Exploring the Use of Iris Species: Antioxidant Properties, Phytochemistry, Medicinal and Industrial Applications
Abstract
:1. Introduction
2. Botany (Taxonomy, Geographic Distribution and Edaphic Conditions)
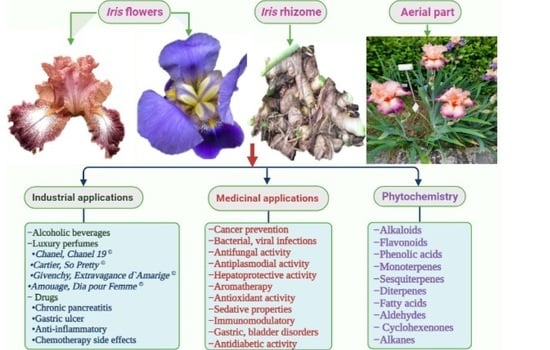
3. Uses and Applications
3.1. Ethnobotanical Uses
3.2. Ethnoveterinary Uses
| Botanical Name | Country | Parts Used | Ethno-Preparation | Mode of Administration | Ethnobotanical Uses | References |
|---|---|---|---|---|---|---|
| I. albicans Lange | Portugal | Fl | Nr | Nr | Ornamental, religious rituals (church, processions) | [45] |
| I. caucasica Hoffm. | Turkey | Fl | Raw | Oral | Food purposes (Eaten fresh) | [32,33] |
| I. clarkei Baker ex. Hook.f. | Nepal | R | Paste | Topical | Alleviate joint pain | [46] |
| India | Fl, Le, St, R | Paste | Topical | Muscle pains | [25] | |
| I. dichotoma Pall. | China | R | The root bark cut into small pieces | Topical | Gum swelling and toothache | [30] |
| I. domestica L. | Vietnam | Rh | Decoction | Oral | Cough | [47] |
| Bhutan | Nr | Liquide extract | Oral | Appetizers | [48] | |
| I. douglasiana Herb. | United states | R | Decoction, burned root | Oral, inhalation | Cathartic and emetic; to relieve dizziness, roots were burnt and the smoke inhaled. | [49] |
| I.drepanophylla Aitch. & Baker | Iran | Fl, R | Lily flower tea | Oral | Liver stimulant, cough, diuretic, expectorant | [50] |
| I. ensata Thunb | India | R | Nr | Oral | Blood cleanser, venereal infection | [51] |
| India | Sd | Powder | Oral | 10 g of seeds powder is used orally to eliminate stomach worms and tranquilize stomach ulcers | [28] | |
| India | Sd | Powder | Oral | 10 g of seeds powder is taken by oral route to treat gastric ulcers and stomach problems | [52] | |
| Pakistan | R | Nr | Nr | Medicinal purposes | [53] | |
| Pakistan | R | Decoction, raw | Oral | Blood purifier and to make green rice | [54] | |
| I. florentina L. | Morocco | Fl, St | Nr | Oral and topical | Ophthalmological agent, digestive and metabolic disorders | [55] |
| Bosnia and Herzegovina | Rh | Decoction, syrup | Oral | Cough and stomach disorders | [56] | |
| I. foetidissima L. | Portugal | Fl | Nr | Nr | Ornamental, religious rituals (Church, processions) | [45] |
| I. germanica L. | Morocco | Le | Nr | Nr | Neurological diseases | [55] |
| Italy | Rh | Raw | Oral and topical | Strengthen children teeth, chilblains, respiratory diseases, vomiting agent. | [35] | |
| Turkey | Rh | Peeled rhizomes | Oral | The rhizomes are dug out and peeled before being eaten with bread. | [34] | |
| Bosnia and Herzegovina | Rh | Decoction, Syrup | Oral | Cough and stomach problems | [56] | |
| I. germanica L. | Pakistan | R | Nr | Nr | Roots are used to reduce body pain. The plant is also cultivated in cemeteries | [57] |
| Pakistan | R | Decoction | Oral | Diuretic, intestinal obstruction in cattle | [58] | |
| I. goniocarpa Baker | Nepal | R | Paste | Topical | Root paste is used externally to alleviate itching and decrease joint pains. | [59] |
| I. hookeriana Foster | Pakistan | R | Nr | Topical, oral | Skin diseases, milk production in livestock | [60] |
| Pakistan | Le, Bu | Raw | Oral | The raw or cooked bulbs and leaves are consumed as vegetables | [61] | |
| Pakistan | Le | Nr | Oral | Anthelmintic for goat and sheep | [62] | |
| India | R | Paste | Oral | Sore throat treatment | [60] | |
| I. kashmiriana Baker | India | Rh, Le | Paste, raw | Topical | Raw rhizomes are applied to relieve joint pain, while flowers are appreciated for their antiseptic value. The infected eyes are also treated with flower paste. | [63] |
| India | WP | Powder | Topical | Dried herb powder is mixed with oil and applied to the affected area | [64] | |
| India | Rh | Nr | Nr | Eczema, wounds, body weakness, and repellent for rodents | [65] | |
| I. kopetdagensis (Vved.) B. Mathew & Wendelbo | Iran | Fl, R | Lily flower tea | Oral | Cough, diuretic, expectorant | [50] |
| I. lactea Pall. | China | Rh, Le, Se | Nr | Nr | Cold and cough, liver diseases | [29] |
| India | Fl, WP | Nr | Nr | The plant is used as fodder, to increase milk production in cattle, while the flowers are used for decorative purposes. | [66] | |
| India | WP | Decoction | Oral | Intestinal cramps, stomach cramps, boost appetite, food poisoning | [26] | |
| I. nepalensis Wall Ex Lindle. | India | Rh | Juice | Topical | The rhizome is crushed to extract the sap, and then applied to pimples daily for ten days. | [67] |
| India | R | Paste | Topical | Rheumatic pain | [68] | |
| I. persica L. | Turkey | WP | Nr | Nr | Grown in gardens for ornamental purposes | [33] |
| Turkey | Fl | Raw | Oral | Snack | [31] | |
| I. reticulata var. bakeriana (Foster) B. Mathew & Wendelbo | Turkey | Fl | Raw | Oral | Snack | [31] |
| I. songarica Schrenk | Pakistan | R | Crushed roots | Topical | Inflammation | [58] |
| I.sibirica L. | Brazil | R | Nr | Oral | Diarrhea | [69] |
| I.spuria L. | Iran | R | Nr | Nr | Diuretic, Arthrodynia | [70] |
| I. tectorum Maxim. | China | Le | Nr | Nr | The plants’ leaves are utilized by people to wrap zongzi, a traditional Chinese rice dish. | [71] |
| I.xiphium L. | Portugal | Fl | Nr | Nr | Ornamental, religious rituals (church, processions) | [45] |
3.3. Pharmaceutical Uses
3.4. Potential Application in the Food Industry
4. Phytochemistry
4.1. Phenolic Acids
| Polyphenolic Acids | Activities and Functions | Species Resources | Plant Part | References |
|---|---|---|---|---|
| Hydroxybenzoic acid derivatives | ||||
| Gallic acid (1) | Anticancer, cardioprotective, neurodegenerative diseases prevention, ameliorative for metabolic diseases. | I. hungarica Waldst. I. Variegata L., I. schachtii Markgr., I. lactea Pall., I. pseudacorus L. | Rh | [75,76,77,78,79] |
| p-hydoxybenzoic acid (2) | Keratolytic agent, antimicrobial, antioxidant, cytotoxic activities. | I. schactii Markgr., I. flavissima Pall., I. dichotoma Pall., I. germanica L., I. versicolor L., I. lactea Pall. | Rh, R | [76,78,80] |
| Protocatechuic acid (3) | Neuroprotective, brain injury attenuation, ameliorative for metabolic diseases, cardiovascular protection, liver injury, antineoplastic agent, anti-asthma, antispasmodic, antiulcer properties. | I. schachtii Markgr., I. flavissima Pall., I. dichotoma Pall., I. germanica L., I. pseudacorus L. | Rh, L | [76,77,79,80] |
| Syringic acid (4) | Anti-inflammatory, antimicrobial, hepatoprotective, antiendotoxic, neuroprotective effects, prevention and alleviation of oxidative stress, prevention of diabetes; cerebral ischemia, cancer, and cardiovascular diseases. | I. schactii Markgr., I. flavissima Pall., I. dichotoma Pall., I. lactea Pall., I. bungei Maxim. | Rh, L | [76,79,81,82,83] |
| Vanillic acid (5) | Neuroprotective, hepatoprotective, antimicrobial, anti-inflammatory effects (anti-ulcerative colitis effects). | I. schactii Markgr., I. flavissima Pall., I. dichotoma Pall., I. bungei Maxim., I. tenuifolia Pall., I. lactea Pall., I. florentina L., I. germanica L., I. versicolor L., I. carthaliniae Fomin | L, R, Rh | [76,78,79,80,83,84,85,86] |
| Hydroxycinnamic acid derivatives | ||||
| Ferulic acid (6) | Ultraviolet absorption, antioxidant, anti-aging for skin, anti-inflammatory, cardioprotective. | I. schactii Markgr., I. flavissima Pall., I. dichotoma Pall., I. germanica L., I. carthaliniae Fomin, I. lactea Pall. | Rh, R, L | [73,78,80,86] |
| Caffeic acid (7) | Ultraviolet absorption, antioxidant (prevents oxidative stress and DNA damage), food preservation, antimicrobial, anti-cancer, anti-inflammatory. | I. hungarica Waldst., I. variegata L., I. schachtii Markgr., I. pallida Lam., I. sibirica L., I. flavissima Pall., I. dichotoma Pall. | L, R | [75,76,78,79,86,87] |
| Chlorogenic acid (8) | Antioxidant, antihypertensive, chemopreventive, neuroprotective effects, cardiovascular benefits. | I. pseudacorus L. | Rh, L | [80,88] |
| Neochlorogenic acid (9) | Chemopreventive, anticarcinogenics, and as a laxative | I. halophila Pall., I. pseudacorus L., I. sibirica L. | Rh | [75] |
| trans-Cinnamic acid (10) | Anti-oxidant, anti-obesity, antitumor (colon cancer), antimicrobial, anti-inflammatory. | I. pallida Lam., I. versicolor L., I. lactea Pall., I. carthaliniae Fomin, I. germanica L. | Rh, R, L | [78,89] |
| p-coumaric acid (11) | Food preservation, skin-lightening, antimicrobial properties. | I. bungei Maxim, I. flavissima Pall., I. dichotoma Pall., I. lactea Pall., I. tenuifolia Pall. | L | [79,87] |
| Sinapic acid (12) | Antioxidant, anticancer, antidiabetic, neuroprotective, anti-inflammatory, antibacterial, antimutagenic effects. | I. schachtii Markgr. | Rh | [76,90] |
4.2. Flavonoids
| Flavonoids | Activities and Functions | Species Resources | Plant Part | References |
|---|---|---|---|---|
| Flavones and flavone glycosides | ||||
| Luteolin (13) | Anticancer, chemopreventive, antioxidant, neuroprotector, anti-inflammatory, molluscicidal, immunomodulatory effects. | I. schachtii Markgr., I. pseudacorus L. | Rh, L | [76,77,104,105] |
| Apigenin (14) | Antioxidant (↑ CAT, SOD, GSH), anti-amyloidogenic, analgesic, anti-inflammatory, anticancer, anti-hyperglycemic, hepatoprotective effects. | |||
| Vitexin (apigenin-8-C-glucoside) (15) | Prevention of hypoxia and ischemia injury, antidiabetic (α-glucosidase inhibitor), anti-inflammatory, anti-hyperalgesic, anti-inflammatory, molluscicidal, and neuroprotective properties. | I. pseudacorus L. | L | [77,106] |
| Iso-vitexin (apigenin-6-C-glucoside) (16) | Anti-oxidant, antidiabetic (α-glucosidase inhibitor), antilipase, anti-inflammatory, molluscicidal, antinociceptive, protective effects against hypoxia and ischemia injury. | |||
| Isovitexin 2″-O-glucoside (17) | Antioxidant, protective against UV-B radiation | I. sanguinea var. Tobataensis, I. sanguinea var. sanguinea | F, L | [107] |
| Orientin (18) | Antioxidant, antiviral, anti-inflammatory, antibacterial, cardioprotective, radiation protective, antiaging, neuroprotective, antiadipogenesis, antinociceptive, and antidepressant-like effects. | I. pseudacorus L. | L | [77,108] |
| Iso-orientin (19) | Antioxidant, anti-inflammatory, antinociceptive, and hepatoprotective properties. | I. pseudacorus L. | L | [77,109] |
| Swertisin (20) | Antidiabetic | I. germanica L., I. biflora L., I. albicans Lange, I. setina Colas., I. marsica I. Ricci & Colas. | Rh, L., F | [91,110] |
| Swertisin 2″-O-rhamnoside (21) | Antioxidant | I. pallida Lam. | L | [91] |
| Embinin (22) | Antioxidant, anticancer (ovarian BG-1, SkBr3 and MCF7 breast, lung A549 cells, and mesothelioma IST-MES1) | I. germanica L., I. pallida Lam., I. japonica Thunb., I. persica L., I. tectorum Maxim. | L, F | [91,111] |
| Swertiajaponin (23) | Anti-atherosclerosis (prevents the in vitro LDL oxidation), and anti-oxidant activity | I. germanica L., I. albicans Lange | L | [91,112] |
| 5-hydroxy-4′-methoxyflavone (24) | Antioxidant, neuroprotective | I. ensata Thunb. | CT | [113] |
| 5-hydroxy-3′-methoxyflavone (25) | ||||
| 5-hydroxy-2′-methoxyflavone (26) | ||||
| Vicenin-2 (27) | α-glucosidase inhibitor, antioxidant, hepatoprotective, anti-inflammatory, molluscicidal. | I. pseudacorus L. | L | [77] |
| Hispidulin (28) | Antioxidant, anticonvulsant, anti-inflammatory, and antineoplastic. | I. bungei Maxim. | L | [83] |
| Isoflavones | ||||
| Tenuifodione (29) | Antioxidant | I. tenuifolia Pall. | WP | [92] |
| Tenuifone (30) | ||||
| Irisone A (31) | Antioxidant, estrogenic effects | I. missouriensis Nutt., I. tenuifolia Pall. | R, WP | [92,94] |
| Irisone B (32) | Antioxidant, estrogenic effects | I. missouriensis Nutt., I. tenuifolia Pall., I. songarica Schrenk | ||
| Irilin B (33) | Antioxidant, estrogenic effects | I. songarica Schrenk | Rh, R | [94] |
| Irilin D (34) | Antioxidant, cholinesterase inhibitory activity | I. dichotoma Pall. | Rh | [93] |
| Genistein (35) | Antioxidant, anti-inflammatory, antiviral, antibacterial, estrogen-like functions. | I.germanica L., I. carthaliniae Fomin, I. lactea Pall., I. lactea Pall. | Rh, R, L | [78] |
| Genistein-7-O-glucoside (36) | Antioxidant | I. tectorum Maxim., I. dichotoma Pall. | Rh | [93] |
| Irisflorentin (37) | Estrogenic | I. adriatica Trinajstic ex Mitic, I. florentina L. | Rh | [100] |
| Dichotomitin (38) | Antioxidant | I. dichotoma Pall. | Rh | [93] |
| Dichotomitin 3′-O-glucoside (39) | ||||
| Irigenin S (40) | Estrogenic, anti-inflammatory | I. adriatica Trinajstic ex Mitic, I. germanica L. | Rh | [12,100] |
| Irilone (41) | Immunomodulatory, antineoplastic, α-amylase inhibitory potency | |||
| Iriskumaonin methyl ether (42) | Cytotoxic | I. adriatica Trinajstic ex Mitic, I. germanica L., I. pallida Lam. | Rh | [100,114] |
| Irigenin (43) | Estrogenic activity, α-amylase inhibitory, anti-inflammatory, and inhibitor of cytochrome P450 1A. | I. adriatica Trinajstic ex Mitic, I. germanica L., I. pallida Lam., I. germanica L. | Rh | [12,100,114] |
| Iristectorigenin A (44) | Weak anti-inflammatory, hepatoprotective | |||
| Iristectorin B (45) | Estrogenic, anticancer activity (Breast cancer) | I. tectorum Maxim., I. dichotoma Pall. | Rh | [93] |
| Irisolone (nigricin) (46) | Anti-inflammatory, cytotoxic. | I. adriatica Trinajstic ex Mitic, I. germanica L., I. pallida Lam. | Rh | [100,114] |
| Irisolidone (47) | Antioxidant, anti-inflammatory, antidiabetic, CyP1A inhibitor, and immunomodulatory activity. | I. germanica L. | Rh | [12] |
| 8-Hydroxyirigenin (48) | α-amylase inhibitory, antioxidant | I. germanica L., I. pallida Lam. | Rh | [111,114] |
| Germanaism A (49) | Cytotoxic | I. germanica L | Rh | [12] |
| 5,7-Dihydroxy-3-(3′-hydroxy-4′,5′-dimethoxy)-8-methoxy-4H-1-benzopyran-4-one (50) | Potent anti-inflammatory | |||
| Germanaism B (51) | Antioxidant | I. hungarica Waldst. & Kit. I. variegata L., I. pallida Lam. I. sibirica L | Rh | [75,100] |
| Germanaism E (52) | Antioxidant | I. adriatica Trinajstic ex Mitic | Rh | [100] |
| Tectorigenin (53) | Antioxidant, antiproliferative, anti-hyperalgesic, antineoplastic, hepatoprotective, cardiovascular protector, estrogenic, and antithrombotic effects. | I. adriatica Trinajstic ex Mitic, I. germanica L. | Rh | [12,100] |
| Tectorigenin-7-O-glucosyl-4′-O-glucoside (54) | Antioxidant | I. tectorum Maxim. | Rh | [93] |
| Irifloside (55) | Cytotoxic | I. germanica L. | Rh | [12] |
| Iriskashmirianin A (56) | [115] | |||
| Germanaism H (57) | ||||
| 8-Hydroxyirilone 5-methyl ether (58) | α-amylase inhibitory, antioxidant | I. germanica L. | Rh | [12] |
| Irilone 4′-O-β-D-glucopyranoside (59) | Anti-inflammatory | |||
| Irisolidone 7-O-β-D-glucopyranoside (60) | Antioxidant, CyP1A inhibitor | |||
| Iridin (61) | Anti-inflammatory | |||
| Iridin A (62) | α-amylase inhibitory, antioxidant | |||
| Iridin S (63) | Cytotoxic | I. germanica L. | Rh | [116] |
| Dichotomitin 3′-O-(6″-hexosyl)hexoside (64) | Antioxidant | I. humilis Georgi | R | [102] |
| Irisolone-O-sinapoylhexoside (65) | ||||
| 5,6-Dihydroxy-7,8,3′,5′-tetramethoxyisoflavone (66) | Antioxidant | I. pseudacorus L., I. pallida Lam., I. versicolor L., I. hungarica Waldst | Rh | [75] |
| Dalspinosin (67) | Antioxidant | I. dichotoma Pall. | [93] | |
| Homotectoridin (68) | I. tectorum Maxim, I. dichotoma Pall. | |||
| Ayamenin A (69) | Estrogenic, fungitoxic | I. pseudacorus L. | L | [83] |
| Ayamenin B (70) | I. pseudacorus L., I. bungei Maxim. | |||
| Ayamenin C (71) | Fungitoxic | I. pseudacorus L. | ||
| Ayamenin E (72) | ||||
| Daidzein (73) | Antineoplastic, estrogenic activity | I. hungarica Waldst. | Rh | [75] |
| Formononetin (74) | Antiadipogenic, bone loss protection, anti-osteoporosis activity | |||
| Tectoridin (75) | Anti-inflammatory, a platelet agglutination inhibitor. | |||
| Iriflogenin (76) | Cytotoxic | I. dichotoma Pall. | Rh | [93] |
| Tectorigenin 7-O-glucosyl-(1→3)-glucoside (77) | Hepatoprotective | I. japonica Thunb. | WP | [117] |
| Iristectorigenin B 7-O-glucoside (78) | Antioxidant | Iris dichotoma Pall. | Rh | [93] |
| Irigenin 7-O-glucoside (79) | Antimutagenic, antioxidant | I. tectorum Maxim, I. dichotoma Pall. | ||
| Iristectorigenin A 7-O-gentiobioside (80) | Antioxidant | I. adriatica Trinajstic ex Mitic | Rh | [100] |
| Flavonols | ||||
| Irisoid A (81) | Antioxidant, anticancer | I. songarica Schrenk, I. bungei Maxim. | Rh, R | [94,96] |
| Irisoid B (82) | Antioxidant | I. bungei Maxim | Rh, R | [96] |
| Irisoid C (83) | ||||
| Irisoid D (84) | ||||
| Irisoid E (85) | ||||
| Irisflavone A (86) | Antioxidant, estrogenic | I. bungei Maxim., I. songarica Schrenk | Rh, R | [94,96] |
| Irisflavone B (87) | Antioxidant, estrogenic | I. bungei Maxim. | Rh, R | [93] |
| Irisflavone C (88) | ||||
| Irisflavone D (89) | ||||
| Rhamnocitrin (kaempferol-7-methylether) (90) | Antioxidant, cytotoxicity, antiviral (inhibition of Influenza A Jiangsu/10/2003 virus) | I. tectorum Maxim. | Rh | [93] |
| Kaempferol 3-O-glucoside (91) | Antiproliferative | I. humilis Georgi | Rh, F | [102] |
| Kaempferol 3-O-galactoside (92) | Antioxidant, anti-cancer, anti-inflammatory | F | ||
| Isorhamnetin 3-O-glucoside(93) | Antioxidant, anti-cancer, anti-inflammatory, antiviral. | |||
| Embigenin (94) | Anticancer. | I. tectorum Maxim. | L | [118] |
| Quercetin-3-glucoside (95) | Hepatoprotective, antiproliferative, antioxidant, cardioprotective, anti-allergic, and neuroprotective. | I. pallida Lam., I. germanica L. | L, R | [78,119] |
| Quercetin 3-O-galactoside (96) | ||||
| Quercetin 3-O-rhamnoside (97) | Antioxidant, anti-cancer, anti-viral, anti-inflammatory. | I. sanguinea var. Tobataensis, I. sanguinea var. sanguinea | F, L | [107,119] |
| Myricetin 3-O-rhamnoside (98) | Antioxidant; anticancer, antidiabetic, anti-HIV, anti-Alzheimer, anti-inflammatory. | I. sanguinea var. Tobataensis, I. sanguinea var. sanguinea | F, L | [107,120] |
| Hyperoside (quercetin-3-O-galactoside) (99) | Anti-inflammatory, hepatoprotective | I. humilis Georgi | F | [102] |
| Irisdichotin B (100) | Antioxidant | I. humilis Georgi, I. dichotoma Pall., I. pumila L. | Rh, R | [97,102] |
| Kaempferol (101) | Antioxidant, anticancer, anti-inflammatory, chemo-preventative, geroprotector. | I. schachtii Markgr. | Rh, L | [79,121] |
| Rutin (102) | Antioxidant, anti-inflammatory, antimicrobial, improving blood flow, cardioprotective. | I. schachtii Markgr. | Rh | [76] |
| Izalpinin (103) | Potent inhibitor of bladder contractions | I. tenuifolia Pall. | WP | [92,122] |
| Flavan-3-ols | ||||
| (+)-Catechin (104) | Potent antioxidant, molluscicidal, antimicrobial, chemopreventive, anticancer. | I. germanica L., I. schachtii Markgr. | Rh, AGP | [76,77] |
| (-)-Epicatechin (105) | Rh, L | |||
| Isoflavanones | ||||
| 2,3-Dihydroirigenin (106) | Antioxidant | I. germanica L., I. pallida Lam. | Rh | [114] |
| Dihydroflavonol | ||||
| Songaricol (107) | Antioxidant | I. songarica Schrenk | Rh, R | [94] |
| Coumaronochromone | ||||
| Irisbungin (108) | Antibacterial | I. bungei Maxim. | L | [83] |
| Flavanone | ||||
| 5,7,2′-Trihydroxy-6-methoxyflavanone (109) | Molluscicidal | I. germanica L | Rh, L | [123] |
| Flavanonol | ||||
| Irisdichotin B (110) | Antioxidant | I. dichotoma Pall. | Rh | [97] |
| Irisdichotin C (111) | ||||
| Alpinone (112) | Antioxidant, immunostimulant, antiviral. | I. tenuifolia Pall. | WP | [92] |
| Dihydrokaempferide (113) | Antimicrobial activity against Staphylococcus aureus, Coniophora puteana, antioxidant | I. tectorum Maxim. | Rh | [93] |
| Xanthones | ||||
| Mangiferin (114) | Antibacterial, anti-inflammatory, antioxidant, analgesic, anticancer. | I. pallida Lam., I. hungarica Waldst. & Kit., I. sibirica L., I. variegata L., I. humilis Georgi, | Rh, F | [75,102] |
| Neomangiferin (115) | Antidiabetic and antiosteoporotic properties. | I. adriatica Trinajstic ex Mitic | Rh | [100] |
| Irisxanthone (116) | Potent antioxidant, antihyperglycemic | I. albicans Lange, I. adriatica Trinajstic ex Mitic, I. germanica L. | L, Rh | [97,100,124] |
| 7-O-methyl(iso)mangiferin-O-hexoside (117) | Potent antioxidant, anti-inflammatory | I. adriatica Trinajstic ex Mitic | Rh | [100] |
| 7-o-methyl(iso)mangiferin-O-hexoside (118) | ||||
| 7-O-Methylmangiferin (119) | Analgesic, antioxidant | I. pumila L., I. variegata L. | R | [102] |
| Isomangiferin (120) | Antioxidant, anti-inflammatory, chemoprotective, hepatoprotective, anticancer. | I. humilis Georgi, I. pumila L., I. variegata L. | R, F, AGP | [102,125] |
| 7-O-Methylisomangiferin (121) | Antioxidant | I. humilis Georgi, I. pumila L., I. variegata L. | R, F, AGP | [102] |
| Iriflophenone (122) | I. humilis Georgi, I. pumila L., I. variegata L. | R, F | ||
| Polygalaxanthone III (123) | Antioxidant, anxiolytic, sedative. | I. humilis Georgi | R | |
| Nigricanside (124) | Antioxidant, antihyperglycemic, antihyperlipidemic | I. variegata L., I. nigricans Dinsm. | R, Rh | [102,103] |
| Bellidifolin (125) | Anti-hyperalgesic | I. pumila L. | F | [102] |
| Iriflophenone (126) | Antioxidant | I. pumila L., I. variegata L., I. humilis Georgi | R, F | |
| 4-O-methyliriflophenone (127) | Antibacterial | I. pallida Lam., I. lactea Pall. | Rh, R | |
| Iriflophenone 4-O-hexoside (128) | Antioxidant | I. pallida Lam, I. versicolor L., I. lactea Pall. | Rh, R, L | [78,102] |
| Iriflophenone 2-O-hexoside (129) | Antioxidant | I. pallida Lam, I. versicolor L., I. lactea Pall. | Rh, R, L | [78] |
| 1,3,5,8-Tetrahydroxyxanthone ((Desmethylbellidifolin) (130) | Antioxidant, acetylcholinesterase inhibitor | I. nigricans Dinsm. | Rh | [103] |
| Anthocyanins | ||||
| Delphinidin 3-O-[acetyl-(p-coumaroyl)]rutinoside-5-O-glucoside (132) | Antioxidant, anti-inflammatory, anti-aging skin | I. domestica L., I. dichotoma Pall | F | [98,126] |
| Delphinidin 3-O-(p-coumaroyl)rutinoside (133) | ||||
| Delphinidin 3-O-(p-coumaroyl)rutinoside (133) | ||||
| Delphinidin 3-O-(feruloyl)rutinoside-5-O-glucoside (134) | Antioxidant, anti-inflammatory, anti-aging skin | I. domestica L., I. dichotoma Pall | F | [98,126] |
| Delphinidin 3-O-(trans-p-coumaroyl)rutinoside-5-O-glucoside (135) | Antioxidant, anti-inflammatory, anti-aging skin | I. domestica L., I. dichotoma Pall | F | [98,126] |
| Delphinidin 3-O-(cis-p-coumaroyl)rutinoside-5-O-glucoside (136) | ||||
| Delphinidin 3-O-(caffeoyl)rutinoside-5-O-glucoside (137) | ||||
| Delphinidin 3-O-rutinoside (138) | ||||
| Delphinidin 3-O-(acetyl)rutinoside-5-O-glucoside (139) | ||||
| Delphinidin 3-O-rutinoside-5-O-glucoside (140) | ||||
4.3. Alkaloids
4.4. Primary Metabolites
4.5. Essential Oils
| Compounds | Plant Parts | Method of Identification | Plant Resource | Country | References |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | |||||
| α-Pinene (141) | Rh | GC-MS | I. bulleyana Dykes | China | [131] |
| Camphene (142) | |||||
| β-Pinene (143) | |||||
| Limonene (144) | |||||
| trans-β-Ocimene (145) | |||||
| Oxygenated monoterpenes | |||||
| Linalool (146) | Rh | GC-MS, GC–FID | I. bulleyana Dykes, I. nigricans Dinsm | China | [131,132] |
| Camphor (147) | GC-MS | I. bulleyana Dykes | China | [131] | |
| (-)-Terpinen-4-ol (148) | GC-MS | ||||
| Linalool oxide (149) | GC-MS | I. bulleyana Dykes, I. carthaliniae Fomin, I. medwedewii Fomin | [131] | ||
| α-Terpineol (150) | GC-MS, GC–FID | I. bulleyana Dykes, I. nigricans Dinsm | China, Jordan | [131,132] | |
| 1,8-Cineol (151) | GC-MS, GC–FID | I. nigricans Dinsm | Jordan | [131] | |
| Borneol (152) | |||||
| Piperitenone oxide (153) | |||||
| Sesquiterpene hydrocarbons | |||||
| β-Elemene (154) | Rh | GC-MS, GC–FID | I. bulleyana Dykes, I. nigricans Dinsm | China, Jordan | [131,132] |
| α-Humulene (155) | Rh | GC-MS, GC–FID | I. nigricans Dinsm | Jordan | [132] |
| α-Muurolene (156) | Rh | GC-MS | I. bulleyana Dykes | China | [131] |
| γ-Muurolene (157) | |||||
| β-Gurjunene (158) | |||||
| α-Himachalene (159) | |||||
| α-Longipinene (160) | |||||
| Germacrene D (161) | Rh | GC-MS | I. bulleyana Dykes, I. carthaliniae Fomin, I. medwedewii Fomin | China, Azerbaïdjan | [129,131] |
| γ-Elemene (162) | Rh | GC-MS | I. bulleyana Dykes | China | [131] |
| α-Gurjunene (163) | |||||
| δ-Amorphene (164) | |||||
| α-Elemene (165) | |||||
| Alloaromadendrene (166) | |||||
| Cuparene (167) | |||||
| α-Bulnesene (168) | |||||
| δ-Cadinene (169) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin | Azerbaïdjan | [129] |
| Calamenene (170) | |||||
| β-Farnesene (171) | |||||
| Oxygenated sesquiterpenes | |||||
| Spathulenol (172) | Rh | GC-MS | I. bulleyana Dykes, I. carthaliniae Fomin, I. medwedewii Fomin | China, Azerbaïdjan | [129,131] |
| 1-Hydroxy-1,7-dimethyl-4-isopropyl-2,7-cyclodecadiene (173) | Rh | GC-MS | I. bulleyana Dykes | China | [131] |
| τ-Cadinol (174) | |||||
| α-Cadinol (175) | Rh | GC-MS, GC–FID | I. bulleyana Dykes, I. carthaliniae Fomin, I. medwedewii Fomin | China, Azerbaïdjan, Jordan | [129,131,132] |
| β-Cadinol (176) | Rh | GC-MS | I. bulleyana Dykes, I. carthaliniae Fomin, I. medwedewii Fomin | China, Azerbaïdjan | [129,131] |
| Aristolone (177) | Rh | GC-MS | I. bulleyana Dykes | China | [131] |
| β-Bisabolene epoxide (178) | I. carthaliniae Fomin | Azerbaïdjan | [129] | ||
| Diterpenes hydrocarbons | |||||
| Neophytadiene (179) | L | GC-MS | I. germanica L., I. versicolor L. | Ukraine | [133] |
| Oxygenated diterpenes | |||||
| Phytol (180) | L | GC-MS | I. versicolor L. | Ukraine | [133] |
| Triterpenes hydrocarbons | |||||
| Squalene (181) | Rh, L | GC-MS | I. pallida Lam., I. germanica L., I. versicolor L., I. graminea L., I. halophila Pall. | Ukraine | [11,133] |
| Fatty acids | |||||
| Stearic acid (182) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin | Azerbaïdjan | [129] |
| Oleic acid (183) | |||||
| Linoleic acid (184) | |||||
| Linolenic acid (185) | |||||
| Palmitic acid (186) | Rh, L | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. germanica L., I. versicolor L., I. graminea L., I. halophila Pall. | Azerbaïdjan, Ukraine | [129,133] |
| Palmitoleic acid (187) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin | Azerbaïdjan | [129] |
| Pentadecanoic acid (188) | |||||
| Ethylpalmitate (189) | I. carthaliniae Fomin | ||||
| Myristic acid (190) | Rh, L | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. pallida Lam, I. versicolor L., I. graminea L., I. halophila Pall. | Azerbaïdjan, Ukraine | [13,129,133] |
| Lauric acid (191) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. graminea L., I. halophila Pall. | Azerbaïdjan, Ukraine | [129,133] |
| Capric acid (192) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. graminea L. | Azerbaïdjan, Ukraine | [129,133] |
| Caprylic acid (193) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin | Azerbaïdjan | [129] |
| Nonanoic acid (194) | |||||
| Palmitic acid (195) | Rh | GC-MS | I. pallida Lam. | Ukraine | [13] |
| Caprylic acid (196) | |||||
| Cerotic acid (197) | |||||
| Alkanes | |||||
| Nonacosane (198) | Rh, L | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. pallida Lam., I. germanica L., I. versicolor L., I. graminea L., I. halophila Pall. | Azerbaïdjan, Ukraine | [13,129,133] |
| Heptacosane (199) | Rh, L | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. pallida Lam. | Azerbaïdjan, Ukraine | [13,129] |
| Hexacosane (200) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. germanica L., I. versicolor L., I. graminea L., I. halophila Pall. | Azerbaïdjan, Ukraine | [13,129,133] |
| Pentacosane (201) | Rh, L | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. pallida Lam., I. germanica L., I. versicolor L., I. graminea L., I. halophila Pall. | Azerbaïdjan, Ukraine | [13,129,133] |
| Tetracosane (202) | |||||
| Tricosane (203) | |||||
| Heneicosane (204) | Rh | GC-MS | I. pallida Lam. | Ukraine | [13] |
| Untriacontane (205) | L | GC-MS | I. germanica L., I. versicolor L., I. graminea L., I. halophila Pall. | Ukraine | [133] |
| Eicosane (206) | I. germanica L., | ||||
| Aldehydes | |||||
| Dodecanal (207) | Rh, L | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin, I. germanica L. | Azerbaïdjan, Ukraine | [129,133] |
| Nonanal (208) | Rh | GC-MS | I. carthaliniae Fomin, I. medwedewii Fomin | Azerbaïdjan | [129] |
| Decanal (209) | |||||
| Phenylacetaldehyde (210) | L | GC-MS | I. germanica L., I. versicolor L. | Ukraine | [133] |
| Cyclohexenones | |||||
| Megastigmatrienone 2 (211) | L | GC-MS | I. pallida Lam. | Ukraine | [13] |
5. Pharmacological Properties of Iris spp.
5.1. Antioxidant Activity
5.2. Anticancer Activity
| Species | Parts | Extract | Cancer Type | Cell Line | Method | IC50 | Results | References | |
|---|---|---|---|---|---|---|---|---|---|
| I.nertschinskia Lodd. | Rhizomes | EtOH | Breast | MCF-7 | TBE | - | Induced apoptosis; triggered cell cycle block at G1 phase; ↑ p53 phosphorylation in a dose-dependent fashion; ↑ Bax expression; induced caspase-7 cleavage. | [17] | |
| I.nertschinskia Lodd. | Whole plant | EtOH | Breast | Hs578T | TBE | - | Triggered apoptosis hallmarked by cells accumulation in the sub-G 1 phase. | [145] | |
| MDA-MB-231 | |||||||||
| I. pseudopumila Tineo | Rhizomes | PET | Breast | MCF-7 | SRB | 48 h | 96.79 µg/mL | Induced potent cytotoxic effects against the three cell lines. | [146] |
| Skin | C32 | 57 ± 1.04 µg/mL | |||||||
| Kidney | ACHN | 99 ± 1.95 µg/mL | |||||||
| I. variegata L. | Rhizomes | H2O | Skin | IGR39 | MTT | 0.53 mg/mL | Reduced significantly cell viability; the ethanolic extract was shown to be more efficient against both cell lines. | [75] | |
| Breast | MDA-MB-231 | 0.33 mg/mL | |||||||
| I. hungarica Waldst. & Kit. | H2O | Skin | IGR39 | 1.15 mg/mL | |||||
| Breast | MDA-MB-231 | 0.57 mg/mL | |||||||
| 70% EtOH | Skin | IGR39 | 0.53 mg/mL | ||||||
| Breast | MDA-MB-231 | 0.33 mg/mL | |||||||
| I. pseudopumila Tineo | Rhizomes | MeOH | lung | CORL-23 | MTT | 31.5 ± 2.6 µg/mL | Both extracts revealed strong antiproliferative effects towards both cell lines. | [147] | |
| Skin | C32 | 48.7 ± 2.6 µg/mL | |||||||
| Flowers | lung | CORL-23 | 25.4 ± 2.6 µg/mL | ||||||
| Skin | C32 | 50.9 ± 2.6 µg/mL | |||||||
| I. Spuria L. | Rhizomes | MeOH | Lung | A549 | MTT | 123.04 µg/mL | All extracts displayed a dose dependent inhibitory potential against both cell lines A549, and Caco-2. | [148] | |
| Colon | Caco-2 | 302.94 µg/mL | |||||||
| I. kashmiriana Baker | Lung | A549 | 128.7µg/mL | ||||||
| Colon | Caco-2 | 237.76 µg/mL | |||||||
| I. germanica L. | Lung | A549 | 134.72 µg/mL | ||||||
| Colon | Caco-2 | 230.82 µg/mL | |||||||
| I. crocea Jacquem. ex R.C.Foster | Lung | A549 | 149.80 µg/mL | ||||||
| Colon | Caco-2 | 368.88µg/mL | |||||||
| I. ensata Thunb. | Lung | A549 | 137.98 µg/mL | ||||||
| Colon | Caco-2 | 358.81 µg/mL | |||||||
| I. kashmiriana Baker | Whole plant | MeOH | Lung | A549 | MTT | 128.7 µg/mL | The ethanol extract exhibited a dose-dependent selective antiproliferative effect on epithelial cancers. | [149] | |
| Colon | Caco-2 | 237.76 µg/mL | |||||||
| I. hungarica | Rhizomes | H2O | Colon | HCT116 | MTT | 42.3 µg/mL | Cell lines HCT116, HeLa, HL-60 were sensitive to the plant aqueous extract. The highest cytotoxicity was noticed against HL-60. | [150] | |
| Cervical | HeLa | 78.7 µg/mL | |||||||
| Leukemia | HL-60 | 3.6 µg/mL | |||||||
5.3. Neuroprotective Activity
5.4. Hepatoprotective Activity
5.5. Anthelmintic Activity
5.6. Antibacterial Activity
5.7. Antifungal Activity
5.8. Antiviral Activity
5.9. Antidiabetic Activity
6. Toxicity
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kicel, A. An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, biological activity, and toxicology. Antioxidants 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs, and Poisons; The University of Chicago Press: Chicago, IL, USA, 2015; pp. 1–304. [Google Scholar]
- Zougagh, S.; Belghiti, A.; Rochd, T.; Zerdani, I.; Mouslim, J. Medicinal and Aromatic Plants Used in Traditional Treatment of the Oral Pathology: The Ethnobotanical Survey in the Economic Capital Casablanca, Morocco (North Africa). Nat. Prod. Bioprospect. 2019, 9, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Gao, Y.; Hasenstein, K.H.; Wang, L. ‘Flower Angel’: A New Iris sanguinea Cultivar. HortScience 2021, 56, 617–618. [Google Scholar] [CrossRef]
- Roguz, K.; Gallagher, M.K.; Senden, E.; Bar-Lev, Y.; Lebel, M.; Heliczer, R.; Sapir, Y. All the Colors of the Rainbow: Diversification of Flower Color and Intraspecific Color Variation in the Genus Iris. Front. Plant Sci. 2020, 11, 1519. [Google Scholar] [CrossRef] [PubMed]
- Crişan, I.; Cantor, M. New perspectives on medicinal properties and uses of Iris sp. Hop. Med. Plants 2016, 24, 24–36. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants: Modified Stems, Roots, Bulbs; Springer International Publishing: Cham, Switzerland, 2016; Volume 11, pp. 1–392. [Google Scholar] [CrossRef]
- Crișan, I.; Vidican, R.; Olar, L.; Stoian, V.; Morea, A.; Ştefan, R. Screening for changes on Iris germanica L. rhizomes following inoculation with arbuscular mycorrhiza using Fourier transform infrared spectroscopy. Agronomy 2019, 9, 815. [Google Scholar] [CrossRef] [Green Version]
- Austin, C. Irises. A Gardener’s Encyclopedia; Timber Press: Portland, OR, USA, 2005. [Google Scholar]
- Xie, G.; Qin, X.; Chen, Y.; Wen, R.; Wu, S.; Qin, M. Alkaloids from the Rhizomes of Iris germanica. Chem. Nat. Compd. 2017, 53, 196–198. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Hussain, F.H.S.; Najmaldin, S.K.; Thu, Z.M.; Ibrahim, M.F.; Gilardoni, G.; Vidari, G. Phytochemistry and Biological Activities of Iris Species Growing in Iraqi Kurdistan and Phenolic Constituents of the Traditional Plant Iris postii. Molecules 2021, 26, 264. [Google Scholar] [CrossRef]
- Mykhailenko, O. Composition of volatile oil of Iris pallida Lam. from Ukraine. Turk. J. Pharm. Sci. 2018, 15, 85–90. [Google Scholar] [CrossRef]
- Wang, H.; Cui, Y.; Zhao, C. Flavonoids of the genus Iris (Iridaceae). Mini Rev. Med. Chem. 2010, 10, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Sieniawska, E.; Widelski, J.; Urjin, O.; Głowniak, P.; Skalicka-Woz’niak, K. Major secondary metabolites of Iris spp. Phytochem. Rev. 2015, 14, 51–80. [Google Scholar] [CrossRef]
- Wollenweber, E.; Stevens, J.F.; Klimo, K.; Knauft, J.; Frank, N.; Gerhäuser, C. Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica. Planta Med. 2003, 69, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Hong, S.W.; Lee, J.G.; Lee, Y.M.; Kim, D.W.; Kim, J.E.; Jung, D.J.; An, S.K.; Hong, N.J.; Kim, D.; et al. An ethanol extract of Iris nertschinskia induces p53-dependent apoptosis in the MCF7 human breast cancer cell line. Int. J. Mol. Med. 2011, 27, 401–405. [Google Scholar] [CrossRef]
- Bensari, S.; Ouelbani, R.; Yimaz, M.A.; Bensouici, C.; Gokalp, E.; Khelifi, D. Phytochemical profiles of Iris unguicularis Poir. with antioxidant, antibacterial, and anti-Alzheimer activities. Acta Nat. Sci. 2020, 7, 74–87. [Google Scholar] [CrossRef]
- Benoit-Vical, F.; Imbert, C.; Bonfils, J.P.; Sauvaire, Y. Antiplasmodial and antifungal activities of iridal, a plant triterpenoid. Phytochemistry 2003, 62, 747–751. [Google Scholar] [CrossRef]
- Nazir, N. Immunomodulatory activity of isoflavones isolated from Iris kashmiriana: Effect on T-lymphocyte proliferation and cytokine production in Balb/c mice. Biomed. Prev. Nutr. 2013, 3, 151–157. [Google Scholar] [CrossRef]
- Qi, X.Y.; Fan, L.J.; Gao, Y.; Shang, Y.; Liu, H.Y.; Wang, L. ‘NEFU-1′: A new Iris sanguine cultivar. HortScience 2020, 55, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.A. Subgeneric classification in Iris re-examined using chloroplast sequence data. Taxon 2011, 60, 27–35. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Green, I.R.; Rehman, U. Iris (Iris germanica) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: London, UK, 2016; pp. 481–486. [Google Scholar] [CrossRef]
- Kaššák, P. Secondary metabolites of the choosen genus Iris species. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 60, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, T.; Saha, S.; Bisht, N.S. First Report on the Ethnopharmacological Uses of Medicinal Plants among Monpa Tribe Living in the Zemithang Region of the Arunachal Pradesh, Eastern Himalayas, India. Plants 2016, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Haq, S.M.; Yaqoob, U.; Calixto, E.S.; Rahman, I.U.; Hashem, A.; Abd_Allah, E.F.; Alakeel, M.A.; Alqarawi, A.A.; Abdalla, M.; Hassan, M.; et al. Plant Resources Utilization among Different Ethnic Groups of Ladakh in Trans-Himalayan Region. Biology 2021, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.Y.; Yaqoob, U.; Hassan, M.; Bashir, F.; Zanit, S.B.; Haq, S.M.; Bussmann, R.W. Ethnopharmacology and phenology of high-altitude medicinal plants in Kashmir, Northern Himalaya. Ethnobot. Res. Appl. 2021, 22, 1–15. [Google Scholar] [CrossRef]
- Singh, K.N. Traditional knowledge on ethnobotanical uses of plant biodiversity: A detailed study from the Indian western Himalaya. Biodivers. Conserv. 2012, 28, 63. [Google Scholar] [CrossRef]
- Chang, N.; Luo, Z.; Li, D.; Song, H. Indigenous uses and pharmacological activity of traditional medicinal plants in Mount Taibai, China. Evid.-Based Complement. Altern. Med. 2017, 2017, 8329817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurchaih, H.; Menggenqiqig, K. Medicinal wild plants used by the Mongol herdsmen in Bairin Area of Inner Mongolia and its comparative study between TMM and TCM. J. Ethnobiol. Ethnomed. 2019, 15, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeşil, Y.; İnal, İ. Traditional knowledge of wild edible plants in Hasankeyf (Batman Province, Turkey). Acta Soc. Bot. Pol. 2019, 88, 3633. [Google Scholar] [CrossRef]
- Polat, R.; Güner, B.; Yüce Babacan, E.; Çakılcıoğlu, U. Survey of wild food plants for human consumption in Bingöl (Turkey). Indian J. Tradit. Knowl. 2016, 16, 378–384. [Google Scholar]
- Korkmaz, M.; Alpaslan, Z.; Turgut, N.; Ilhan, V. Ethnobotanical aspects of some geophytes from Ergan Mountain, Turkey. Bangladesh J. Bot. 2014, 43, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Kargıoğlu, M.; Cenkci, S.; Serteser, A.; Konuk, M.; Vural, G. Traditional uses of wild plants in the middle Aegean region of Turkey. Int. J. Hum. Ecol. 2010, 38, 429–450. [Google Scholar] [CrossRef]
- Menale, B.; De Castro, O.; Cascone, C.; Muoio, R. Ethnobotanical investigation on medicinal plants in the Vesuvio National Park (Campania, southern Italy). J. Ethnopharmacol. 2016, 4, 320–349. [Google Scholar] [CrossRef] [PubMed]
- Nsuala, B.N.; Enslin, G.; Viljoen, A. “Wild cannabis”: A review of the traditional use and phytochemistry of Leonotis leonurus. J. Ethnopharmacol. 2015, 174, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.S.; Pljevljakušić, D.S.; Nikolić, B.M.; Miladinović, D.L.; Djokić, M.M.; Rakonjac, L.B.; Jovanović, V.P.S. Ethnoveterinary knowledge in Pirot County (Serbia). S. Afr. J. Bot. 2021, 137, 278–289. [Google Scholar] [CrossRef]
- McGaw, L.J.; Eloff, J.N. Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. J. Ethnopharmacol. 2008, 119, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Miara, M.D.; Bendif, H.; Ouabed, A.; Rebbas, K.; Hammou, M.A.; Amirat, M.; Greene, A.; Teixidor-Toneu, I. Ethnoveterinary remedies used in the Algerian steppe: Exploring the relationship with traditional human herbal medicine. J. Ethnopharmacol. 2019, 244, 112164. [Google Scholar] [CrossRef] [PubMed]
- Gradé, J.T.; Tabuti, J.R.; Van Damme, P. Ethnoveterinary knowledge in pastoral Karamoja, Uganda. J. Ethnopharmacol. 2009, 122, 273–293. [Google Scholar] [CrossRef]
- Lans, C.; Khan, T.E.; Curran, M.M.; McCorkle, C.M. Ethnoveterinary medicine: Potential solutions for large-scale problems? In Veterinary Herbal Medicine; Wynn, S.G., Fougere, B.J., Eds.; Mosby: St. Louis, MO, USA, 2007; pp. 17–32. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Lone, P.A.; Dar, M.; Parray, J.A.; Shah, K.W. Ethnoveterinary medicinal uses of plants of district Bandipora of Jammu and Kashmir, India. Int. J. Trad. Nat. Med. 2013, 2, 164–178. [Google Scholar]
- Kirmani, N.R.; Banday, M.T.; Abdullah, M. Ethno-medicinal plants used by Bakarwals for treatment of livestock. J. Entomol. Zool. Stud. 2020, 8, 1742–1745. [Google Scholar]
- Shoaib, G.; Shah, G.M.; Shad, N.; Dogan, Y.; Siddique, Z.; Shah, A.H.; Farooq, M.; Khan, K.R.; Nedelcheva, A. Traditional practices of the ethnoveterinary plants in the Kaghan Valley, Western Himalayas-Pakistan. Rev. biol. Trop. 2021, 69, 1–11. [Google Scholar] [CrossRef]
- Vinagre, C.; Vinagre, S.; Carrilho, E.; García, D.; Vázquez, F.M.; Pinto-Gomes, C. Ethnobotanical study in the protected landscape “Serra de Montejunto” (Portugal). J. Med. Plants 2017, 5, 110–124. [Google Scholar]
- Malla, B.; Gauchan, D.P.; Chhetri, R.B. An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 2015, 165, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.S.; Xia, N.H.; Van Chu, T.; Van Sam, H. Ethnobotanical study on medicinal plants in traditional markets of Son La province, Vietnam. For. Soc. 2019, 3, 171–192. [Google Scholar] [CrossRef]
- Chetri, B.K.; Ghalley, L.R.; Penjor, D.; Dechen, K.; Gyeltshen, T. Ethnobotanical study on wealth of home gardens in gosiling gewog of Tsirang District. Asian J. Plant Sci. 2018, 1, 45775. [Google Scholar] [CrossRef]
- USDA; NRCS. Iris douglasiana. In The Plants Database; National Plant Data Team: Greensboro, NC, USA. Available online: plants.usda.gov (accessed on 10 February 2022).
- Kiasi, Y.; Forouzeh, M.R.; Mirdeilami, S.Z.; Niknahad-Gharmakher, H. Ethnobotanical study on the medicinal plants in khosh Yeilagh rangeland, Golestan province, Iran. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, S. An ethnobotanical study of local plants and their medicinal importance in Tons river area, Dehradun, Uttarakhand. Indian J. Trop. Biodiv. 2015, 23, 227–231. [Google Scholar]
- Singh, K.N.; Lal, B. Ethnomedicines used against four common ailments by the tribal communities of Lahaul-Spiti in western Himalaya. J. Ethnopharmacol. 2008, 115, 147–159. [Google Scholar] [CrossRef]
- Sher, Z.; Hussain, F.; Ibrar, M. Traditional knowledge on plant resources of Ashezai and Salarzai valleys, District Buner, Pakistan. Afr. J. Plant Sci. 2013, 8, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Shuaib, M.; Hussain, F.; Rauf, A.; Jan, F.; Romman, M.; Parvez, R.; Zeb, A.; Ali, S.; Abidullah, S.; Bahadur, S.; et al. Traditional knowledge about medicinal plant in the remote areas of Wari Tehsil, Dir Upper, Pakistan. Braz. J. Biol. 2021, 83, 2023. [Google Scholar] [CrossRef]
- Fatiha, B.A.; Souad, S.; Ouafae, B.; Jamila, D.; Allal, D.; Lahcen, Z. Ethnobotanical study of medicinal plants used in the region of Middle Oum Rbia (Morocco). Plant Arch. 2019, 19, 2005–2017. [Google Scholar]
- Redzić, S.S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar]
- Ahmad, I.; Ibrar, M.; Ali, N. Ethnobotanical study of tehsil kabal, swat district, KPK, Pakistan. J. Bot. 2011, 2011, 368572. [Google Scholar] [CrossRef] [Green Version]
- Kifayatullah, J.A.; Ali, H.; Ahmad, H.; Muhammad, S. The traditional knowledge of some phenorogames of Molkhow-Valley district Chitral. J. Biol. Sci. 2017, 3, 16–31. [Google Scholar]
- Rokaya, M.B.; Münzbergová, Z.; Timsina, B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. J. Ethnopharmacol. 2010, 130, 485–504. [Google Scholar] [CrossRef]
- Khan, K.U.; Shah, M.; Ahmad, H.; Khan, S.M.; Rahman, I.U.; Iqbal, Z.; Khan, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; et al. Exploration and local utilization of medicinal vegetation naturally grown in the Deusai plateau of Gilgit, Pakistan. Saudi J. Biol. Sci. 2018, 25, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Barki, A.; Yousaf Khan, M.; Fazal, H. Ethnobotanical studies of the flora of tehsil Birmal in South Waziristan Agency, Pakistan. Pak. J. Weed Sci. Res. 2012, 18, 277–291. [Google Scholar]
- Sher, H.; Inamuddin, I.; Khan, Z.; Bussmann, R.W.; Rahman, I.U. Medicinal plant diversity of Hindubaig Mountain, Lalku Valley, District Swat, Pakistan. Ethnobot. Res. Appl. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Trak, T.H.; Giri, R.A. Inventory of the plants used by the tribals (Gujjar and bakarwal) of district kishtwar, Jammu and Kashmir (India). Indian J. Sci. Res. 2017, 13, 104–115. [Google Scholar]
- Mala, F.A.; Lone, M.A.; Lone, F.A.; Arya, N. Ethno-medicinal survey of Kajinaag range of Kashmir Himalaya, India. Int. J. Pharm. Biol. Sci. 2012, 3, 442–449. [Google Scholar]
- Lone, P.A.; Bhardwaj, A.K.; Bahar, F.A. Study of indigenous/traditional medicinal plant knowledge-An endeavour towards new drug discovery. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, O.P.; Singh, B. An Ethnobotanical Profile on Cold Desert Flora of Nubra Valley (Ladakh). Bull. Bot. Surv. India 2000, 42, 81–90. [Google Scholar] [CrossRef]
- Khanday, Z.H.; Singh, S. Ethnobotanical study of some important medicinal plants of Shopian district of Jammu and Kashmir (India). Asian J. Sci. Technol. 2017, 8, 5088–5091. [Google Scholar]
- Lone, M.A.; Lone, M.A. Traditional Plant Remedies from Bungus Valley of District Kupwara Kashmir. Int. J. Innov. Sci. Res. Technol. 2018, 3, 120–125. [Google Scholar]
- Silva, J.D.; Nascimento, M.G.; Castro, K.N.; Andrade, I.M. Ethnobotanical survey of medicinal plants used by the community of Sobradinho, Lus Correia, Piau, Brazil. J. Med. Plant Res. 2015, 9, 872–883. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna J. Phytomed. 2013, 3, 254–271. [Google Scholar]
- Lin, F.; Luo, B.; Long, B.; Long, C. Plant leaves for wrapping zongzi in China: An ethnobotanical study. J. Ethnobiol. Ethnomed. 2019, 15, 63. [Google Scholar] [CrossRef]
- Kovalev, V.M.; Mykhailenko, O.O.; Krechun, A.V.; Osolodchenko, T.P. Antimicrobial activity of extracts of Iris hungarica and Iris sibirica. Ann. Mechnikov’s Inst. 2017, 2, 57–64. [Google Scholar] [CrossRef]
- Minina, S.A.; Pryakhina, N.I.; Chemesova, I.I.; Chizhikov, D.V. A pediatric medicinal preparation containing an extract of the milk-white iris (Iris lactea). Pharm. Chem. J. 2008, 42, 37–39. [Google Scholar] [CrossRef]
- Stansbury, J.; Saunders, P.; Winston, D. Promoting healthy thyroid function with iodine, Bladderwrack, Guggul and Iris. J. Restore Med. 2012, 1, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Mykhailenko, O.; Korinek, M.; Ivanauskas, L.; Bezruk, I.; Myhal, A.; Petrikaitė, V.; El-Shazly, M.; Lin, G.H.; Lin, C.H.; Yen, C.H.; et al. Qualitative and Quantitative Analysis of Ukrainian Iris Species: A Fresh Look on Their Antioxidant Content and Biological Activities. Molecules 2020, 25, 4588. [Google Scholar] [CrossRef]
- Mocan, A.; Zengin, G.; Mollica, A.; Uysal, A.; Gunes, E.; Crişan, G.; Aktumsek, A. Biological effects and chemical characterization of Iris schachtii Markgr. extracts: A new source of bioactive constituents. Food Chem. Toxicol. 2020, 112, 448–457. [Google Scholar] [CrossRef]
- Sary, H.G.; Ayoub, N.A.; Singab, A.B.; Ahmed, A.H.; Al-Azizi, M.M. Chemical constituents and molluscicidal activity of Iris pseudacorus L. cultivated in Egypt. Bull. Pharm. Sci. Assiut Univ. 2004, 27, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Hoang, L.; Beneš, F.; Fenclová, M.; Kronusová, O.; Švarcová, V.; Řehořová, K.; Švecová, E.B.; Vosátka, M.; Hajšlová, J.; Kaštánek, P.; et al. Phytochemical Composition and In Vitro Biological Activity of Iris spp. (Iridaceae): A New Source of Bioactive Constituents for the Inhibition of Oral Bacterial Biofilms. Antibiotics 2020, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Machalska, A.; Skalicka-Woźniak, K.; Widelski, J.; Głowniak, K.; Purevsuren, G.; Oyun, Z.; Khishgéé, D.; Urjin, B. Screening for phenolic acids in five species of iris collected in Mongolia. Acta Chromatogr. 2008, 20, 259–267. [Google Scholar] [CrossRef]
- Basgedik, B.; Ugur, A.; Sarac, N. Antimicrobial, antioxidant, antimutagenic activities, and phenolic compounds of Iris germanica. Ind. Crops Prod. 2014, 61, 526–530. [Google Scholar] [CrossRef]
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant Activity of Syringic Acid Prevents Oxidative Stress in l-arginine-Induced Acute Pancreatitis: An Experimental Study on Rats. Int. Surg. 2015, 100, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Shu, P.; Qin, M.; Shen, W.; Wu, G. A new coumaronochromone and phenolic constituents from the leaves of Iris bungei Maxim. Biochem. Syst. Ecol. 2009, 37, 20–23. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef]
- Fujita, M.; Inoue, T. Studies on the constituents of Iris florentina L. II. C-glucosides of xanthones and flavones from the leaves. Chem. Pharm. Bull. 1982, 30, 2342–2348. [Google Scholar] [CrossRef] [Green Version]
- Horbury, M.D.; Baker, L.A.; Quan, W.D.; Greenough, S.E.; Stavros, V.G. Photodynamics of potent antioxidants: Ferulic and caffeic acids. Phys. Chem. Chem. Phys. 2016, 18, 17691–17697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Mykchailenko, O.O.; Kovalyov, M.V. Phenolic compounds of the genus Iris plants (Iridaceae). Čes. Slov. Farm. 2016, 65, 70–77. [Google Scholar]
- Yılmaz, S.; Ergün, S. Trans-cinnamic acid application for rainbow trout (Oncorhynchus mykiss): I. Effects on haematological, serum biochemical, non-specific immune and head kidney gene expression responses. Fish Shellfish Immunol. 2018, 78, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.A.; Harborne, J.B.; Colasante, M. Flavonoid and xanthone patterns in bearded Iris species and the pathway of chemical evolution in the genus. Biochem. Syst. Ecol. 1997, 25, 309–325. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Hareem, S.; Siddiqui, H.; Anjum, S.; Ali, S.; Atta-ur-Rahman; Zaidi, M.I. A benzil and isoflavone from Iris tenuifolia. Phytochemistry 2008, 69, 1880–1885. [Google Scholar] [CrossRef]
- Xie, G.Y.; Zhu, Y.; Shu, P.; Qin, X.Y.; Wu, G.; Wang, Q.; Qin, M.J. Phenolic metabolite profiles and antioxidants assay of three Iridaceae medicinal plants for traditional Chinese medicine “She-gan” by on-line HPLC–DAD coupled with chemiluminescence (CL) and ESI-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2014, 98, 40–51. [Google Scholar] [CrossRef]
- Moein, M.R.; Khan, S.I.; Ali, Z.; Ayatollahi, S.A.; Kobarfard, F.; Nasim, S.; Choudhary, M.I.; Khan, I.A. Flavonoids from Iris songarica and their antioxidant and estrogenic activity. Planta Med. 2008, 74, 1492–1495. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.C.Y.; Emery, P.W.; Preedy, V.R.; Wiseman, H. Health benefits of isoflavones in functional foods? Proteomic and metabonomic advances. Inflammopharmacology 2008, 16, 235–239. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Nur-e-Alam, M.; Baig, I.; Akhtar, F.; Khan, A.M.; Ndögnii, P.Ö.; Badarchiin, T.; Purevsuren, G.; Nahar, N.; Atta-ur-Rahman. Four new flavones and a new isoflavone from Iris bungei. J. Nat. Prod. 2001, 64, 857–860. [Google Scholar] [CrossRef]
- Huang, L.; Ma, W.H.; Liu, Y.Z.; Yang, J.S.; Peng, Y.; Xiao, P.Y. Irisdichotins A–C, three new flavonoid glycosides from the rhizomes of Iris dichotoma Pall. J. Asian Nat. Prod. Res. 2011, 13, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, G.; Yu, F.; Jia, Q.; Zheng, Y.; Bi, X.; Lei, J. Characterization of anthocyanins in the hybrid progenies derived from Iris dichotoma and I. domestica by HPLC-DAD-ESI/MS analysis. Phytochemistry 2018, 150, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alperth, F.; Mitic, B.; Mayer, S.; Maleš, Ž.; Kunert, O.; Hruševar, D.; Bucar, F. Metabolic profiling of rhizomes of native populations of the strictly endemic Croatian species Iris adriatica. Plant Biosyst. 2019, 153, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone glucosides: Isolation, bioactivity and synthesis. Molecules 2021, 26, 5575. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Gašić, U.M.; Pešić, M.B.; Stanojević, S.P.; Barać, M.B.; Mačukanović-Jocić, M.P.; Avramov, S.N.; Tešić, Z.L. Phytochemical analysis and total antioxidant capacity of rhizome, above-ground vegetative parts and flower of three Iris species. Chem. Biodivers. 2019, 16, e1800565. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalil, S.; Tosa, H.; Iinuma, M. A xanthone C-glycoside from Iris nigricans. Phytochemistry 1995, 38, 729–731. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Abdulai, I.L.; Kwofie, S.K.; Gbewonyo, W.S.; Boison, D.; Puplampu, J.B.; Adinortey, M.B. Multitargeted Effects of Vitexin and Isovitexin on Diabetes Mellitus and Its Complications. Sci. World J. 2021, 2021, 6641128. [Google Scholar] [CrossRef]
- Mizuno, T.; Okuyama, Y.; Iwashina, T. Flavonoids from Iris sanguinea var. tobataensis and chemotaxonomic and molecular phylogenetic comparisons with Iris sanguinea var. sanguinea. Bull. Natl. Sci. Mus. Tokyo Ser. B 2018, 44, 135–145. [Google Scholar]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Wang, J.; Wu, W.; Liu, Q.; Liu, X. Effect of isoorientin on intracellular antioxidant defence mechanisms in hepatoma and liver cell lines. Biomed. Pharmacother. 2016, 81, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, K.; Mubashir, K.; Dar, S.A.; Nazir, T.; Hameed, I.; Ganai, B.A.; Akbar, S.; Masood, A. Gentiana kurroo Royle attenuates the metabolic aberrations in diabetic rats; Swertiamarin, swertisin and lupeol being the possible bioactive principles. J. Complement. Integr. 2017, 14. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Amin, A.A.; Tosi, S.; Mellerio, G.G.; Hussain, F.H.S.; Picco, A.M.; Vidari, G. Chemical composition and antifungal activity of essential oils from flowers, leaves, rhizomes, and bulbs of the wild Iraqi Kurdish plant Iris persica. Nat. Prod. Commun. 2017, 12, 1934578X1701200334. [Google Scholar] [CrossRef] [Green Version]
- Orrego, R.; Leiva, E.; Cheel, J. Inhibitory effect of three C-glycosylflavonoids from Cymbopogon citratus (Lemongrass) on human low density lipoprotein oxidation. Molecules 2009, 14, 3906–3913. [Google Scholar] [CrossRef]
- Boltenkov, E.V.; Rybin, V.G.; Zarembo, E.V. Flavones from callus tissue of Iris ensata. Chem. Nat. Compd. 2005, 41, 539–541. [Google Scholar] [CrossRef]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and quantification of flavonoids in Iris germanica L. and Iris pallida Lam. resinoids from Morocco. Phytochem. Anal. 2012, 23, 450–455. [Google Scholar] [CrossRef]
- Xie, G.Y.; Qin, X.Y.; Liu, R.; Wang, Q.; Lin, B.B.; Wang, G.K.; Wen, R.; Qin, J. New isoflavones with cytotoxic activity from the rhizomes of Iris germanica L. Nat. Prod. Res. 2013, 27, 2173–2177. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Ross, S.A. New ceramides and isoflavone from the Egyptian Iris germanica L. rhizomes. Phytochem. Lett. 2013, 6, 340–344. [Google Scholar] [CrossRef]
- Shi, G.R.; Wang, X.; Liu, Y.F.; Zhang, C.L.; Ni, G.; Chen, R.Y.; Yu, D.Q. Bioactive flavonoid glycosides from whole plants of Iris japonica. Phytochem. Lett. 2017, 19, 141–144. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Lin, B.; Wang, G.; Qin, M. C-glycosylflavones from the leaves of Iris tectorum Maxim. Acta Pharm. Sin. B. 2012, 2, 598–601. [Google Scholar] [CrossRef] [Green Version]
- Appleton, J. Evaluating the bioavailability of isoquercetin. Nat. Med. J. 2010, 2, 1–6. [Google Scholar]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Tan, Y.F.; Xu, P.; Li, H.; Li, Y.H.; Chen, W.Y.; Zhang, J.Q.; Chen, F.; Huang, G.J. Izalpinin from fruits of Alpiniaoxyphylla with antagonistic activity against the rat bladder contractility. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singab, A.N.B.; Ahmed, A.H.; Sinkkonen, J.; Ovcharenko, V.; Pihlaja, K. Molluscicidal activity and new flavonoids from Egyptian Iris germanica L. (var. alba). Z. Nat. C J. Biosci. 2006, 61, 57–63. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Zuo, J.; Olatunde, O.O. Securidaca inappendiculata stem extract confers robust antioxidant and antidiabetic effects against high fructose/streptozotocin induced type 2 diabetes in rats. Exploration of bioactive compounds using UHPLC-ESI-QTOF-MS. Arch. Physiol. Biochem. 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Yue, S.; Xue, N.; Li, H.; Chen, Z.; Huang, B.; Wang, X. Isomangiferin Attenuates Renal Injury in Diabetic Mice via Inhibiting Inflammation. Diabetes Metab. Syndr. Obes. 2020, 2020, 4273–4280. [Google Scholar] [CrossRef]
- Watson, R.R.; Schönlau, F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: A review. Minerva Cardioangiol. 2015, 63, 1–12. [Google Scholar]
- Okba, M.M.; Baki, P.M.A.; Khaleel, A.E.K.; El-Sherei, M.M.; El-Sherei, M.A. Discrimination of common Iris species from Egypt based on their genetic and metabolic profiling. Phytochem. Anal. 2020, 32, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Isaev, D.I.; Mikhailenko, O.A.; Gurbanov, G.M.; Kovalev, V.N. Constituents of essential oils from Azerbaijan Iris medwedewii and I. carthaliniae rhizomes. Chem. Nat. Compd. 2016, 52, 748–750. [Google Scholar] [CrossRef]
- Chikhi, I.; Allali, H.; Dib, M.E.; Halla, N.; Muselli, A.; Tabti, B.; Costa, J. Free radical scavenging and antibacterial activity of essential oil and solvent extracts of Iris planifolia (Mill) from Algeria. J. Med. Plant Res. 2012, 6, 1961–1968. [Google Scholar] [CrossRef]
- Deng, G.B.; Zhang, H.B.; Xue, H.F.; Chen, S.N.; Chen, X.L. Chemical composition and biological activities of essential oil from the rhizomes of Iris bulleyana. Agric. Sci. China 2009, 8, 691–696. [Google Scholar] [CrossRef]
- Al-Jaber, H.I. Variation in essential oil composition of Iris nigricans Dinsm. (Iridaceae) endemic to Jordan at different flowering stages. Arab. J. Chem. 2016, 9, 1190–1196. [Google Scholar] [CrossRef] [Green Version]
- Mykhailenko, O.; Kovalyov, V.; Orlova, T. Chemical composition of the essential oil of several Iris species. Thai. J. Pharm. Sci. 2020, 44, 179–185. [Google Scholar]
- Henriksen, E.J. Role of oxidative stress in the pathogenesis of insulin resistance and type 2 diabetes. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: London, UK; Oxford, UK; San Diego, CA, USA; Cambridge, MA, USA, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Huwaitat, S.; Al-Khateeb, E.; Finjan, S.; Maraqa, A. Antioxidant and antimicrobial activities of Iris nigricans methanolic extracts containing phenolic compounds. Eur. Sci. J. 2018, 9, 83–91. [Google Scholar] [CrossRef]
- Mahdinezhad, M.R.; Hooshmand, S.; Soukhtanloo, M.; Jamshidi, S.T.; Ehtiati, S.; Ghorbani, A. Protective effects of a standardized extract of Iris germanica on pancreas and liver in streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Res. 2021, 16, 71. [Google Scholar] [CrossRef]
- Hacıbekiroğlu, I.; Kolak, U. Antioxidant and anticholinesterase constituents from the petroleum ether and chloroform extracts of Iris suaveolens. Phytother. Res. 2011, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Nadaroğlu, H.; Demir, Y.; Demir, N. Antioxidant and radical scavenging properties of Iris germanica. Pharm. Chem. J. 2007, 41, 409–415. [Google Scholar] [CrossRef]
- Ganaie, A.A.; Mishra, R.P.; Allaie, A.H. Antioxidant activity of some extracts of Iris ensata. J. Pharmacogn. Phytochem. 2018, 7, 230–235. [Google Scholar]
- Deyno, S.; Eneyew, K.; Seyfe, S.; Wondim, E. Efficacy, safety and phytochemistry of medicinal plants used for the management of diabetes mellitus in Ethiopia: A systematic review. Clin. Phytoscience 2021, 7, 16. [Google Scholar] [CrossRef]
- Alam, A.; Jaiswal, V.; Akhtar, S.; Jayashree, B.S. Isolation of isoflavones from Iris kashmiriana Baker as potential anti proliferative agents targeting NF-κB. Phytochemistry 2017, 136, 70–80. [Google Scholar] [CrossRef]
- Tantry, M.A.; Ghazanfar, K.; Zargar, U.R. New alkylated benzoquinone from Iris nepalensis. Nat. Prod. Res. 2013, 27, 1832–1836. [Google Scholar] [CrossRef]
- Fang, R.; Houghton, P.J.; Hylands, P.J. Cytotoxic effects of compounds from Iris tectorum on human cancer cell lines. J. Ethnopharmacol. 2008, 118, 257–263. [Google Scholar] [CrossRef]
- Shin, J.S.; Maeng, H.G.; Hong, S.W.; Moon, J.H.; Kim, J.S.; Suh, Y.A.; Kim, E.S.; Choi, E.K.; Kim, I.; Lee, S.K.; et al. Iris Nertschinskia ethanol extract differentially induces cytotoxicity in human breast cancer cells depending on AKT1/2 activity. Asian Pac. J. Cancer Prev. 2012, 13, 6511–6516. [Google Scholar] [CrossRef] [Green Version]
- Rigano, D.; Conforti, F.; Formisano, C.; Menichini, F.; Senatoter, F. Comparative free radical scavenging potential and cytotoxicity of different extracts from Iris pseudopumila Tineo flowers and rhizomes. Nat. Prod. Res. 2009, 23, 17–25. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, F.; Rigano, D.; Senatore, F. Antiproliferative activity on human cancer cell lines after treatment with polyphenolic compounds isolated from Iris pseudopumila flowers and rhizomes. Z. Nat. C 2009, 64, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.H.; Padder, B.A.; Mokhdomi, T.; Mir, J.I.; Bhat, H.A.; Hassan, Q.P.; Qadri, R.A. Antiproliferative activity of methanolic extracts of different Iris plant species against A549 and Caco-2 cell lines. J. Pharmacogn. Phytochem. 2017, 6, 1034–1037. [Google Scholar] [CrossRef]
- Amin, A.; Wani, S.H.; Mokhdomi, T.A.; Bukhari, S.; Wafai, A.H.; Mir, J.I.; Hassan, Q.P.; Qadri, R.A. Investigating the pharmacological potential of Iris kashmiriana in limiting growth of epithelial tumors. Pharmacogn J. 2013, 5, 170–175. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Lesyk, R.; Finiuk, N.; Stoika, R.; Yushchenko, T.; Ocheretniuk, A.; Vaschuk, V.; Mishchenko, V.; Georgiyants, V. In vitro anticancer activity screening of Iridaceae plant extracts. J. Appl. Pharm. Sci. 2020, 10, 59–63. [Google Scholar] [CrossRef]
- Jalsrai, A.; Numakawa, T.; Numakawa, Y.; Adachi, N.; Kunugi, H. Phosphatase-mediated intracellular signaling contributes to neuroprotection by flavonoids of Iris tenuifolia. Am. J. Chin. Med. 2014, 42, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Jalsrai, A.; Reinhold, A.; Becker, A. Ethanol Iris tenuifolia extract reduces brain damage in a mouse model of cerebral ischaemia. Phytother. Res. 2018, 32, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Wang, Y.; Liu, Y.F.; Ni, G.; Liang, D.; Luo, H.; Song, X.Y.; Zhang, W.Q.; Chen, R.Y.; Chen, N.H.; et al. Iridal-type triterpenoids with neuroprotective activities from Iris tectorum. J. Nat. Prod. 2014, 77, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Akther, N.; Andrabi, K.; Nissar, A.; Ganaie, S.; Chandan, B.K.; Gupta, A.P.; Khuswant, M.; Sultana, S.; Shawl, A.S. Hepatoprotective activity of LC–ESI-MS standardized Iris spuria rhizome extract on its main bioactive constituents. Phytomedicine 2014, 21, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.R.; Wang, X.; Liu, Y.F.; Zhang, C.L.; Ni, G.; Chen, R.Y.; Chen, D.Q. Novel iridal metabolites with hepatoprotective activities from the whole plants of Iris japonica. Tetrahedron Lett. 2016, 57, 5761–5763. [Google Scholar] [CrossRef]
- Romero-González, R.; Garrido Frenich, A.; Martínez Vidal, J.L. Veterinary Drugs Residues: Anthelmintics. Encycl. Food Saf. 2014, 45–54. [Google Scholar] [CrossRef]
- Khan, A.; Tak, H.; Nazir, R.; Lone, B.A. In vitro and in vivo anthelmintic activities of Iris kashmiriana Linn. J. Saudi Soc. Agric. Sci. 2018, 17, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F.; Shawl, A.S.; Tantray, M.A. Evaluation of anthelmintic activity of Iris hookeriana against gastrointestinal nematodes of sheep. J. Helminthol. 2008, 82, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mykhailenk, O.; Kovalyov, V.; Kovalyov, S.; Krechun, A. Isoflavonoids from the rhizomes of Iris hungarica and antibacterial activity of the dry rhizomes extract. Ars Pharm. 2017, 58, 39–45. [Google Scholar] [CrossRef]
- Rigano, D.; Grassia, A.; Formisano, C.; Basile, A.; Sorbo, S.; Sorbo, F. Antibacterial and allelopathic activity of methanolic extract from Iris pseudopumila rhizomes. Fitoterapia 2006, 77, 460–462. [Google Scholar] [CrossRef]
- Sofiane, G.; Wafa, N.; Loubna, A. Evaluation of antioxidant and antifungal activities of methanolic aerial part extract of Iris unguicularis Poiret. Asian J. Plant Sci. Res. 2016, 6, 18–23. [Google Scholar]
- Tikhomirova, L.I.; Ilyicheva, T.N. Preparation of biotechnological raw materials of Iris sibirica L. with a given content of mangiferin and antiviral activity. IOP Conf. Ser. Earth Environ. 2020, 421, 022049. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Suresh, D.K.; Ahemad, W.; Khalid, M.S.; Aasim, S.M. Anti-hyperglycemic activity of iris ensata Thunb root extracts in normal, glucose fed and streptozotocin induced diabetic rats. Adv. Pharmacol. Toxicol. 2010, 11, 93. [Google Scholar]
- Lin, A.H.M.; Nichols, B.L.; Quezada-Calvillo, R.; Avery, S.E.; Sim, L.; Rose, D.R.; Naim, H.Y.; Hamaker, B.R. Unexpected high digestion rate of cooked starch by the Ct-maltase-glucoamylase small intestine mucosal α-glucosidase subunit. PLoS ONE 2012, 7, e35473. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.; Mohamed, G.A.; Zayed, M.F.; Ross, S.A. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017, 70, 192–198. [Google Scholar] [CrossRef]



| Taxonomic Hierarchy | Classification |
|---|---|
| Kingdom | Plantae |
| Subkingdom | Viridiplantae |
| Infrakingdom | Streptophyta |
| Superdivision | Embryophyta |
| Division | Tracheophyta |
| Subdivision | Spermatophytina |
| Class | Magnoliopsida |
| Superorder | Lilianae |
| Order | Asparagales |
| Family | Iridaceae |
| Genus | Iris L.—Iris |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatib, S.; Faraloni, C.; Bouissane, L. Exploring the Use of Iris Species: Antioxidant Properties, Phytochemistry, Medicinal and Industrial Applications. Antioxidants 2022, 11, 526. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11030526
Khatib S, Faraloni C, Bouissane L. Exploring the Use of Iris Species: Antioxidant Properties, Phytochemistry, Medicinal and Industrial Applications. Antioxidants. 2022; 11(3):526. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11030526
Chicago/Turabian StyleKhatib, Sohaib, Cecilia Faraloni, and Latifa Bouissane. 2022. "Exploring the Use of Iris Species: Antioxidant Properties, Phytochemistry, Medicinal and Industrial Applications" Antioxidants 11, no. 3: 526. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11030526