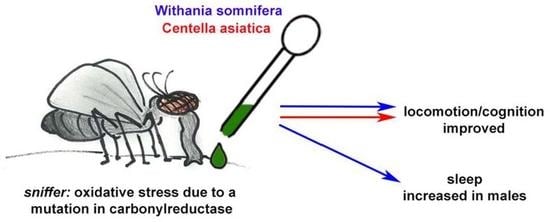
Withania somnifera and Centella asiatica Extracts Ameliorate Behavioral Deficits in an In Vivo Drosophila melanogaster Model of Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Stocks
2.2. Interventions
2.2.1. Raw Plant Materials
2.2.2. Preparation of Extracts
2.2.3. Fractionation of CAW
2.2.4. Analysis of A1 and Preparation of Matching CQA and TT Mixtures
2.2.5. Preparation of Test Food for D. melanogaster Flies
2.2.6. Administration to D. melanogaster Flies
2.3. Effects of WS Extract on NRF2 Activation in HepG2-ARE Reporter Cells
2.4. Phototaxis
2.5. Sleep Experiments
2.6. Statistical Analyses
3. Results
3.1. NRF2 Activation
3.2. Phototaxis
3.2.1. Sniffer Flies Show Reduced Performance in the Phototaxis Test Compared to CS Wild Type Flies
3.2.2. CAW at Higher Concentrations in Drosophila Food Improves Phototaxis in Female Sniffer Flies
3.2.3. CAW Mixed into Yeast Paste Improves Phototaxis in Sniffer Flies
3.2.4. CAW A1 Fraction and CQAs Improve Phototaxis in Sniffer Flies
3.2.5. WS Improves Phototaxis in Sniffer Flies
3.3. Sleep
4. Discussion
4.1. WS and Phototaxis
4.2. WS and Sleep
4.3. CAW and Phototaxis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations; Department of Economic and Social Affairs. World Population Prospects: The 2010 Revision. Volume I: Comprehensive Tables. 2011. Available online: https://www.un.org/en/development/desa/news/population/population-exceed-10-billion.html (accessed on 6 December 2021).
- He, W.; Goodkind, D.; Kowal, P. An Aging World: 2015 (International Population Reports, P95/16-1); U.S. Census Bureau/U.S. Government Publishing Office: Washington, DC, USA, 2016. [Google Scholar]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Rocca, W.A.; Petersen, R.C.; Knopman, D.S.; Hebert, L.E.; Evans, D.A.; Hall, K.S.; Gao, S.; Unverzagt, F.W.; Langa, K.M.; Larson, E.B.; et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011, 7, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Blazer, D.G.; Yaffe, K.; Karlawish, J. Cognitive aging: A report from the Institute of Medicine. JAMA 2015, 313, 2121–2122. [Google Scholar] [CrossRef]
- Zdanys, K.F.; Steffens, D.C. Sleep Disturbances in the Elderly. Psychiatr. Clin. N. Am. 2015, 38, 723–741. [Google Scholar] [CrossRef]
- Benca, R.M.; Teodorescu, M. Sleep physiology and disorders in aging and dementia. Handb. Clin. Neurol. 2019, 167, 477–493. [Google Scholar] [CrossRef]
- Gooneratne, N.S.; Vitiello, M.V. Sleep in older adults: Normative changes, sleep disorders, and treatment options. Clin. Geriatr. Med. 2014, 30, 591–627. [Google Scholar] [CrossRef] [Green Version]
- Casey, D.A. Depression in Older Adults: A Treatable Medical Condition. Prim. Care 2017, 44, 499–510. [Google Scholar] [CrossRef]
- Almeida, O.P. Prevention of depression in older age. Maturitas 2014, 79, 136–141. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [Green Version]
- Sanford, A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef]
- Cooper, C.; Li, R.; Lyketsos, C.; Livingston, G. Treatment for mild cognitive impairment: Systematic review. Br. J. Psychiatry 2013, 203, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, S.; Fujishiro, H.; Takechi, H. Efficacy and Safety of Cholinesterase Inhibitors for Mild Cognitive Impairment:A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 71, 513–523. [Google Scholar] [CrossRef]
- Dar, N.J.; MuzamilAhmad. Neurodegenerative diseases and Withania somnifera (L.): An update. J. Ethnopharmacol. 2020, 256, 112769. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Hossain, M.; Khalil, M.I.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochem. Rev. 2012, 11, 97–112. [Google Scholar] [CrossRef]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Banerjee, S.; Biswas, S.; Das, B.; Kar, A.; Katiyar, C.K. Withania somnifera (L.) Dunal—Modern perspectives of an ancient Rasayana from Ayurveda. J. Ethnopharmacol. 2021, 264, 113157. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D.; Acharya, S.; Ankari, A.B.; Nanakram, V.; Raghavendra, A.S. Metabolomics of Withania somnifera (L.) Dunal: Advances and applications. J. Ethnopharmacol. 2021, 267, 113469. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, U.G.; Prasad Kumarab, P.A. Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits. Adv. Food Nutr. Res. 2015, 76, 125–157. [Google Scholar] [CrossRef] [PubMed]
- Lokanathan, Y.; Omar, N.; Ahmad Puzi, N.N.; Saim, A.; Hj Idrus, R. Recent Updates in Neuroprotective and Neuroregenerative Potential of Centella asiatica. Malays. J. Med. Sci. 2016, 23, 4–14. [Google Scholar]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef]
- Gray, N.E.; Alcazar Magana, A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asaiatica- Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging-matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [Green Version]
- Durg, S.; Dhadde, S.B.; Vandal, R.; Shivakumar, B.S.; Charan, C.S. Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: A systematic review and meta-analysis. J. Pharm. Pharmacol. 2015, 67, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol. 2016, 180, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Kalonia, H. Effect of Withania somnifera on sleep-wake cycle in sleep-disturbed rats: Possible GABAergic mechanism. Indian J. Pharm. Sci. 2008, 70, 806–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, B.H. Drosophila—A versatile model in biology & medicine. Mater. Today 2011, 14, 190–195. [Google Scholar] [CrossRef]
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef] [Green Version]
- Dissel, S. Drosophila as a Model to Study the Relationship Between Sleep, Plasticity, and Memory. Front. Physiol. 2020, 11, 533. [Google Scholar] [CrossRef]
- Botella, J.A.; Ulschmid, J.K.; Gruenewald, C.; Moehle, C.; Kretzschmar, D.; Becker, K.; Schneuwly, S. The Drosophila carbonyl reductase sniffer prevents oxidative stress-induced neurodegeneration. Curr. Biol. 2004, 14, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Maser, E. Neuroprotective role for carbonyl reductase? Biochem. Biophys. Res. Commun. 2006, 340, 1019–1022. [Google Scholar] [CrossRef]
- Dutta, S.; Rieche, F.; Eckl, N.; Duch, C.; Kretzschmar, D. Glial expression of Swiss cheese (SWS), the Drosophila orthologue of neuropathy target esterase (NTE), is required for neuronal ensheathment and function. Dis. Model. Mech. 2016, 9, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Bolkan, B.J.; Triphan, T.; Kretzschmar, D. β-secretase cleavage of the fly amyloid precursor protein is required for glial survival. J. Neurosci. 2012, 32, 16181–16192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzer, S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, R.; Heisenberg, M. A higher control center of locomotor behavior in the Drosophila brain. J. Neurosci. 1993, 13, 1852–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassar, M.; Law, A.D.; Chow, E.S.; Giebultowicz, J.M.; Kretzschmar, D. Disease-Associated Mutant Tau Prevents Circadian Changes in the Cytoskeleton of Central Pacemaker Neurons. Front. Neurosci. 2020, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Metaxakis, A.; Tain, L.S.; Grönke, S.; Hendrich, O.; Hinze, Y.; Birras, U.; Partridge, L. Lowered insulin signalling ameliorates age-related sleep fragmentation in Drosophila. PLoS Biol. 2014, 12, e1001824. [Google Scholar] [CrossRef]
- Zweig, J.A.; Caruso, M.; Brandes, M.S.; Gray, N.E. Loss of NRF2 leads to impaired mitochondrial function, decreased synaptic density and exacerbated age-related cognitive deficits. Exp. Gerontol. 2020, 131, 110767. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhansi, D.; Kola, M. The antioxidant potential of Centella asiaitica: A review. J. Med. Plants Stud. 2019, 7, 18–20. [Google Scholar]
- Ghosh, T.; Suke, S.; Yadav, C.; Ahmed, R.; Banerjee, B. Ashwagandha Reverses the Dierldrin-induced Cognitive Impairment by Modulation of Oxidative Stress in Rat Brain. Pharmacogn. Res. 2019, 11, 92–97. [Google Scholar] [CrossRef]
- Khan, M.A.; Subramaneyaan, M.; Arora, V.K.; Banerjee, B.D.; Ahmed, R.S. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J. Complement. Integr. Med. 2015, 12, 117–125. [Google Scholar] [CrossRef]
- Shah, N.; Singh, R.; Sarangi, U.; Saxena, N.; Chaudhary, A.; Kaur, G.; Kaul, S.C.; Wadhwa, R. Combinations of Ashwagandha leaf extracts protect brain-derived cells against oxidative stress and induce differentiation. PLoS ONE 2015, 10, e0120554. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Guerra-Araiza, C.; Álvarez-Mejía, A.L.; Sánchez-Torres, S.; Farfan-García, E.; Mondragón-Lozano, R.; Pinto-Almazán, R.; Salgado-Ceballos, H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013, 47, 451–462. [Google Scholar] [CrossRef]
- Manjunath, M.J.; Muralidhara. Standardized extract of Withania somnifera (Ashwagandha) markedly offsets rotenone-induced locomotor deficits, oxidative impairments and neurotoxicity in Drosophila melanogaster. J. Food Sci. Technol. 2015, 52, 1971–1981. [Google Scholar] [CrossRef] [Green Version]
- De Rose, F.; Marotta, R.; Poddighe, S.; Talani, G.; Catelani, T.; Setzu, M.D.; Solla, P.; Marrosu, F.; Sanna, E.; Kasture, S.; et al. Functional and Morphological Correlates in the Drosophila LRRK2 loss-of-function Model of Parkinson’s Disease: Drug Effects of Withania somnifera (Dunal) Administration. PLoS ONE 2016, 11, e0146140. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.L.M.; Brogan, B.; Whitworth, A.J.; Okello, E.J. Effects of Five Ayurvedic Herbs on Locomotor Behaviour in a Drosophila melanogaster Parkinson’s Disease Model. Phytother. Res. 2014, 28, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ma, W.W.; Peng, I.F. Screening of sleep assisting drug candidates with a Drosophila model. PLoS ONE 2020, 15, e0236318. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Flick, G.; Rozpedowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piskur, J.; Witzgall, P.; et al. Yeast, not fruit volatiles, mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Gray, N.E.; Sampath, H.; Zweig, J.A.; Quinn, J.F.; Soumyanath, A. Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. J. Alzheimers Dis. 2015, 45, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol. Cell Biochem. 2019, 457, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Niveditha, S.; Deepashree, S.; Ramesh, S.R.; Shivanandappa, T. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B 2017, 187, 899–909. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Naz, F.; Jyoti, S.; Fatima, A.; Khanam, S.; Rahul; Ali, F.; Mujtaba, S.F.; Faisal, M. Effect of Centella asiatica Leaf Extract on the Dietary Supplementation in Transgenic Drosophila Model of Parkinson’s Disease. Parkinsons Dis. 2014, 2014, 262058. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Compound | Concentration of Compounds in Test Solutions (mg/mL) (10× That Present in Drosophila Food) | |||
|---|---|---|---|---|
| CAW 100 mg/mL | A1 11.35 mg/mL | CQA Mix | CAST 2 mg/mL | |
| CQAs: | ||||
| Neochlorogenic acid | 0.21 | 0.02 | 0.02 | - |
| Chlorogenic acid | 0.21 | 0.06 | 0.06 | - |
| Cryptochlorogenic acid | 0.1 | 0.02 | 0.02 | - |
| 1,3-dicaffeoylquinic acid | 0.23 | 0.08 | 0.08 | - |
| 1,5-dicaffeoylquinic acid | 0.53 | 0.50 | 0.50 | |
| Isochlorogenic acid A | 0.54 | 0.24 | 0.24 | - |
| Isochlorogenic acid B | 0.34 | 0.08 | 0.08 | - |
| Isochlorogenic acid C | 0.18 | 0.00 | 0.00 | - |
| Triterpenes: | ||||
| Asiatic acid | nd | 0.01 | - | 0.46 |
| Madecassic acid | nd | 0.03 | - | 0.69 |
| Asiaticoside | 2.1 | 0.79 | - | 0.74 |
| Madecassoside | 0.56 | 0.21 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabey, K.; Long, D.M.; Law, A.; Gray, N.E.; McClure, C.; Caruso, M.; Lak, P.; Wright, K.M.; Stevens, J.F.; Maier, C.S.; et al. Withania somnifera and Centella asiatica Extracts Ameliorate Behavioral Deficits in an In Vivo Drosophila melanogaster Model of Oxidative Stress. Antioxidants 2022, 11, 121. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11010121
Cabey K, Long DM, Law A, Gray NE, McClure C, Caruso M, Lak P, Wright KM, Stevens JF, Maier CS, et al. Withania somnifera and Centella asiatica Extracts Ameliorate Behavioral Deficits in an In Vivo Drosophila melanogaster Model of Oxidative Stress. Antioxidants. 2022; 11(1):121. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11010121
Chicago/Turabian StyleCabey, Kadine, Dani M. Long, Alexander Law, Nora E. Gray, Christine McClure, Maya Caruso, Parnian Lak, Kirsten M. Wright, Jan F. Stevens, Claudia S. Maier, and et al. 2022. "Withania somnifera and Centella asiatica Extracts Ameliorate Behavioral Deficits in an In Vivo Drosophila melanogaster Model of Oxidative Stress" Antioxidants 11, no. 1: 121. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11010121