Immunohistochemical Analysis of 4-HNE, NGAL, and HO-1 Tissue Expression after Apocynin Treatment and HBO Preconditioning in Postischemic Acute Kidney Injury Induced in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
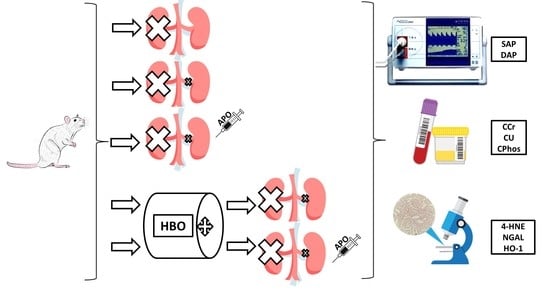
2.3. Experimental Design
2.4. Haemodynamic Measurements
2.5. Sample Collection
2.6. Glomerular Filtration
2.7. Immunohistochemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Hemodynamic Parameters
3.2. Glomerular Filtration Rate
3.3. Immunohistochemical Analysis
3.3.1. 4-Hydroxy-2-Nonenal (4-HNE) Expression
3.3.2. Neutrophil Gelatinase-Associated Lipocalin (NGAL) Expression
3.3.3. Heme Oxygenase-1 (HO-1) Expression
3.3.4. Immunohistochemical Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Tanaka, T.; Nagaku, M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Renal Physiol. 2014, 307, F1187–F1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Deng, A.; Baylis, C. Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient. Am. J. Physiol. Renal Physiol. 1993, 264, F212–F215. [Google Scholar] [CrossRef]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Schaur, R.; Siems, W.; Bresgen, N.; Ecki, P.E. 4-Hydroxy-nonenal—A bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- Jinsmaa, Y.; Florang, V.R.; Rees, J.N.; Anderson, D.G.; Strack, S.; Doorn, J.A. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem. Res. Toxicol. 2009, 22, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Bradley, M.A.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic. Biol. Med. 2010, 48, 1570–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflam. 2011, 514623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174. [Google Scholar] [CrossRef]
- Shinmura, K.; Bolli, R.; Liu, S.Q.; Tang, X.L.; Kodani, E.; Xuan, Y.T.; Srivastava, S.; Bhatnagar, A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ. Res. 2002, 91, 240–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Y. Effect of NADPH oxidase inhibitor-apocynin on the expression of Src homology-2 domain-containing phosphatase-1 (SHP-1) exposed renal ischemia/reperfusion injury in rats. Toxicol. Rep. 2015, 2, 1111–1116. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.W.; Cahill, J.; Zhang, J.H. Hyperbaric oxygen and cerebral physiology. Neurol. Res. 2007, 29, 132–141. [Google Scholar] [CrossRef]
- Lavrnja, I.; Parabucki, A.; Brkic, P.; Jovanovic, T.; Dacic, S.; Savic, D.; Pantic, I.; Stojiljkovic, M.; Pekovic, S. Repetitive hyperbaric oxygenation attenuates reactive astrogliosis and suppresses expression of inflammatory mediators in the rat model of brain injury. Mediat. Inflamm. 2015, 2015, 498405. [Google Scholar] [CrossRef]
- Parabucki, A.B.; Bozic, I.D.; Bjelobaba, I.M.; Lavrnja, I.C.; Brkic, P.D.; Jovanovic, T.S.; Savic, D.Z.; Stojiljkovic, M.B.; Pekovic, S.M. Hyperbaric oxygenation alters temporal expression pattern of superoxide dismutase 2 after cortical stab injury in rats. Croat. Med. J. 2012, 53, 586–597. [Google Scholar] [CrossRef] [Green Version]
- Hentia, C.; Rizzato, A.; Camporesi, E.; Yang, Z.; Muntean, D.M.; Săndesc, D.; Bosco, G. An overview of protective strategies against ischemia/reperfusion injury: The role of hyperbaric oxygen preconditioning. Brain Behav. 2018, 8, e00959. [Google Scholar] [CrossRef]
- Simsek, K.; Sadir, K.; Oter, S. The relation of hyperbaric oxygen with oxidative stress—reactive molecules in action. Oxid. Antioxid. Med. Sci. 2015, 4, 17–22. [Google Scholar] [CrossRef]
- Krzeminska, E.; Wyczalkowska-Tomasik, A.; Korytowska, N.; Paczek, L. Comparison of two methods for determination of NGAL levels in urine: ELISA and CMIA. J. Clin. Lab. Anal. 2016, 30, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Gagneux-Brunon, A.; Delanaye, P.; Legrand, D.; Cavalier, E.; Mariat, C. NGAL, biomarqueur de lésion rénale: Point d’étape en 2012. Néphrol. Thér. 2012, 8, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Wang, L.; He, H.Y.; Chen, J.; Yu, Y.R. Expression of neutrophil gelatinase-associated lipocalin inlow osmolar contrast-induced nephropathy in rats and the effect of N-acetylcysteine. Exp. Ther. Med. 2016, 12, 3175–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goleg, F.A.; Kong, N.C.; Sahathevan, R. Dialysis-treated end-stage kidney disease in Libya: Epidemiology and risk factors. Int. Urol. Nephrol. 2014, 46, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.R.; Li, Y.; Williams, D.E. Racial and ethnic differences in trends of end-stage renal disease: United States, 1995 to 2005. Adv. Chronic Kidney Dis. 2008, 15, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative stress and acute kidney injury in critical illness: Pathophysiological mechanisms—Biomarcers—Interventions, and future perspectives. Oxid. Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef] [Green Version]
- Abraham, N.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Inoue, K.; Li, X.; Drummond, G.; Abraham, N.G. Physiological significance of heme oxygenase in hypertension. Int. J. Biochem. Cell Biol. 2009, 41, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolisetty, S.; Zarjou, A.; Agarwal, A. Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury. Am. J. Kidney Dis. 2017, 69, 531–545. [Google Scholar] [CrossRef] [Green Version]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taille, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Durevil, B.; et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: Implications for protection against endotoxic shock in rats. FASEB J. 2005, 19, 1890–1892. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Itabe, H.; Maruyama, Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol. Cell. Biochem. 2006, 291, 21–28. [Google Scholar] [CrossRef]
- WHO. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 21 May 2021).
- Cartin-Ceba, R.; Kashiouris, M.; Plataki, M.; Kor, D.J.; Gajic, O.; Casey, E.T. Risk factors for development of acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Crit. Care Res. Pract. 2012, 2012, 691013. [Google Scholar] [CrossRef]
- Brkic, P.; Mitrovic, A.; Rakic, M.; Grajic, M.; Jovanovic, T. Hyperbaric oxygen therapy of angiopathic changes in patients with inherited gene imbalance. Srp. Arh. Celok. Lek. 2007, 135, 669–671. [Google Scholar] [CrossRef]
- Weaver, L.K. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report, 13th ed.; Best Publishing Company: North Palm Beach, FL, USA, 2014. [Google Scholar]
- Kovacevic, S.; Ivanov, M.; Miloradovic, Z.; Brkic, P.; Vajic, U.J.; Zivotic, M.; Mihailovic-Stanojevic, N.; Jovovic, D.; Karanovic, D.; Jeremic, R.; et al. Hyperbaric oxygen preconditioning and the role of NADPH oxidase inhibition in postischemic acute kidney injury induced in spontaneously hypertensive rats. PLoS ONE 2020, 15, e0226974. [Google Scholar] [CrossRef] [PubMed]
- Petras, T.; Siems, W.; Henke, W.; Jung, K.; Olbricht, C.J.; Gwinner, W.; Grune, T. Metabolic rates of 4-hydroxynonenal in tubular and mesangial cells of the kidney. Exp. Nephrol. 1999, 7, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. 4-Hydroxynonenal–protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol. Asp. Med. 2008, 29, 67–71. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, J.J.; Rabb, H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004, 66, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Invest. 2018, 48, 12951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heumuller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schroder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.G.; Yi, H.J.; Kim, N.H.; Rhee, S.G.; Woo, H.A. Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice. Antioxidants 2020, 9, 769. [Google Scholar] [CrossRef]
- Welch, W.J.; Baumgartl, H.; Lubbers, D.; Wilcox, C.S. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001, 59, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Bowmer, C.J.; Nichols, A.J.; Warren, M.; Yates, M.S. Cardiovascular responses in rats with glycerol-induced acute renal failure. Br. J. Pharmacol. 1983, 79, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Virdis, A.; Gesi, M.; Taddei, S. Impact of apocynin on vascular disease in hypertension. Vascul. Pharmacol. 2016, 87, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Buonafine, M.; Martinez-Martinez, E.; Jaisser, F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin. Sci. 2018, 132, 909–923. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesovic Ostojic, J.; Ivanov, M.; Mihailovic-Stanojevic, N.; Karanovic, D.; Kovacevic, S.; Brkic, P.; Zivotic, M.; Vajic, U.J.; Jovovic, D.j.; Jeremic, R.; et al. Hyperbaric oxygen preconditioning upregulates heme oxygenase-1 and anti-apoptotic Bcl-2 protein expression in spontaneously hypertensive rats with induced postischemic acute kidney injury. Int. J. Mol. Sci. 2021, 22, 1382. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, P.; Halabian, R.; Rouhbakhsh, M.; Roushandeh, A.M.; Masroori, N.; Ebrahimi, M.; Samadikuchaksaraei, A.; Shokrgozar, M.A.; Roukenar, M.H. Neutrophil gelatinase-associated lipocalin induces the expression of heme oxygenase-1 and superoxide dismutase 1, 2. Cell Stress Chaperones 2010, 15, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Roudkenar, M.; Halabian, R.; Bahmani, P.; Roushandeh, A.; Kuwahara, Y.; Fukumoto, M. Neutrophil gelatinase-associated lipocalin: A new antioxidant that exerts its cytoprotective effect independent on heme oxygenase-1. Free Radic. Res. 2011, 45, 810–819. [Google Scholar] [CrossRef]
- Mori, K.; Lee, H.T.; Rapoport, D.; Drexler, I.R.; Foster, K.; Yang, J.; Schmidt-Ott, K.M.; Chen, X.; Li, J.Y.; Weiss, S.; et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig. 2005, 115, 610–621. [Google Scholar] [CrossRef]
- Mishra, J.; Mori, K.; Ma, Q.; Kelly, C.; Yang, J.; Mitsnefes, M.; Barasch, J.; Devarajan, P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2004, 15, 3073–3082. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Bao, G.; Clifton, M.; Hoette, T.M.; Mori, K.; Deng, S.X.; Qiu, A.; Viltard, M.; Williams, D.; Paragas, N.; Leete, T.; et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 2010, 6, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Jung, H.; Kwak, K.H.; Yi, S.J.; Lim, J.A.; Park, S.H.; Park, J.M.; Kim, S.; Jee, D.L.; Lim, D.G. Inhibition of oxidative stress in renal ischemia-reperfusion injury. Anesth. Analg. 2017, 124, 204–213. [Google Scholar] [CrossRef]
- Sponsel, H.T.; Alfrey, A.C.; Hammond, W.S.; Durr, J.A.; Ray, C.; Anderson, R.J. Effect of iron on renal tubular epithelial cells. Kidney Int. 1996, 50, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, K.; Ohta, K.; Yachie, A.; Yang, Y.; Shimizu, M.; Goto, C.; Toma, T.; Kasahara, Y.; Yokoyama, H.; Miyata, T.; et al. Cytoprotective Role of Heme Oxygenase (HO)-1 in Human Kidney with Various Renal Diseases. Kidney Int. 2001, 60, 1858–1866. [Google Scholar] [CrossRef] [Green Version]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31, S170–S180. [Google Scholar] [CrossRef] [Green Version]
- Grundwald, A.; Roumenina, L.T.; Frimat, M. Heme oxygenase 1: A defensive mediator in kidney diseases. Int. J. Mol. Sci. 2021, 22, 2009. [Google Scholar] [CrossRef] [PubMed]
- Mosley, K.; Wembridge, D.E.; Cattell, V.; Cook, H.T. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998, 53, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]







| Title | CCr (mL/min/kg) | Cu (mL/min/kg) | CPhos (mL/min/kg) |
|---|---|---|---|
| SHAM | 4.03 ± 1.68 | 1.88 ± 0.89 | 0.71 ± 0.40 |
| AKI | 0.30 ± 0.18 *** | 0.09 ± 0.03 *** | 0.22 ± 0.10 ** |
| AKI + APO AKI + HBO AKI + APO + HBO | 1.70 ± 0.98 ## 1.33 ± 1.25 # 1.44 ± 0.077 # | 0.38 ± 0.21 ## 0.27 ± 0.22 0.30 ± 0.18 # | 0.62 ± 0.16 ### 0.54 ± 0.32 ## 0.48 ± 0.20 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacevic, S.; Ivanov, M.; Zivotic, M.; Brkic, P.; Miloradovic, Z.; Jeremic, R.; Mihailovic-Stanojevic, N.; Vajic, U.J.; Karanovic, D.; Jovovic, D.; et al. Immunohistochemical Analysis of 4-HNE, NGAL, and HO-1 Tissue Expression after Apocynin Treatment and HBO Preconditioning in Postischemic Acute Kidney Injury Induced in Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 1163. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10081163
Kovacevic S, Ivanov M, Zivotic M, Brkic P, Miloradovic Z, Jeremic R, Mihailovic-Stanojevic N, Vajic UJ, Karanovic D, Jovovic D, et al. Immunohistochemical Analysis of 4-HNE, NGAL, and HO-1 Tissue Expression after Apocynin Treatment and HBO Preconditioning in Postischemic Acute Kidney Injury Induced in Spontaneously Hypertensive Rats. Antioxidants. 2021; 10(8):1163. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10081163
Chicago/Turabian StyleKovacevic, Sanjin, Milan Ivanov, Maja Zivotic, Predrag Brkic, Zoran Miloradovic, Rada Jeremic, Nevena Mihailovic-Stanojevic, Una Jovana Vajic, Danijela Karanovic, Djurdjica Jovovic, and et al. 2021. "Immunohistochemical Analysis of 4-HNE, NGAL, and HO-1 Tissue Expression after Apocynin Treatment and HBO Preconditioning in Postischemic Acute Kidney Injury Induced in Spontaneously Hypertensive Rats" Antioxidants 10, no. 8: 1163. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10081163