion on the Antioxidant Activities and Some Haemo-Indices In Healthy Goats. Int. J. Complement. Altern. Med. 2018, 11, 281–286. [Google Scholar]
- Nasuti, C.; Gabbianelli, R.; Falcioni, G.; Cantalamessa, F. Antioxidative and Gastroprotective Activities of Anti-Inflammatory Formulations Derived from Chestnut Honey in Rats. Nutr. Res. 2006, 26, 130–137. [Google Scholar] [CrossRef]
- Nemoseck, T.M.; Carmody, E.G.; Furchner-Evanson, A.; Gleason, M.; Li, A.; Potter, H.; Lauren, M.R.; Kelly, J.L.; Kern, M. Honey Promotes Lower Weight Gain, Adiposity, and Triglycerides Than Sucrose in Rats. Nutr. Res. 2011, 31, 55–60. [Google Scholar] [CrossRef]
- Samat, S.; Kanyan Enchang, F.; Nor Hussein, F.; Wan Ismail, W.I. Four-Week Consumption of Malaysian Honey Reduces Excess Weight Gain and Improves Obesity-Related Parameters in High Fat Diet Induced Obese Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 1342150. [Google Scholar] [CrossRef]
- Chepulis, L.; Starkey, N. The Long-Term Effects of Feeding Honey Compared with Sucrose and a Sugar-Free Diet on Weight Gain, Lipid Profiles, and DEXA Measurements in Rats. J. Food Sci. 2007, 73, H1–H7. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Tanvir, E.M.; Afroz, R.; Sulaiman, S.A.; Gan, S.H. Cardioprotective Effects of Tualang Honey: Amelioration of Cholesterol and Cardiac Enzymes Levels. BioMed Res. Int. 2015, 2015, 286051. [Google Scholar] [CrossRef] [Green Version]
- Samat, S.; Enchang, F.K.; Abd Razak, A.; Hussein, F.N.; Ismail, W.I.W. Adulterated Honey Consumption Can Induce Obesity, Increase Blood Glucose Level and Demonstrate Toxicity Effects. Sains Malays. 2018, 47, 353–365. [Google Scholar]
- Öztaşan, N.; Altinkaynak, K.; Akçay, F.; Göçer, F.; Dane, Ş. Effects of Mad Honey on Blood Glucose and Lipid Levels in Rats with Streptozocin-Induced Diabetes. Turk. J. Vet. Anim. Sci. 2005, 29, 1093–1096. [Google Scholar]
- Abdulrhman, M.A. Honey as a sole treatment of type 2 diabetes mellitus. Endocrinol. Metab. Syndr. 2016, 5, 1000232. [Google Scholar] [CrossRef]
- Whitfield, P.; Parry-Strong, A.; Walsh, E.; Weatherall, M.; Krebs, J.D. The Effect of a Cinnamon-, Chromium-and Magnesium-Formulated Honey on Glycaemic Control, Weight Loss and Lipid Parameters in Type 2 Diabetes: An Open-Label Cross-Over Randomised Controlled Trial. Eur. J. Nutr. 2016, 55, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M. Honey Therapy in a Patient Volunteer with Type 2 Diabetes Mellitus: Case Report. J. Clin. Trials 2013, 3, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Abdulrhman, M.M.; El-Hefnawy, M.H.; Aly, R.H.; Shatla, R.H.; Mamdouh, R.; Mahmoud, D.M.; Mohamed, W.S. Metabolic Effects of Honey in Type 1 Diabetes Mellitus: A Randomized Crossover Pilot Study. J. Med. Food. 2013, 16, 66–72. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abdel-Rahman, E.H.; Abd-Allah, F.; Abdou, A.M. Influence of Honey on Immune Status in Mice-Bearing Ehrlich Carcinoma. J. Clin. Cell. Immunol. 2015, 6, 1000295. [Google Scholar]
- Mohamed, Z.B.H.; Alfarisi, H.A.H.; Abdullah, N.Z.; Harun, N.; Muhammad, N.; Rahim, R.A. Renoprotective Role of Tualang Honey Against High Cholesterol Diet Induced Acute Kidney Diseases in an Animal Model. J. Appl. Pharm. Sci. 2017, 7, 97–101. [Google Scholar]
- Cheng, N.; Wu, L.; Zheng, J.; Cao, W. Buckwheat Honey Attenuates Carbon Tetrachloride-Induced Liver and DNA Damage in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 987385. [Google Scholar] [CrossRef] [Green Version]
- Mansouri-Tehrani, H.A.; Rabbani-Khorasgani, M.; Hosseini, S.M.; Mokarian, F.; Mahdavi, H.; Roayaei, M. Effect of Supplements: Probiotics and Probiotic Plus Honey on Blood Cell Counts and Serum IgA in Patients Receiving Pelvic Radiotherapy. J. Res. Med Sci. 2015, 20, 679–683. [Google Scholar]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A. Defining the Standards for Medical Grade Honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Scepankova, H.; Saraiva, J.A.; Estevinho, L.M. Honey Health Benefits and Uses in Medicine. Bee Prod. Chem. Biol. Prop. 2017, 83–96. [Google Scholar] [CrossRef]
- Molan, P. Why Honey Effective as a Medicine? Its Use in Modern Medicine. Bee World 1999, 80, 80–92. [Google Scholar] [CrossRef]
- Söğüt, Ö.; Sayhan, M.B.; Mordeniz, C.; Gökdemir, M.T.; Al, B. Deli Bal Zehirlenmesi: Olgu Sunumu Ve Literatürün Gözden Geçirilmesi. Anatol. J. Clin. Investig. 2009, 3, 100–102. [Google Scholar]
- Stangaciu, S. Internet Apitherapy Course Notes. (Lesson 25: Honey Composition; Lesson: 46: Honey indications). Available online: http://www.apitherapy.com (accessed on 28 January 2021).
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in Oral Health and Care: A Mini Review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Compton, B.J.; Marshall, A.; Anderson, R.; Li, Y.; van der Woude, H.; Hermans, I.F.; Painter, F.G.; Gasser, O. Mānuka Honey-Derived Methylglyoxal Enhances Microbial Sensing by Mucosal-Associated Invariant T Cells. Food Func. 2020, 11, 5782–5787. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.N.; Khaledi, E.M. Antibacterial Activity and Mechanism of Action of Some Iranian Honeys Compared to Manuka Honey Against Multidrug-Resistant Respiratory and Urinary Infections. Food Biosci. 2021, 41, 101003. [Google Scholar] [CrossRef]
- Majtan, J.; Majtan, V. Is Manuka Honey the Best Type of Honey for Wound Care? J. Hosp. Infect. 2010, 74, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K. Growth Inhibition by Caffeic Acid, One of the Phenolic Constituents of Honey, in HCT 15 Colon Cancer Cells. Sci. World J. 2012, 2012, 372345. [Google Scholar] [CrossRef] [Green Version]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz Baiomy, A.A.; Rady, H.M.; Amer, M.A.; Kiwan, H.S. Effect of Some Honey Bee Extracts on the Proliferation, Proteolytic and Gelatinolytic Activities of the Hepatocellular Carcinoma Hepg2 Cell Line. Aust. J. Basic Appl. Sci. 2009, 3, 2754–2769. [Google Scholar]
- Bogdanov, S. Honey as Nutrient and Functional Food. Honey; Bee Product Science: 2017; Chapter 8. Available online: www.bee-hexagon.net (accessed on 13 February 2021).
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Asokan, M.K.; Subramanian, A.P.; John, A.A.; Supriyanto, E.; Razak, S.I.; Marvibaigi, M. A Review on Antiproliferative and Apoptotic Activities of Natural Honey. Anticancer Agents Med. Chem. 2015, 15, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Waheeda, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and Cancer: A Mechanistic Review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.K.; Rao, S.; Rao, P.; Pais, M.; Adnan, M.; Pai, K.S.; Baliga, M.S. Evidence for Anticancer Properties of Honey with Emphasis on Mechanistic Overview. Funct. Foods Cancer Prev. Ther. 2020, 121–135. [Google Scholar] [CrossRef]
| Geographical Origin/Provenance | Monofloral Honey Taxa | Reference | ||
|---|---|---|---|---|
| Family | Specie | Common Name | ||
| Turkey | Apiaceae | Pimpinella sp. | Anise | [22,23,24,25,26,27,28,29,30,31] |
| Asteraceae | Centaurea solstitialis L. | Yellow star-thistle | ||
| Centaurea cyanus L. | Cornflower or bachelor’s button | |||
| Helianthus annuus L | Common sunflower | |||
| Taraxacum farinosum Hausskn. & Bornm. Ex Hand.-Mazz. | Turkish cırtlık | |||
| Brassicaceae | Brasssica sp. | Canola | ||
| Ericaceae | Calluna vulgaris (L.) Hull | Common heather | ||
| Rhododendron sp. | Rhododendron | |||
| Fabaceae | Astragalus microcephalus Willd | Milkvetch | ||
| Robinia pseudoacacia L. | Black locust | |||
| Trifolium sp. | Clover | |||
| Vicia cracca L. | Tufted vetch or blue vetch | |||
| Fagaceae | Castanea sativa Mill. | Chestnut | ||
| Quercus cerris L. | Turkey oak or Austrian oak | |||
| Lamiaceae | Thymus sp. | Thyme | ||
| Vitex agnus-castus L. | Vitex or chaste tree | |||
| Lavandula stoechas L. | Spanish lavender or topped lavender | |||
| Lavandula pedunculata Mill. | French lavender | |||
| Malvaceae | Gossypium barbadense L. | Sea island cotton | ||
| Gossypium hirsutum L., | Upland cotton or Mexican cotton | |||
| Tilia tomentosa Moensch | Silver linden | |||
| Pinaceae | Cedrus sp. | Cedar | ||
| Pinus brutia Ten. | Turkish pine | |||
| Rhamnaceae | Paliurus spina-christi Mill. | Jerusalem thorn | ||
| Rosaceae | Prunuscerasus L. | Sour cherry | ||
| Rutaceae | Citrus sp. | Citrus | ||
| Bulgaria | Apiaceae | Coriandrum sativum L., Daucus sp. | [32,33,34,35] | |
| Asteraceae | Carduus nutans L. | Musk thistle or nodding thistle | ||
| Centaurea cyanus L. | ||||
| Helianthus annuus L, | ||||
| Onopordum acanthium L. | Cotton thistle | |||
| Brassicaceae | B. napus | |||
| Fabaceae | R. pseudoacacia, Styphnolobium japonicum (L.) Schott | Japanese pagoda tree | ||
| Vicia sp. | ||||
| Honeydew honey | Forest honey | |||
| Polygonaceae | Fagopyrum esculentum Moench | Common buckwheat | ||
| Rosaceae | Prunus sp. | |||
| Salicaceae | Salix sp. | |||
| Saxifragaceae | Saxifraga adscendens L. | Wedgeleaf saxifrage | ||
| Tiliaceae | T. cordata | |||
| Romania | Asteraceae | H. annuus, Taraxacum officinale (L.) Weber ex F.H.Wigg. | Common dandelion | [36] |
| Betulaceae | Corylus avellana L. | Common hazel | ||
| Brassicaceae | Brassica napus L. | Canola | ||
| Cornaceae | Cornus mas L. | European cornel or Cornelian cherry | ||
| Fabaceae | R. pseudoacacia | |||
| Fagaceae | C. sativa | |||
| Lamiaceae | Thymus serpyllum L. | Breckland thyme or wild thyme | ||
| Rosaceae | Rubus idaeus L. | European red raspberry | ||
| Malus domestica Borkh. | ||||
| Malus floribunda Siebold ex Van Houtte | Japanese crabapple or purple chokeberry | |||
| Prunus padus L. | Bird cherry | |||
| Salicaceae | Salix caprea L. | Goat willow | ||
| Tiliaceae | Tilia platyphyllos Scop. | Large-leaved linden | ||
| Tilia cordata Mill. | Small-leaved lime or little-leaf linden | |||
| Ukraine | Asteraceae | H. annuus | [37,38] | |
| Brassicaceae | B.napus | |||
| Fabaceae | R. pseudoacacia | |||
| Polygonaceae | Fagopyrum esculentum Moench. | |||
| Tiliaceae | Tilia platyphyllos Scop. | Large-leaved lime or large-leaved linden | ||
| Italy | Apiaceae | Coriandrum sativum L. | Chinese parsley or cilantro | [39,40,41,42,43] |
| Asteraceae | Dittrichia viscosa (L.) Greuter | False yellowhead | ||
| H.annuus | ||||
| Ericaceae | Erica arborea L. | Tree heather | ||
| Erica scoparia L. | ||||
| Arbutus unedo L. | Arbutus or strawberry tree | |||
| Rhododendron ferrugineum L. | Snow-rose or rusty-leaved alpenrose | |||
| Fabaceae | Trifolium pratense L. | Red clover | ||
| Hedysarum coronarium L. | French honeysuckle or sulla | |||
| Medicago sativa L. | Alfalfa | |||
| Fagaceae | C. sativa | |||
| Honeydew honey | Abies alba Mill. and/or Picea abies L. | Fir honeydew | ||
| Forest Honeydew, Insect: Metcalfa pruinosa Say | Forest honeydew | |||
| Myrtaceae | Eucalyptus camaldulensis Dehnh. | River red gum | ||
| Rhamnaceae | Paliurus spina-christi Mill. | Marruca | ||
| Rutaceae | Citrus sp. | |||
| Tiliaceae | R. pseudoacacia L. | |||
| T. cordata | ||||
| Xanthorrhoeaceae | Asphodelus microcarpus Salzm. et Viv. | Asphodel | ||
| Portugal | Aamaryllidaceae | Narcissus triandrus L. (Angel’s tears) | [44,45,46] | |
| Ericaceae | Arbutus unedo L., | |||
| Erica sp. | Heather | |||
| Erica umbellata L. | Dwarf Spanish heather | |||
| Rhododendron ponticum L. | Common rhododendron | |||
| Fabaceae | Cytisus scoparius L. (Link) | Common broom or Scotch broom | ||
| Ceratonia siliqua L | Carob | |||
| Fagaceae | Fagus sylvatica L. | European beech | ||
| Lamiaceae | Thymus sp. (thyme), | |||
| Lavandula stoechas L. | Spanish lavender or topped lavender | |||
| Lavandula latifolia Medik. | Portuguese lavender or broadleaved lavender | |||
| Myrtaceae | Eucalyptus globulus Labill. | Southern blue gum | ||
| Rhamnaceae | Frangula azorica Grubov | Azorean buckthorn | ||
| Rosaceae | Prunus lusitanica L. | Portugal laurel | ||
| Rutaceae | Citrus sinensis (L.) Osbeck | Common sweet orange | ||
| Santalaceae | Viscum cruciatum Sieber ex Boiss. | Red-berry mistletoe | ||
| Hungary | Amaryllidaceae | Allium ursinum L. | Wild garlic | [47,48,49] |
| Apocynaceae | Asclepias sp. | Milkweeds | ||
| Apiaceae | Coriandrum sativum L., | |||
| Daucus spp. | ||||
| Asteraceae | H. annuus | |||
| Solidago sp. | ||||
| Boraginaceae | Phacelia tanacetifolia Benth | Lacy phacelia, blue tansy or purple tansy | ||
| Brassicaceae | B. napus | |||
| Honeydew honey | Forest Honeydew | |||
| Fabaceae | Vicia sp. | |||
| Fagaceae | C. sativa | |||
| Rosaceae | Crataegus sp. | Hawthorn | ||
| Tiliaceae | R. pseudoacacia | |||
| T. cordata | ||||
| Morocco | Euphorbiaceae | Euphorbia resinifera O. Berg. | Resin spurge | [50,51,52] |
| Fabaceae | Acacia raddiana Savi | Twisted acacia | ||
| Lamiaceae | Lavandula sp.; Thymus sp. | |||
| Lamiaceae | Rosmarinus officinalis L. | Rosemary | ||
| Leguminosae | Ceratonia siliqua L. | Carob | ||
| Myrtaceae | Eucalyptus sp. | |||
| Nitrariaceae | Peganum harmala L. | Harmal or wild rue | ||
| Resedaceae | Reseda villosa Coss. | Mignonette | ||
| Rhamnaceae | Zizipus jujuba Mill | Jujube or Chinese date | ||
| Rutaceae | Citrus reticulate Blanco | Mandarin orange | ||
| Malaysia | Fabaceae | Koompassia excelsa (Becc.) Taub | Tualang | [53,54] |
| Acacia sp. | ||||
| Myrtacaeae | Melaleuca cajuputi Powell | Gelam | ||
| New Zealand | Asteraceae | Carduus nutans L. | Musk thistle | [55,56,57,58] |
| Boraginaceae | Echium vulgare L. | Viper’s bugloss or blueweed | ||
| Cunoniaceae | Weinmannia racemosa L.f. | Kāmahi | ||
| Fabaceae | Trifolium sp. | |||
| Lamiaceae | Thymus vulgaris L. | German thyme | ||
| Myrtaceae | Leptospermum scoparium J.R.Forst. & G.Forst. | Manuka or New Zealand teatree | ||
| Metrosideros umbellate Cav. | Southern rata | |||
| Proteaceae | Knightia excelsa R. Br. | Rewarewa | ||
| Strasburgeriaceae | Ixerba brexioides A.Cunn. | Tawari and Whakou when in flower | ||
| Phenolic Compounds | Honey Type | Extraction Technique/Extraction Solvent | Spectral Analysis | Referencces |
|---|---|---|---|---|
| Flavonols | ||||
| Quercetin | Heather, Lavender; Jelly bush (Leptospermum polygalifolium), manuka; Clover, Thyme; Liven vine; Christmas vine; Chestnut, Eucalyptus, Citrus, Sulla; Sesame, Coriander, sunflower, Savory, Sage | MeOH; MeCN | RP-HPLC; HPLC-PDA; HPLC-UV; UHPLC-DAD MS/MS | [62,63,64,65] |
| Quercetin-O-rhamnoside | Liven vine | MeOH | HPLC-DAD-ESI-MS/MS | [66] |
| Quercetin 3-orutinoside (rutin) | Kelulut, Tualang; Chestnut, Eucalyptus, Citrus, Sulla, Sesame | MeOH/water 1:1; MeCN | LC-ESI-MS/MS; HPLC-UV | [64,67,68] |
| Quercetin 3′,3′-dimethyl eter | Rosemary | MeOH:water (1:1) | CE-ESI-MS | [69] |
| Quercetin 7,3′-dimethyl eter | Rosemary | MeOH:water (1:1) | CE-ESI-MS | [69] |
| Quercetin rhamnosyl-hexosyl-rhamnoside | Sulla, Dill, Lemon, Orange | MeOH | HPLC-DAD-MS | [70] |
| Apigenin | Buckwheat, manuka, tualang, chaste, strawberry tree; Sesamum indicum, jujube, longan, Black locust, sunflower, linden, basil, goldenrod, sulla, Citrus, Ziziphus Spina-Christi, Kelulut, Tualang; Sesamum indicum; Black locust, sunflower, linden, sulla, thistle, basil, buckwheat, rapeseed and goldenrod; Ziziphus Spina-Christi; ailanthus, savory; Jujube, longan and chaste | MeOH; MeOH/water 1:1 | HPLV-UV; LC-ESI-MS/MS | [64,67,71,72,73,74] |
| Kaempferol | Buckwheat, manuka, Liven vine; Christmas vine; Rosemary; Tualang, manuka, Black locust, chestnut, savory, sulla, ailanthus, thymus and orange | MeOH; MeOH:water (1:1) | HPLC-DAD-ESI-MS/MS; CE–ESI-MS; LC-ESI-MS/MS | [66,67] |
| Kaempferid | Rosemary | MeOH:water (1:1) | CE–ESI-MS | [69] |
| Isorhamnetin | Liven vine; Christmas vine, manuka; | MeOH | HPLC-DAD-ESI-MS/MS | [66,75] |
| 8-methoxykaempferol | Liven vine; Christmas vine | MeOH | HPLC-DAD-ESI-MS/MS | [66] |
| Galangin | Buckwheat, manuka, black locust, chestnut, savory, sulla, ailanthus, thymus; sage | MeOH; 40/50/10 (v/v/v) water/TFA/MeCN | HPLV-UV; UHPLC-DAD MS/MS | [65,71,76] |
| Genistein | Acacia, Thymus, Black locust, chestnut, savory, sulla, ailanthus, thymus and orange | MeOH; 40/50/10 (v/v/v) water/TFA/MeCN | HPLC-PDA; HPLC-UV | [75,76,77] |
| Myricetin | Rosemary; Chestnut, eucalyptus, citrus and sulla; Sesamum indicum, black locust, thistle, Lavender, orange blossom, heather; ailanthus, Buckwheat | MeOH:water (1:1); MeOH; MeCN | CE–ESI-MS; HPLC-UV | [64,68,69,73,78] |
| Methyl anthranilate | Citrus | MeOH | GC–MS | [43] |
| Flavanols | ||||
| Catechin | Kelulut, Tualang; Chestnut, eucalyptus, citrus and sulla; sage; Jujube, longan and chaste; Tualang, pine | MeOH/water 1:1 | LC-ESI-MS/MS; HPLC-UV | [67,68,74,75] |
| Epicatechin | Acacia, Chestnut, eucalyptus, citrus and sulla, manuka, Ziziphus Spina-Christi | MeOH | HPLC-UV | [68,79] |
| Flavanonols | ||||
| Pinobanksin | Black locust, Rosemary, manuka, Sulla, Thistle, Citrus, Eucalyptus, Sage; Dill; Jujube, Longan and Chaste | MeOH:water (1:1); MeOH; MeCN; | CE–ESI-MS; HPLC-PDA; UHPLC–UV; UHPLC-DAD MS/MS | [65,69,70,77,80] |
| Pinocembrin | Rosemary, Sage; Dill; Jujube, Longan And Chaste; Acacia, Sunflower, Linden, Basil, Citrus, Buckwheat, Goldenrod, Black Locust, Sulla, Thistle | MeOH:water (1:1); MeCN | CE–ESI-MS; UHPLC-DAD MS/MS | [65,69,70,74] |
| Pinostrobin | Sage | MeCN | UHPLC-DAD MS/MS | [65] |
| Flavones | ||||
| Chrysin | Rosemary; Kelulut, Tualang; Buckwheat, Manuka; Sulla, Thistle, Black locust, Citrus; Lavender, Eucalyptus, Thyme, Chestnut; Sage; Sunflower, Linden, Basil, Buckwheat; Ziziphus spina-christi | MeOH/water (1:1); MeOH; MeCN; 40/50/10 (v/v/v) water/TFA/MeCN | CE–ESI-MS; LC-ESI-MS/MS; UHPLC–UV; HPLC-DAD-TOF-MS | [65,67,69,71,72,76,80] |
| Acacetin | Acacia | MeOH | HPLV-UV | [77] |
| Luteolin | Black locust, Sulla, Thistle, Citrus, Manuka, Tualang, Sunflower; Rhododendron, Rosemary; Raspberry, Orange, Cherry Blossom, Dandelion, Melon, Lavender, Sage, Rapeseed, Sunflower, Linden, Basil, Buckwheat, Thyme, Pine, Sage | MeOH; MeOH/water (20:80,v:v) | UHPLC–UV; HPLC-CEAD HPLC-ESI-MS | [72,80,81] |
| Baicalein | Lavender, Orange Blossom, Rosemary, Heather, Eucalyptus, Chestnut and Thyme | MeCN | HPLC-DAD-TOF-MS | [78] |
| Flavanones | ||||
| Hesperetin | Citrus; Lavender, Orange Blossom, Rosemary, Heather, Eucalyptus, Chestnut;Thyme; Sage, Sulla, Thistle, Rhododendron; Phacelia, Pumpkin, Raspberry | MeOH; MeCN | HPLC-ECD; HPLC-DAD-TOF-MS; UHPLC-DAD MS/MS | [65,78,81,82] |
| Naringenin | Lavender, Orange Blossom, Rosemary, Heather, Eucalyptus, Chestnut and Thyme; Ziziphus Spina-Christi | MeCN | HPLC-DAD-TOF-MS | [78,83] |
| Eriodictyol | Sunflower | MeCN | UHPLC-HESI-MS | [72] |
| Dihydroflavonols | ||||
| 5-methoxy pinobanksin | Acacia, Black locust | MeOH | HPLC-PDA | [77] |
| Phenolic acids | ||||
| P-Hydroxybenzoic acid | Acacia, Clover, Heather, Manuka, Buckwheat; Wild Chrysanthemum, Milk Vetch, Jujube, Sage; Sulla, Dill; Black locust, Rapeseed, Lime, Goldenrod, Heather, Buckwheat; Cornflower | MeOH MeCN | HPLV-UV; HPLC-DAD; HPLC–ECD-DAD | [65,71,77,84,85,86] |
| Vanillic acid | Black locust, Heather, Liven vine; Christmas vine; Rapeseed, Lime, Heather, Cornflower, Buckwheat, Black Locust | MeOH | HPLC-DAD-ESI-MS/MS; HPLC–ECD-DAD | [66,77,85,86] |
| Phenylacetic acid | Sweet chestnut; Sage; Chestnut, Eucalyptus, Sulla; Black Locust, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | GC–MS; HPLC-UV | [79,87] |
| L-β-phenyllactic acid | Chestnut, Eucalyptus, Sulla; Black Locust, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | HPLC-UV | [87] |
| Dl-p-hydroxy-phenyllactic acid | Chestnut, Eucalyptus, Sulla; Black Locust, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | HPLC-UV | [88] |
| Gentisic acid | Sage | MeCN | UHPLC-DAD MS/MS | [65] |
| Rosmarinic acid | Sage, Rapeseed, Lime, Heather, Cornflower, Buckwheat and Black Locust | MeCN; MeOH | UHPLC-DAD MS/MS; HPLC–ECD-DAD | [65,85,86] |
| Phenyllactic acid | Heather, Thistle, Manuka, Cornflower | MeCN | HPLC–DAD | [79] |
| Lumichrome | Cornflower | MeCN | HPLC–DAD | [79] |
| Hydroxycinnamic acid | ||||
| Caffeic acid | Black locust, Liven vine; Christmas vine, Buckweat, Manuka, Citrus; Acacia, Milk vetch, Wild Chrysanthemum, Jujube flower; Chestnut, Eucalyptus, Sulla; Coriandrum, Gelam, Pine, Rapeseed, Lime, Heather, Cornflower, Buckwheat And Black locust | MeOH/water 1:1; MeOH 43%:HCOOH (57%, v/v) | HPLC-DAD-ESI-MS/MS; HPLC-ECD; HPLC-DAD; HPLC-UV | [66,68,82,84,85,86] |
| Caffeic acid phenethyl ester | Kelulut | MeOH/water 1:1 | LC-ESI-MS/MS | [67] |
| P-Coumaric acid | Liven vine; Christmas vine; Tualang, kelulut; Citrus; Acacia, Milk vetch, Wild chrysanthemum, Jujube flower; Chestnut, Eucalyptus, Citrus, Sulla, Mint, Thymus | MeOH; MeOH/water 1:1; MeOH 43%:HCOOH (57%, v/v) | HPLC-DAD-ESI-MS/MS; HPLC-ECD; HPLC-DAD; HPLC-UV | [66,68,82,84] |
| O-coumaric acid | Chestnut, Eucalyptus, Sulla; Black locust, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | HPLC-UV | [87] |
| M-coumaric acid | Chestnut, Eucalyptus, Sulla; Black Locust, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | HPLC-UV | [87] |
| Ferulic acid | Liven vine; Christmas vine; Citrus; Acacia, Milk vetch, Wild Chrysanthemum, Jujube flower; Black locust, Buckweat; Chestnut, Eucalyptus, CitrusSulla; Sesamum indicum | MeOH; MeOH/water 1:1; MeCN | HPLC-DAD-ESI-MS/MS; LC-ESI-MS/MS; HPLC-ECD; HPLC-PDA; LC-DAD; HPLC-DAD | [64,66,67,68,77,79,82,85] |
| Cinnamic acid | Black locust, Tualang, kelulut; Rapeseed, Lime, Heather, Cornflower, Buckwheat, Black locust | MeOH/water 1:1 | LC-ESI-MS/MS; HPLC-PDA; HPLC–ECD-DAD | [67,85,86] |
| Trans-cinnamic acid | Chestnut, Eucalyptus, Sulla; Acacia, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | HPLC-UV | [87] |
| 2-Hydroxycinamic acid | Tualang, Kelulut | MeOH/water 1:1 | LC-ESI-MS/MS | [67] |
| 3,4-dimethoxycinnamic acid | Black locust | MeOH | HPLC-PDA | [77] |
| T-cinnamic acid | Black locust | MeOH | LC-MS; | [77,88] |
| Isoferulic acid | Black locust | MeOH | HPLC-PDA | [77] |
| Sinapic acid | Rapeseed, Lime, Heather, Cornflower, Buckwheat and Black locust | MeOH | HPLC–ECD-DAD | [85,86] |
| Hydroxybenzoic acids | ||||
| Syringic acid | Linen vine, Kelulut, Tualang; Kanuka, Acacia, Milk vetch, Wild Chrysanthemum, Jujube flower, Sulla, Thistle, Citrus | Metanol; MeOH/water 1:1; MeOH/water 1:1; MeOH 43%:HCOOH (57%, v/v) | HPLC-DAD-ESI-MS/MS; LC-ESI-MS/MS; HPLC-DAD; UHPLC–UV | [66,67,80,84,89] |
| Gallic acid | Tualang, kelulut; Ziziphus Spina-Christi, Acacia, Prosopis juliflora (mesquite); Acacia, Chestnut, Savory, Sulla, Ailanthus, Thymus, Orange, Cornlfower, Rapeseed, Citrus, Heather, Eucalyptus | MeOH/water 1:1; MeOH 43%:HCOOH (57%, v/v); MeCN | LC-ESI-MS/MS; HPLC-DAD; HPLC-UV | [67,76,84] |
| Vanillic acid | Black locust, Sulla, Thistle, citrus, Prosopis juliflora (mesquite), Manuka, Ziziphus Spina-Christi; Lavender, Rosemary, Sulla, Rapeseed, Lime, Heather, Cornflower, Buckwheat, Thistle | MeOH | UHPLC–UV | [81,85,86,87] |
| Ellagic acid | Rapeseed, Lime, Heather, Cornflower, Buckwheat, Black locust | MeOH | HPLC–ECD-DAD | [85,86] |
| Benzoic acid | Black locust, Buckweat, Manuka; Chestnut, Eucalyptus, Sulla; Acacia, Lime, Lavender, Rapeseed, Lavender, Sunflower, Rosemary, Orange, Lemon | MeOH | HPLV-UV; HPLC-PDA | [68,77,87] |
| P-hydroxybenzoic acid | Acacia, Buckwheat, Cornflower, Milk Vetch, Dill, Citrus, wild chrysanthemum, Jujube Flower; Sage, Sulla | MeOH/water 1:1; MeOH 43%:HCOOH (57%, v/v); MeCN | HPLC-DAD; UHPLC-DAD MS/MS | [65,70,84] |
| 3-Hydroxybenzoic acid | Buckwheat; Chestnut, Eucalyptus, Sulla; Acacia, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH | LC-DAD | [79,87] |
| 4-Hydroxybenzoic acid | Kelulut, Paliurus spina-christi Mill.; Chestnut, Eucalyptus, Sulla; Acacia, Lime, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon | MeOH/water 1:1; MeOH | LC-ESI-MS/MS; GC–MS | [67,79,87] |
| 4-methoxybenzoic acid | Paliurus spina-christi Mill.; Heather; Manuka, Kanuka | MeOH | GC–MS; HPLC-MS/MS | [79,89] |
| Dihydroxybenzoic acids | ||||
| Protocatechuic acid | Acacia, Buckweat, Cornflower, Manuka, Heather, Pine; Milk Vetch, Wild Chrysanthemum, Jujube Flower; Chestnut, Eucalyptus, Lavender, Rapeseed, Sunflower, Rosemary, Orange, Lemon, Black Locust, Sulla, Echium plantagineum | MeOH 43% (v/v) and HCOOH (aq), pH 2.54 (57%, v/v) | HPLV/UV; HPLC-DAD | [71,75,84,87] |
| Benzoic acids derivatives | ||||
| Methyl syringate | Asphodel, Manuka, Kanuka, Sulla, Dill, Lemon, Orange, And Medlar | MeCN 2 Water:MeCN 60:40 (v/v) | HPLC-DAD; HPLC-MS/MS | [70,79] |
| Tanins | ||||
| Monogalloyl-glucose | Rosemary | MeOH:water (1:1) | CE–ESI-MS | [69] |
| Monoterpenoids | ||||
| Carvacrol | Ziziphus Spina Christi | MeOH | HPLC-DAD | [90] |
| Thymol | Ziziphus Spina Christi | MeOH | HPLC-DAD | [90] |
| Other polyphenols | ||||
| Chlorogenic acid | Buckweat, Manuka; Acacia, Milk Vetch, Wild Chrysanthemum, Jujube Flower; Acacia, Chestnut, Savory, Sulla, Ailanthus, Thymus, Orange | MeOH; MeOH 43% (v/v) and HCOOH (aq), pH 2.54 (57%, v/v); 40/50/10 (v/v/v) water/TFA/MeCN | HPLV-UV | [68,71,76,84] |
| Gallocatechin | Sweet chestnut, Eucalyptus, Citrus, Sulla, Sage | MeOH; MeCN | HPLC-MS/MS; UHPLC-DAD MS/MS | [65,89] |
| Epigallocatechin | Sage | MeCN | UHPLC-DAD MS/MS | [65] |
| Epigallocatechin gallate | Sage | MeCN | UHPLC-DAD MS/MS | [65] |
| Resveratrol | Sage | MeCN | UHPLC-DAD MS/MS | [65] |
| Other compounds | ||||
| Phenyllactic acid | Cornflower, Manuka, Kanuka, Thistle, Mint, Heather, Sulla, Dill | MeOH | HPLC-MS/MS | [70,85,86,89] |
| 2-cis,4-trans-abscisic acid | Strawberry tree, manuka, Black Locust, Buckwheat, Basil, Goldenrod, Linden, Sunflower, Rapeseed | MeCN Water/MeCN 60:40 (v/v) | HPLC-DAD HPLC-MS/MS | [79] |
| 2-trans,4-trans-abscisic acid | Strawberry tree, Manuka, Cornflower | MeCN Water/MeCN 60:40 (v/v) | HPLC-DAD HPLC-MS/MS | [79,91] |
| Fisetin | Lavender, Orange Blossom, Rosemary, Heather, Eucalyptus, Chestnut, Thyme | MeCN | HPLC-DAD-TOF-MS | [78] |
| Honey Type | Origin | Moisture Content (%) | Acidity (meq/kg) | Sugar Composition (%) | Ash (%) | HMF (mg/kg) | Reference |
|---|---|---|---|---|---|---|---|
| Allium ursinum L. | Hungary | 18.3–19.7 | 19.0–23.5 | n.i. | n.i. | 15.3–20.6 | [98,99] |
| Arbutus unedo | Portugal | 15.8–19.8 | 20.17–61.10 | Fructose: 35.96 Glucose: 26.743 | 0.70 | 8.2 | [45,100] |
| Asclepias sp. | Hungary | 16.9–20.0 | 29.0–34.0 | Fructose: 41.1 Glucose:30.8 | n.i. | 20.3–25.0 | [98,99] |
| B. napus | Romania | 18.4 | 16 | Fructose: 35.26 Glucose: 31.78 F/G ratio:1.11 | n.i. | 13.3 | [36] |
| Bulgaria | n.i. | n.i. | Fructose: 36.70 Glucose: 37.46 | n.i. | 40.65 | [101] | |
| Hungary | 17.2–19.8 | 21.5–27.5 | Fructose: 38.5 Glucose:40.5 | n.i. | 0.8–17.9 | [98,99] | |
| Calluna vulgaris L. | Portugal | 19.0 | 35.23 | Fructose: 35.963 Glucose: 26.743 | 0.48 | 22.80 | [100] |
| Turkey | 20.86 | n.i. | Fructose: 45.11 Glucose: 25.00 F/G ratio: 1.80 | n.i. | 62.24 | [25] | |
| Carlina racemosa | Portugal | 17.2 | 41.37 | Fructose: 35.703 Glucose: 25.020 | 0.55 | n.i. | [100] |
| Castanea sativa Mill. | Hungary | 17.0–17.4 | 26.0–32.0 | n.d. | n.d. | 12.6–33.1 | [98,99] |
| Turkey | 19.70 | n.i. | Fructose: 38.44 Glucose: 19.35 F/G ratio: 1.98 | n.i. | 9.28 | [25] | |
| Ceratonia siliqua | Portugal | 15.40–18.6 | 31.72–56.20 | Fructose: 37.964 Glucose: 26.372 | 0.43 | 41.80 | [45,100] |
| Cistus sp. | Portugal | 15.90 | 32.0 | n.i. | 0.25 | 20.0 | [102] |
| Citrus sinensis | Portugal | 15.8–18.2 | 19.40–30.50 | Fructose: 40.180 Glucose: 27.110 | 0.13 | 28.20 | [45,100] |
| Coriandrum sp. | Hungary | 19.1–19.3 | 40.2–43.5 | n.i. | n.i. | 8.2–14.5 | [98,99] |
| Bulgaria | 16.27 | 4.71–16.09 | Fructose: 40.25 Glucose: 26.11 | n.i. | 17.38–22.72 | [101,103] | |
| Cytisus scoparius | Portugal | 19.70 | 30.0 | n.i. | 0.37 | 6.56 | [46] |
| Echium sp. | Portugal | 16.80 | 25.0 | n.i. | 0.23 | 94.0 | [102] |
| Erica sp. | Portugal | 17.31–20.60 | 15.5–47.70 | Fructose:34.4–37.1 Glucose:28.0–33.4 | 0.32–0.36 | 4.63–20.40 | [45,46,104] |
| Spain | 18.19 | 35.66 | n.d. | 0.47 | 3.72 | [105] | |
| Eucaliptus sp. | Portugal | 14.30–19.20 | 12.6–29.7 | Fructose: 23.34 Glucose: 35.82 | 0.07–0.46 | 2.54–32.75 | [46,100,105] |
| H. annus | Romania | 16.23–20.39 | 15–94–47.32 | Fructose: 36.74 Glucose: 28.37 F/G ratio: 1.33 | 0.33–0.36 | 2.66–10.96 | [106,107] |
| Bulgaria | n.i. | n.i. | Fructose: 40.91 Glucose: 35.80 | n.i. | 25.47 | [101] | |
| Portugal | 19.2 | 25.50 | Fructose:41.24 Glucose:38.09 | 0.15 | 8.10 | [100] | |
| Hungary | 17.2–19.7 | 33.5–46.5 | Fructose: 40.5 Glucose:36.5 | n.i. | 0.6–25.2 | [98,99] | |
| Honeydew honey | Hungary | 19.9–20.0 | 42.0–48.5 | Fructose: 37.3 Glucose:27.6 | n.i. | 19.6–34.2 | [98,99] |
| Lavandula stoechas L. | Hungary | 19.0–19.1 | 29.5–35.5 | n.i. | n.i. | 1.0–13.0 | [98,99] |
| Portugal | 13.56–19.20 | 16.40–35.10 | Fructose:39.81–-41.66 Glucose: 26.40–28.52 | 0.09–0.18 | 5.35–12.80 | [45,46,100] | |
| Turkey | 17.15 | n.i. | Fructose: 32.65 Glucose: 22.19 F/G ratio: 1.47 | n.i. | 24.42 | [25] | |
| Manuka | New Zealand, Australia, Malaysia | 11.59–20.27% | 42.67 ± 3.01 | Fructose: 33.0–40.0 Glucose: 27.8–36.2 | 0.21 ± 0.01 | 31.53–40.0 | [19,71] |
| Tualang | Malaysia | 16.39–22.32 | 44.92–86.08 | Fructose: 29.60–41.73 Glucose: 30.00–47.13 F/G ratio: 0.88 | 5.08–1226.32 | [19,108] | |
| Gelam | Malaysia | 18.51–20.33 | 46.50–59.75 | Fructose: 44.90 Glucose: 50.44 F/G ratio: 0.89 | 8.52–10.20 | [19,108] | |
| Longan | Thailand | 20.11 | 17.60 | Fructose: 41.02 Glucose: 34.91 F/G ratio: 1.18 | 0.23 | 0.58 | [109] |
| Mentha pulegium | Portugal | 18.10 | 42.17 | Fructose: 36.41 Glucose:24.66 | 0.61 | 4.10 | [100] |
| Mentha x piperita | Romania | 17.7 | 26.9 | Fructose: 36.03 Glucose: 27.87 F/G ratio: 1.30 | 0,15 | 29.2 | [36] |
| Pinus sp. | Turkey | 15.5–15.8 | 26.5- 28.6 | Fructose: 32.1–39.8 Glucose: 23.67–28.2 F/G ratio: 1.17–1.68 | 0.50–0.55 | 3.57 | [25,110,111,112,113] |
| Pinus sp | Greece | n.i. | n.i. | Fructose: 10.33 Glucose: 2.52 F/G ratio: 4 | n.i. | 12.34 | [114] |
| R. pseudoacacia | Bulgaria | n.i. | n.i. | Fructose: 43.22–42.76 Glucose: 27.50–24.13 | n.i. | 17.82 | [102,115] |
| Hungary | 17.1–19.9 | 13.0–27.5 | Fructose: 43.6 Glucose: 29.1 | n.i. | 1.1–29.4 | [98,99] | |
| Rhododendron sp | Italy | n.d. | 28.00 | Fructose: 27.80 Glucose: 38.20 | n.i. | 3.40 | [40] |
| Turkey | 18.89 | 34.33 | Fructose: 43.58 Glucose: 23.16 F/G ratio: 1.88 | n.i. | 3.20 | [25,116] | |
| Rubus sp. | Romania | 18.3 | 27.3 | Fructose: 36.30–33.46 Glucose: 38.15–29.00 F/G ratio: 1.04–1.26 | 0.340 | 18.7 | [36,117] |
| Thymus sp. | Romania | 17.3 | 22.5 | Fructose: 36.77 Glucose: 26.86 F/G ratio: 1.38 | n.i. | 30.8 | [36] |
| Italy | n.d. | 38.50 | Fructose: 37.80 Glucose: 30.50 | n.i. | 30.40 | [16] | |
| Greece | n.i. | n.i. | Fructose: 11.51 Glucose: 5.15 F/G ratio: 2.2 | n.i. | 203 | [114] | |
| Thymus vulgaris | Portugal | 15.80–17.50 | 37.20–69.50 | Fructose:37.04 Glucose:24.79 | 0.45 | 1.60 | [45,100] |
| Tilia sp. | Bulgaria | n.i. | n.i. | Fructose: 40.13 Glucose: 27.07 | n.i. | n.i. | [101] |
| Italy | n.i. | 12.80 | Fructose: 37.20 Glucose: 30.20 | n.i. | 3.50 | [16] | |
| Hungary | 17.6–19.9 | 21.0–41.5 | Fructose: 40.0 Glucose: 30.6 | n.i. | 4.3–37.5 | [89,99] | |
| Ziziphus sp. | Egypt | 15.1–20.20 | 12.5–29.17 | Fructose: 39.5–42.1 Glucose: 29.5–32.0 F/G ratio: 1.30–1.36 | 0.178 | 0.6–1.6 | [118] |
| Hovenia dulcis | South Korea | 17.0–19.73 | 8.50–33.50 | Fructose: 33.1–44.5 Glucose: 20.2–29.3 F/G ratio: 1.27–2.23 | 0.08–0.82 | 0.00 | [119,120] |
| Honey Type | Origin | K (mg/kg) | Mg (mg/kg) | Ca (mg/kg) | Na (mg/kg) | References |
|---|---|---|---|---|---|---|
| Acacia mangium | Malaysia | 1459.33–413.63 | 44.97–21.83 | 119.80–567.27 | 458.95–180.23 | [144] |
| Arbutus unedo | Portugal | 1736.29 | 24.92 | 50.00 | 161.02 | [100] |
| Asclepias sp. | Hungary | 262–342 | 6.81–8.88 | 17.8–24.1 | 4.51–5.92 | [48] |
| Brassica sp. | Romania | 194.17–112.56 | 23.90–23.47 | 88.63–87.14 | 47.96–36.08 | [145] |
| Bulgaria | 105 | 11 | 46 | 8.49 | [146] | |
| Hungary | 162–292 | 11.2–16.9 | 33.4–50.6 | 6.13–9.09 | [48] | |
| Hungary | 160–280 | 11–16 | 36–48 | 5.6–12 | [47] | |
| C. sativa | Bulgaria | 16.28 | 16 | 66 | 9.55 | [146] |
| Spain | 221.2–269.4 | 107.89–962.64 | 122.16–111.42 | 22.30–26.20 | [147] | |
| Italy | 290.00–5300 | 45.0–201.0 | 23.00–352.0 | 64.0–104.0 | [148] | |
| Hungary | 1563–2186 | 30.1–41.2 | 81.9–116 | 10.8–14.6 | [48] | |
| Calluna vulgaris | Portugal | 1196.31 | 31.51 | 45.15 | 155.45 | [100] |
| Carlina racemosa | Portugal | 1341.16 | 46.63 | 42.12 | 208.10 | [100] |
| Ceratonia siliqua | Portugal | 723.28 | 46.89 | 79.09 | 138.10 | [100] |
| Citrus limon L. | Italy | 186–3110 | 59.00–152 | 36.00–289 | 41.00–118.0 | [148] |
| Citrus sinensis | Portugal | 170.07 | 9.81 | 28.18 | 56.34 | [100] |
| Spain | 735.21 | 54.15 | 43.10 | 12.04 | [146] | |
| Citrus sp. Orange blossom | Italy | 168.00–3016.0 | 84.00–202.00 | 177.00–318.00 | 39.00–145.00 | [148] |
| Coriandrum sativum | Bulgaria | 564 | 7.1 | 44 | 14.20 | [149] |
| Cytisus scoparius | Portugal | 160.06 | 31.97 | 71.41 | 101.22 | [46] |
| Erica sp. | Portugal | 166.70 | 36.78 | 35.14 | 174.45 | [46] |
| Eucalyptus sp. | Italy | 112.0–372.0 | 42.00–331.0 | 84.0–232.0 | 293.0–928.0 | [148] |
| Portugal | 397.17–2040.50 | 25.04–48.84 | 19.90–122.45 | 151.62–667.39 | [46,100] | |
| Forest honey | Hungary | 1331–2212 | 39.7–60.2 | 52.4–75.5 | 10.8–17.9 | [48] |
| Gelam | Malaysia | 1363.40 | 31.63 | 275.77 | 196.84 | [19,109] |
| H. annus | Romania | 234.64–1111.1 | 24.9–30.3 | 67.7–97.4 | 13.2–36.4 | [145] |
| Bulgaria | 280–247 | 14 | 71 | 7.58 | [33,149] | |
| Portugal | 276.86 | 24.92 | 68.18 | 87.93 | [100] | |
| Hungary | 502–735 | 21.8–33.3 | 82.9–124 | 6.37–9.32 | [48] | |
| Hedysarum coronarium L | Italy | 227.0–295.0 | 92.0–134.0 | 188.0–291.0 | 519.0–681.30 | [148] |
| Lavandula stoechas | Portugal | 78.09–173.17 | 6.84–14.46 | 13.38–32.43 | 41.47–95.02 | [46,100] |
| Dimocarpus longan Lour. | Malaysia | 906.35 | 35.47 | 118.07 | 95.94 | [144] |
| Mentha pulegium | Portugal | 158.256 | 70.92 | 39.09 | 161.64 | [100] |
| Pauliurus spina christi | Bulgaria | 1198 | 17 | 62 | 11.80 | [146] |
| Persea americana Mill. | Spain | 557.073 | 623.58 | 55.97 | 69.05 | [141] |
| Phacelia tanacetifolia | Hungary | 102–130 | 4.09–5.16 | 9.12–12.5 | 3.02–3.81 | [48] |
| Ananas comosus (L.) Merr. | Malaysia | 473.68 | 36.63 | 74.60 | 111.29 | [144] |
| Pinus sp. | Turkey | 1832–1989 | 54.2- 59.2 | 50.1- 59.9 | n.i. | [110,111,113] |
| R. pseudoacacia | Italy | 400–1150 | 41.0–93.0 | 55.0–182.0 | 60.0–1190 | [148] |
| Romania | 244.58–146.66 | 6.72–3.25 | 6.94–1.02 | 24.32–8.32 | [150,151] | |
| Bulgaria | 250–126 | 6.0 | 32.0 | 8.11 | [34,145,149] | |
| Hungary | 115–176 | 3.83–5.30 | 10.2–15.5 | 3.13–4.62 | [48] | |
| Rosmarinus officinalis L (Rosemary) | Spain | 253.00–553.03 | 9.80–42.11 | 15.14–206.70 | 9.18–36.80 | [141,147] |
| Thymus vulgaris L | Portugal | 341.91 | 74.15 | 68.79 | 61.30 | [100] |
| Spain | 322.45–1502.00 | 40.70–341.74 | 98.10–181.69 | 36.0–151.65- | [147] | |
| Tilia sp. | Bulgaria | 112.3–796 | 21 | 77 | 7.50 | [34,146] |
| Hungary | 921–1280 | 17.1–24.9 | 71.8–98.3 | 10.1–13.9 | [48] | |
| Kompassia excelsa | Malaysia | 1576.40 | 35.03 | 165.10 | 268.23 | [144] |
| Vicia sp. | Bulgaria | 196 | 10 | 33 | 9.62 | [146] |
| Wildflower | Italy | 270–2460 | 85.0–184.0 | 168.0–387.0 | 322.8–1321.4 | [148] |
| Ziziphus sp. | Egypt, Palestina | 1569.3–476.40 | 34.48–22.1 | 136.6–94.56 | 115.04–49.2 | [118] |
| Hovenia dulcis | South Korea | 405.3–409.7 mg/L | 9.9–11.5 | 19.3–20.8 | 1.6–2.0 | [120,150,152] |
| Honey Type | Origin | TPC (mg GAE/100 g) | TFC (mg QE/100 g) | Antioxidant Activity | In Vitro Pharmacological Activity | Reference |
|---|---|---|---|---|---|---|
| Acacia Honey (Acacia sp.) | Burkina Fasan, Pakistan, Malaysia | 93.43–14.70 mg GAE/100 g | 6.14–1.13 mg QE/100 g | DPPH, IC50 (mg/mL): between 10.40–17.97 ABTS (176.66–231.5 μmol TE/g) ORAC (30.62–83.72 μmol TE/g) | ↓ IL 1β level and ↓ TNF-α level after 24 h with increase in honey concentration 1–8% (v/v) ↑ calcium ion level from 24 to 48 hrs incubation periods ↑ anticancer activity on PC-3 cell line after 24 hrs incubation with IC50: 4.43% (v/v) ↓ Cell viability and ↑ Apoptotic cell death in MCF-7 breast cancer cells at 3.12–100% (v/v) for 24–72 hrs ↓ TNF-α, IL-1β, Ca ion in NCI-H460 non-small lung cancer cells | [154,155,156,157,158,159,160,161,162,163,164,165,166,167] |
| Astragalus honey (Astragalus microcephalus Willd) | Turkey, Iran | 198.00 CE/100 g | 23.57 mg QE /100 g | DPPH: 7.2 mg/mL | ↓ in Bcl-2 mRNA expression ↓ Bcl-2 gene in 5637cells ↓ p53 gene in HepG2 cells ↓ expression of the p53 gene by 80% in 5637 cells ↔ to ↓ p53 gene expressions in L929 cells | [158] |
| Berry honey Rubus sp. | Romania | 19.9 mg GAE/100 g | 33.5 mg QE/100 g | DPPH: 79.05% | n.i. | [36] |
| Black locust honey (Robinia pseudoacacia) | Malaysia, Poland, Turkey, Romania | 2.0–39.0 mg GAE/100 g | 0.91–2.42 mg QE/100 g | DPPH: 12.72–29.98 mg GAE/100 g honey; FRAP: 82.39 mg TE/100 g | ↓ LPO in liver ↓ Cell viability in B16-F1 cells after 24 hrs at doses of 0.2 and 0.1 g/mL ↓ Cell viability in A375 line observed after 48- and 72-h exposure of cells to 0.2 and 0.1 g/mL doses of acacia honey ↓ Cell viability and ↓ Bcl-2, p53 in NCI-H460 non-small lung cancer cells at 0.5–8% (w/v) for 48 hrs Arrest cell cycle at G0/G1 phase in B16-F1 and A375 line | [18,24,159,160,161] |
| Italy | 112.99 mg GAE/kg | 67.32 mg QE/kg | DPPH, IC50 (mg/mL): 21.56 FRAP, (mmol Fe(II)/Kg honey): 1.377 | n.i. | [76] | |
| Poland | 142.8 mg GAE/kg | n.a. | DPPH, (mmol TEAC/kg): 0.3 FRAP, (mmol Fe(II)/Kg honey): 0.6 | n.i. | [87] | |
| Buckwheat honey (Fagopyrum esculentum) | Poland | 1113.0 mg GAE/100 g | n.a. | DPPH, (mmol TEAC/kg): 1.2 FRAP, (mmol Fe(II)/Kg honey): 5.7 | ↑ ROS inhibition produced by human PMNs (160 to 130 mL/g activity) ↑ Superoxide anion scavenging inhibition (RIC50: 290 mL/g) ↓ oxidative stress, ↓ LPO in liver | [87,162] |
| Canola (B. napus) | Poland | 47.71−183.0 mg GAE/100 g | 0.72 mg CEQ/100 g | DPPH: 55.4% DPPH: 18.22 μmol TE/ 100 g FRAP: 92.05 μmol TE/100 g TEAC: 67 μmol TE/100 g | ↓ Superoxide radical, ↓ LPO | [47,87,163] |
| Romania | 23.7–19.9 mg GAE/100 g | 20.2–2.5 mg QE/100 g | n.i. | n.i. | [36,164] | |
| Chestnut honey (Castanea sativa) | Italy | 14.26–94.56 mg GAE/100 g | 12.52–143.63 mg QE/100 g | DPPH, I% (%): 75.37 | ↓ Oxidative stress ↑ inhibition of LPS-induced NO ↑ antimutagenic activity on TA98 strain (10 µg/mL to 20 mg/mL honey concentration) ↑ Apoptotis in MCF-7 cell line after 48 h at 2.5 and 5 mg/mL doses ↑ Apoptotis in SKBR-3 cell line at 5 mg/mL dose of chestnut honey (62.05% cell death) ↔ apoptosis at 5 mg/mL dose on MDA-MB-231 (25.87% cell death) ↔ apoptosis at 5 mg/mL dose of the chestnut honey on MCF-10A (31.5% cell death) | [44,45,62,68,76,165,166,167,168] |
| Turkey | 5.49–8.01 mg GAE/100 g | 0.99–2.49 mg QE/100 g | DPPH: 17.66–20.05 mg/mL FRAP: 2.056–4.30 mmol Fe(II)/Kg honey | ↓ MPO, ulcer index, microvascular permeability | [25] | |
| Orange honey (Citrus sinensis) | Portugal | 32.10 mg GAE/100 g | 1.73 mg QE/100 g | n.i. | n.i. | [100] |
| Italy | 84.37 −32.10 mg GAE/100 g | 69.8 mg QE/100 g 9 | DPPH, IC50 (mg/mL): 25.87 FRAP, (mmol Fe(II)/Kg honey): 1.265 TEAC:4.04 mg/mL ORAC: 3.28 mmol TE/g | ↓ Oxidative stress ↑ antibacterial activity | [76] | |
| Citrus sp. honey | Italy, Egypt | 12.08 mg GAE/100 g | 5.82 mg QE/100 g | DPPH, I% (%): 55.06 | ↑ inhibitory activity in MCF-7 (52.53%) ↔ inhibitory activity in Caco-2 (35.68%) and Hep-G2 (32.47%) | [68,168] |
| Clover honey (Trifolium pratense L., T repens L.) | Croatia | 100.4 mg GAE/100 g | 3.9 mg QE/100 g | DPPH: 23.3% FRAP: 89.0 µM Fe(II) | ↓ LPO, inhibits activity of NO, TNF-α and IL-6 ↓ AST, ALP ↔ inhibitory activity in Caco-2 (30.94–49.84%) ↔ inhibitory activity in MCF-7 (15.45–28.14%) ↓ inhibitory activity in Hep-G2 (1.75–24.84%) | [168,169,170] |
| Coriander honey (Coriandrum sp.) | Bulgaria | 68.70 mg GAE/100 g | 8.02 mg QE/100 g | FRAP: 380.66(μmol Fe [II]/100 g) DPPH: 29.94% | ↓ LPO, SOD, ↑ GSH level in liver | [171] |
| Cornflower honey (Centaurea cyanus) | Poland | 44.06 mg GAE/100 g | n.i. | DPPH IC50: 44.40 mg/mL FRAP IC50: 3.85 mg/mL | ↑ antibacterial activity ↑ wound healing | [86] |
| Cotton honey (Gossypium sp.) | Egypt | 45.42 mg GAE/100 g | DPPH (SC50 (µg/mL): 99.40 ABTS+ SC50 (µg/mL): 41.20 | ↔ inhibitory activity in MCF-7 (32.91%) ↓ inhibitory activity in Caco-2 (29.25%) and Hep-G2 (20.08%) | [168] | |
| Eucalyptus honey (Eucalyptus sp.) | Portugal | 54.25 mg GAE/100 g | 5.28 mg QE/100 g | TEAC:2.86 ORAC: 7.40 | n.i. | [51] |
| Australia | 106.7 mg GAE/100 g | 3.6 mg QE/100 g | DPPH:44.3 μmol TE/100 g FRAP:142.97 μmol TE/100 g | ↓ Superoxide radical, ↓ LPO | [169,172,173] | |
| Italy | 11.08 mg GAE/100 g | 6.16 mg QE/100 g | DPPH, I% (%): 73.04 | ↑ inhibition activity in MCF-7 cell line (159.4 ± 3.6 μg/mL) | [68,174] | |
| European goldenrod or woundwort Solidago virgaurea L) | Poland | 11.29–21.03 mg GAE/100 g | 0.93–1.41 mg QE/100 g | DPPH: 31.08−39.46% ABTS: 46.69–56–92% | n.i. | [174] |
| Fennel honey (Foeniculum vulgare) | Egypt | 29.1–102.0 mg GAE/100 g | 17.7− 27.0 mg QE/100 g | Cellular antioxidant activity: 5.66–26.4 µmol of QE/100 g | ↔ inhibitory activity in MCF-7 (45.93%) ↓ inhibitory activity in Caco-2 (25.53%) and Hep-G2 (11.75%) | [168,175] |
| Gelam honey (Kompassia eroxi) | Malaysia | 74.12 mg GAE/100 g | 46.11 mg QE/100 g | DPPH IC50 (6.68 ± 0.28) mg/mL FRAP: 115.61 ± 3.86 μmol Fe [II]/100 g | ↑ Antioxidant enzyme activities ↓ Hypertriglyceridemia and pro-oxidative effects ↑ apoptosis in HepG2 cells at 3% concentration after 24 h ↑ apoptosis in WRL-68 cells at 6% after 24 h ↓ MDA levels in HIT-T15 cells ↑ insulin content | [176,177] |
| Goldenrod honey (Solidago sp.) | Poland | 173.4 mg GAE/100 g | n.a. | DPPH: (mmol TEAC/kg): 0.2 FRAP: (mmol Fe(II)/Kg honey): 1.0 | n.i. | [87] |
| Heather honey (Calluna vulgaris) | Portugal | 117.59 mg GAE/100 g | 21.16 mg QE/100 g | TEAC:0.86 ORAC: 22.58 | ↑ apoptosis in HL-60 cells with 50 mg/mL concentration of heather honey for 48 h (70.4–78.5%) ↑ antiproliferative activity 72 h treatment with 100–250 mg/mL of heather honey (11.9–7.1% of survival) ↓ ROS levels after 24 h ↓ DNA strand breaks (0.1 mg mL−1, 15%) induced by BaP in HepG2 cells | [100,178,179] |
| Poland, Portugal | 306.2−269.03 mg GAE/kg | n.a. | DPPH IC50: (24.6 (SD 0.2) mg/mL, FRAP (1948 mg/kg TEAC/kg): 0.6 FRAP: (mmol Fe(II)/Kg honey): 2.1 | Protection of HepG2 against mutagens-induced DNA damage | [87] | |
| Lavander honey (Lavandula sp.) | Portugal | 31.85–34.13 mg GAE/100 g | 3.09–3.15 mg QE/100 g | DPPH IC50: 5.3 mg/mL TEAC: 3.98–4.03 ORAC: 7.43–7.57 | Diabetic foot ulcers healing activity through reducing ROS | [44,100] |
| Linden honey (Tilia sp.) | Romania, Slovenia, Poland | 16.0–85.8 mg GAE/kg 192.5 mg GAE/100 g | 4.70–6.98 mg QE/100 g | DPPH IC50: 42.77 mg/mL, FRAP: 137.8 mg/kg DPPH: (mmol TEAC/kg): 0.4 FRAP: (mmol Fe(II)/Kg honey): 1.4 | ↓ Oxidative stress and ROS | [27,87,180] |
| Manuka honey Leptospermum scoparium J.R. et G.Forst | New Zealand | 1288.0 GAE µg/g | 37.64 CE µg/g | DPPH: 18.69 µmol TE/g FRAP: 3.68 µmol TE/g; TEAC: 30.72 µmol TE/g | ↑ GPx, ↓ CAT activity, ↓ DNA damage and MDA level, protects proteins and lipids against AAHP-induced stress | [159,181,182] |
| 429.61 mg GAE /kg | 97.62 CE mg/kg | DPPH: 0.06 mmol TE/ 100 g FRAP: 0.14 mmol TE/ 100 g TEAC: 0.22 mmol TE/100 g | ↓ viability of B16 F1 cells (0.3% manuka) after 24 h ↑ apoptosis in CT26 and MCF-7 cells with 2.5% manuka | [129,130,182] | ||
| Oak honey (Quercus sp.) | Turkey | 115.41 mg GAE/100 g | 77.36 mg QE/100 g | Inhibition of ABTS %: 89.36 | 50% inhibition after 24 h incubation of AGS cells with the 1.7% final concentration ↓ ROS generation at the concentration of 0.25% (w/v) in AGS cells DNA damage | [183] |
| Pine honey (Pinus sp.) | Greece, Turkey | 61.42–163.98 mg GAE/100 g | 22.80 mg QE/100 g | DPPH: 44.30 FRAP: 1.48 | ↓ SOD and LPO, ↓ CAT, GPx, GSH in liver ↔ viability on Ishikawa and PC-3 cells and ↑ viability of MCF-7 cells at 0.2–125 μg/mL concentration and incubation for 48 hrs ↑ cytotoxicity on MDAMB 231 cells with a 1 mg/mL dose ↑ cytotoxicity on MCF-7 and SKBR3 cancer cell lines with a 2.5–5 mg/mL dose | [25,108,109,110,166] |
| Pineapple (Ananas comosus) | Malaysia | 27.75 mg GAE/100 g | 24.74 ± 0.35 mg QE/ 100 g | DPPH: 10.86 (IC50 values mg/mL) FRAP (μmol Fe [II]/100 g): 47.92 Total antioxidant capacity (mg AAE/g): 16.12 | ↓ lipid droplet size between 33.78% and 70.36% ↓ lipid accumulation compared to control in 3T3-L1 murine pre-adipocytes | [176,184] |
| Rhododendron honey (Rhododendron sp.) | Turkey | 408.35 mg GAE/100 g | DPPH: 48.95 mg/mL FRAP: 0.0077 mg/100 g honey | [24] | ||
| Rosemary honey (Rosmarinus officinalis L.) | Spain | 102–118 mg GAE/100 g | 2.29–5.85 mg CE/100 g | DPPH: 202 μmol TE/100 g FRAP: 215 μmol TE/100 g | ↑ apoptosis in HL-60 cells through a ROS-independent cell death pathway ↓ DNA strand breaks (0.1–10 mg mL−1, 19–25%) induced by BaP in HepG2 cells | [179,185] |
| Savory honey (Satureja montana L.) | Italy | 253.78 mg GAE/100 g | 211.68 mg QE/100 g | DPPH, IC50 (mg/mL): 10.85 FRAP: (mmol Fe(II)/Kg: 3.702 | ↔ inhibition activity in BJ (IC50 = 27.25–29.70 mg/mL) ↑ inhibition activity in MCF-7 (IC50 = 22.85− 44.60 mg/mL), HeLa (IC50 = 26.70− 44.20 mg/mL) and in SW620 (25.50–49.70 mg/mL) ↑ apoptosis in SW620 after 48 h with savory honey () ↔apoptosis in MCF-7 and HeLa after 48 h | [186] |
| Strawberry tree honey (Arbutus unedo) | Italy | DPPH: 200.83 (µmol TE/100 g) FRAP: 539.01 µmol TE/100 g TEAC: 392.13 µmol TE/100 g | ↓ NF-κB, p-IκBα and Nrf2 expression, ↓ mitochondrial respiration and glycolysis ↑ apoptosis in human colon adenocarcinoma (HCT-116) and metastatic (LoVo) cancer cells ↓ Cell viability ↑ ROS generation ↓ Antioxidant enzyme activity ↓ Nrf2, SOD, catalase, HO-1 ↑ Lipid peroxidation and protein carbonyl content in HCT-116 and LoVo colon cancer cells at 3–12 and 10–40 mg/mL for 48 h ↑ p53, caspase-3, -8, -9, c-PARP1, Bax, Cyto C, FasL ↓ Bcl in HCT-116 and LoVo colon cancer cells at 3–12 and 10–40 mg/mL for 48 h | [187,188] | ||
| Portugal | 91.74–117.65 | 4.09–9.66 mg QE/100 g | DPPH: 40.28–45.20% TEAC:0.39–0.44 mmol TE/100 g ORAC:39.55 | n.i. | [100,189] | |
| Sulla honey (Hedysarum sp.) | Italy | 60.50 mg GAE/100 g | 41.88 mg QE/100 g | DPPH, IC50 (mg/mL): 54.74 FRAP, (mmol Fe(II)/Kg): 1.299 | n.i. | [79,94] |
| Italy | 11.26 mg GAE/100 g | 6.76 mg QE/100 g | DPPH, I%: 66.60 | n.i. | [68] | |
| Sunflower honey (Helianthus annus) | Turkey | 77.64 mg/100 g GAE | n.i. | DPPH: 19.24 mg/mL FRAP: 0.0047 mg/100 g honey | n.i. | |
| Romania | 20.0–45.0 mg GAE/100 g | 11.53–21.1 mg QE/100 g | DPPH: 60.02–76.95% | ↔ inhibition activity on Hep-G2 (32.92%) ↓ inhibition activity onCaco-2 (26.37%) and Hep-G2 (8.47%) | [36,106,171] | |
| Italy | n.i. | n.i. | DPPH IC50: 19.24 mg/mL FRAP: 470 mg/100 g | ↓ Visceral fat percentage, hepatoprotective | [24] | |
| Portugal | 36.69 mg GAE/100 g | 1.93 mg QE/100 g | TEAC: 3.17 ORAC: 0.41 | n.i. | [100] | |
| Thyme honey (Thymus vulgare) | Romania, Portugal | 18.9–62.91 mg GAE/100 g | 17.4–5.62 mg QE/100 g | DPPH IC50: 31.4 mg/mL TEAC: 1.59 ORAC: 10.58 | ↓ viability of Ishikawa and PC-3 cells at 0.2–125 μg/mL concentration and incubation for 48 hrs | [36,100,109] |
| Italy | 126.55 mg GAE/100 g | 73.56 mg QE/100 g | DPPH, IC50 (mg/mL): 31.4 FRAP, (mmol Fe(II)/Kg honey): 1.834 ORAC (415–692 μmol of TE/kg) | ↓ Tartrate-resistant acid peroxidise activity, hydroxyproline level, oxidative and inflammatory stress | [24,76] | |
| Tree of heaven honey (Ailanthus altissima) | Italy | 93.72 | 91.55 mg QE/100 g | DPPH, IC50 (mg/mL): 64.09 FRAP, (mmol Fe(II)/Kg honey): 1.268 | n.i. | [76] |
| Tualang honey (Koompassia excels) | Malaysia | 83.96 mg GAE/ 100 g | 50.45 mg QE/ 100 g | DPPH: 9.65 mg AAE/100 g FRAP: 52.39 mg TE/ 100 g | ↑ apoptosis (51.2%) at 48 h for MDA-MB-231 cells ↑ apoptosis 55.6% MCF-7 and 56.2%, HeLa cells at 72 h ↓ mitochondrial membrane potential (Δψm) in the cancer cell lines after 24 h of treatment ↑ apoptosis (42.8%) in MCF-7 cells after 24 h ↓ Lipid hydroperoxides, ↓ MDA, ↓ pancreatic SOD, ↑ pancreatic CAT apoptotic cell death in OSCC and HOS cell lines when treated with 2% and 10% honey for 24, 48 and 72 h | [190,191,192,193] |
| Willow honey (Salix sp.) | Poland | 288.0 mg GAE/kg | n.i. | DPPH, (mmol TEAC/kg): 2.1 FRAP, (mmol Fe(II)/Kg): 0.5 | n.i. | [94] |
| Ziziphus honey (Ziziphus sp.) | Egypt, Sudan | 81.37−96.99 mg GAE/100 g | 5.43−9.15 mg QE/100 g | DPPH: 32.70–86.18% ABTS (IC50 = mg/mL): 3.60 | ↔ inhibitory activity in Caco-2 (34.22%) ↓ inhibitory activity in MCF-7 (29.87%) and Hep-G2 (15.94%) | [168,194] |
| Honey Type | Animal Model or Human Individuals | Mode/Dosage of Honey Administration | Duration | Effects | References |
|---|---|---|---|---|---|
| Antioxidant effects | |||||
| Tualang honey from Malaysia | Streptozotocin-diabetic Male Sprague-Dawley rats aged 10–12 weeks | Oral administration G1 (Control): Non-diabetic rats received ddH2O (0.5 mL). G2 (Diabetic Control): Diabetic rats received ddH2O (0.5 mL). G3: Diabetic (honey: 0.2 g/kg bw). G4: Diabetic (honey: 1.2 g/kg bw). G5: Diabetic (honey: 2.4 g/kg bw). | 4 weeks | ↓ FPG, ↑ bw gain, ↓ TAS, ↑ activities of CAT, GPx, GR, and GST, ↓ TBARS levels, ↓ thickening of glomerular basement membrane of kidneys | [205] |
| Thirty-six male Sprague-Dawley diabetic rats aged 10–12 weeks | G1: Distilled water (0.5 mL) G2: Tualang honey (1.0 g/kg/bw) G3 (Diabetic Control): Distilled water (0.5 mL) G4: (Diabetic) Tualang honey (1.0 g/kg/bw) G5: (Diabetic) Glibenclamide (0.6 mg/kg/bw) + metformin (100 mg/kg/bw) G6: (Diabetic) Glibenclamide (0.6 mg/kg/bw) + metformin (100 mg/kg/bw) + tualang honey (1.0 g/kg/bw) | 4 weeks | ↓ TBARS levels ↓ superoxide dismutase (SOD) ↓ glutathione peroxidase (GPx) ↑ catalase (CAT) activity ↑ weight gain in G4 and G6 ↓ oxidative stress and damage | [206] | |
| Forty female Sprague-Dawley rats aged between 45 to 48 days old | rats were given 80 mg/kg DMBA then randomly divided into four groups: G1 (Control): distilled water (vehicle) G2: Tualang honey (0.2 g/kg/bw) G3 Tualang honey (1.0 g/kg/bw) G4: Tualang honey (2.0 g/kg/bw) via oral gavage daily | 150 days | ↑ apoptotic index in honey-treated groups ↓ concentration of VEGF Protein | [207] | |
| Mad honey (floral source: Rhododendron | sixty Sprague-Dawley female rats were (6–8 months old; 250–300 g) | Group 1 (control): 1 mL of 0.9% NaCl solution was given via intraperitoneal injection Group 2 (GTX): 0.015 mg/kg/bw of Grayanotoxin-III was given via intraperitoneal injection; (n = 12) Group 3 (RH1): 0.1 g/kg/bw of RH was given via oral gavage; (n = 12) Group 4 (RH2): 0.5 g/kg/bw of RH was given via oral gavage; (n = 12) Group 5 (RH3): 2.5 g/kg/bw of RH was given via oral gavage; (n = 12) | 1 h | ↓ SOD, CAT, GTX activity at high dose of honey (2.5 g/kg/bw) ↑ plasma and various tissue MDA levels at high dose of honey | [208] |
| Pine honey from Turkey | Forty-eight male BALB/c mice, weighing 30–35 g | G1: control G2: 1 g/kg bw/day pine honey G3: 180 mg/kg bw/day (∼1/5LD50) trichlorfon G4: 180 mg/kg bw/day trichlorfon plus 1 g/kg bw/day pine honey | 21 days | ↓ MDA levels, ↑ SOD levels, ↔ CAT and GSH-Px levels in G2 and G4 in liver, kidney, heart and brain tissues | [209] |
| Malicia honey (Mimosa quadrivalvis L) | Thirty-two male Wistar rats at 90 days of age | G1 (control): saline solution via gavage G2 (dyslipidaemic control): saline solution via gavage + dyslipidaemic diet (6% lard, 5% non-hydrolysed vegetable fat, 1% cholesterol and 0.5% cholic acid) G3: honey via gavage G4: honey via gavage + dyslipidaemic diet | 5 weeks | ↓ Food consumption, ↑ glucose tolerance and SOD activity ↓ TC, LDL and AST levels ↑ beneficial bacteria and organic acids ↓ tissue damage in colon and liver induced by the dyslipidaemic diet | [210] |
| Fennel honey | Eight female goats s 4–5 months old weighing about 10–24 kg bw | Intravenous administration 70–80 drops/min as rapid infusion of 20% honey solution, daily | 4 weeks | ↑ GPX, SOD, Lymphocytes (%), Total Leucocytic count (×103/µL), Monocytes (%) ↓ MDA, Plasma globulin (g/dL), Ascorbic acids (mg/dL), | [211] |
| Chestnut honey | Eighteen Male Wistar rats weighing 150 to 175 g | G1: indomethacin (60 mg/kg, orally) + honey G2: indomethacin + Alimento Supervis G3: indomethacin + Alimento Mieleucalipto G4: indomethacin + sucralfate G5: tap water (5 mL/kg) | 7 days | Prevention of indomethacin-induced gastric lesions ↓ ulcer index ↓ microvascular permeability ↓ myeloperoxidase activity of the stomach | [212] |
| Weight control | |||||
| Clover honey | Thirty-six male Sprague-Dawley rats (228.1 ± 12.5 g) | divided by weight into 2 groups (n = 18) and provided free access to 1 of 2 diets (20% carbohydrate (by weight of total diet) from either clover honey or sucrose) | 33 days | ↓ Weight gain and adiposity, ↓ TG ↑ non-HDL-C levels | [213] |
| Acacia and Gelam honey | Seven-week-old male Sprague-Dawley rats, with body weight ranging from 200 to 220 g | G1: normal control G2: high fat diet G3: high fat diet rats fed with Gelam honey, G4: high fat diet rats fed with Acacia honey, G5: high fat diet rats treated with orlistat | 4 weeks | ↓ in excess weight gain and adiposity index ↓ plasma glucose, triglycerides, and cholesterol, plasma leptin and resistin, liver enzymes, renal function test, and relative organ weight | [214] |
| Honeydew honey | Fifty-five Sprague Dawley rats, aged approximately 8 weeks | G1: sugar-free diet G2: 7.9% sucrose G3: 10% honey | 365 days | Similar weight gain and body fat in honey and control group; ↓ HbA1c, ↑ HDL-C | [215] |
| Tualang honey from Malaysia | male Wistar albino rats (n = 40) weighing 160–180 g | G1 (control): standard laboratory diet and drinking water ad libitum G2 (Tualang Honey): Orally administered (3 g/kg) for 45 days. G3 (ISO): Animals were subcutaneously injected with ISO (85 mg/kg) on the 44th and 45th days (at an interval of 24 h) G4 (TH + ISO): Animals were orally treated with TH (3 g/kg) for a period of 45 days followed by subcutaneous injection of ISO (85 mg/kg) on the 44th and 45th days (at an interval of 24 h) | 2 days | ↑ Antioxidant enzyme levels in heart tissue ↓ LPO | [216] |
| Pineapple | Forty-eight healthy Sprague Dawley male rats weighting 280–220 g | G1 (control): water ad libitum G2: 2000 mg/kg bw pineapple honey for 24 h G3: 2000 mg/kg bw adulterated honey A G4: 2000 mg/kg bw adulterated honey B | 14 days | ↓ cholesterol levels (18.94 ± 3.6 mmol/L) ↓ triglycerides (13.5 ± 1.5 mmol/L) and glucose (8.0 ± 1.5 mmol/L) levels Early mortality in G3 and G4 of five rats | [217] |
| Hypercholesterolemia and anti-diabetic | |||||
| Mad honey (floral source: Rhododendron ponticum) from Turkey | Streptozotocin-diabetic rats and non-diabetic rats | Honey given 50 mg/ kg/day (2 mL mad honey dissolved in distilled water) | 3 days | Significant ↓Glucose in both diabetic and non-diabetic rats | [218] |
| Clover and Citrus honey from Egypt, and Ziziphus honey from Yemen and Pakistan | Type 2 diabetics human subjects (n = 38) | Solely treated with honey 2 g/kg/day, orally, before meals twice daily, no antidiabetic medicines were running | Between 0.42–13.5 years | ↑ Glucose, ↔ RBG, ↔ TG, ↔ TC, ↔ HDL, ↔ LDL, ↔ TC/HDL and LDL/HDL ratios, ↓ SBP, ↓ DBP, ↓ bw, prevented ketoacidosis, hyperglycaemic hyperosmolar state, and macrovascular complications (particularly coronary heart disease) | [219] |
| Kanuka honey | Type 2 diabetes human subject, weight and blood samples | G1: 53.5 g (three tablespoons) of kanuka honey G2: mixture of a formulated honey (53.5 g) comprised of 4.5 g food grade cinnamon, 200 µg chromium polynicotinate and 120 mg magnesium citrate mixed with 100% kanuka honey | 40 days | ↓ weight ↓ blood pressure ↓ TC Improve blood lipid profile | [220] |
| Clover honey from Egypt | Type 1 diabetics human subjects (n = 20) aged 4–18 years | Orally administered honey (0.5 mL/kg bw/day) | 12 weeks | ↓ FSG, ↓ Glycosylated hemoglobin, ↓ SSFT, ↓ TC, ↓ LDL, ↑ FCP, ↑ PCP | [221] |
| Single case report of a patient with CHD, hypertension and type 2 diabetes | Given 150 g honey daily, orally | 11 years | Controlled RBG, blood pressure, improved/stabilized CHD, prevented ketoacidosis or hyperosmolar coma. | [222] | |
| Coriander honey from Egypt | 210 male Swiss albino mice weighting 22–25 g with serial intraperitoneal passage Ehrlich ascites carcinoma cells | G1 (normal control): orally dose of 50 µl/ mouse normal saline daily G2 (coriander control): daily dose of 500 mg/kg/mouse through oral administration G3 (5-FU control): daily dose of 20 mg/kg/mouse of 5-flurouracil as standard anticancer G4 (EAC control): was inoculated intraperitoneally with a single dose of EAC cell line (2 × 106 cells/mouse) + normal saline G5: Coriander + EAC G6: 5-FU + EAC | 21 days | ↑ IgM, IgG and IgA levels ↑ Phagocytic activity ↑ skin thickness | [223] |
| Tualang honey from Malaysia | Ten female Sprague-Dawley rats (age 6–8 weeks) weighing 140–170 g | G1 (HCD): 12% cholesterol diet G2 (HCD+TH): 12% cholesterol diet along with oral daily dose of 1.4 g/kg/day of tualang honey by gavage | 6 weeks | ↓ TC and TG compared to the control at 7 day ↓ Serum creatinine level copared to G1 after 48 h No structural effect in the HCD-fed rats | [224] |
| Buckwheat honey | Male Kunming mice (18–22 g) | G1 (Control): distilled water via gavage at 0.22 mL/10 g bw G2 (CCl4-treated mice): distilled water via gavage at 0.22 mL/10 g bw G3: 0.22 g/10 g bw of buckwheat honey G4: 0.5 mg/10 g bw of silymarin via gavage | 10 weeks | serum lipoprotein oxidation inhibition aspartate aminotransferase and alanine aminotransferase activities inhibition ↑ serum oxygen radical absorbance capacity ↓ Hepatic malondialdehyde ↑ GSH-Px and SOD activities lymphocyte DNA damage induced by carbon tetrachloride inhibition | [225] |
| Coriander honey | 21 patients between 20 and 85 years | G1 (n = 22): probiotics G2 (n = 21): probiotic combination with honey G3 (n = 24): placebo | 6 weeks | ↓ RBC, WBC, platelet levels ↓ Iga levels from 236.3 (58.6) to 206.3 (64.3) mg/dL | [226] |
| Antiproliferative | |||||
| Manuka honey | male mice at 8–12 weeks of age | 50% (w/v) manuka honey intravenously, 10 mg/kg taxol twice weekly | 3–4 weeks | ↑ Caspase-3 ↑ Survival rate | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants 2021, 10, 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10071023
Mărgăoan R, Topal E, Balkanska R, Yücel B, Oravecz T, Cornea-Cipcigan M, Vodnar DC. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants. 2021; 10(7):1023. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10071023
Chicago/Turabian StyleMărgăoan, Rodica, Erkan Topal, Ralitsa Balkanska, Banu Yücel, Titanilla Oravecz, Mihaiela Cornea-Cipcigan, and Dan Cristian Vodnar. 2021. "Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine" Antioxidants 10, no. 7: 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10071023