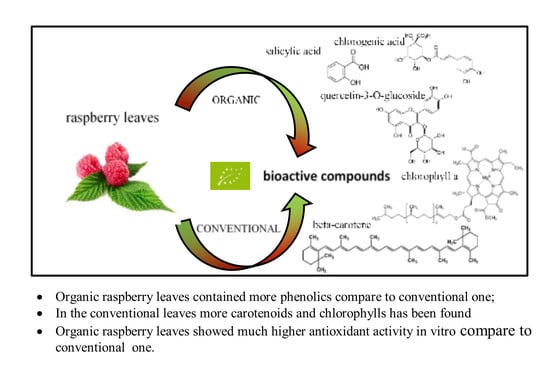
Phenolics and Carotenoid Contents in the Leaves of Different Organic and Conventional Raspberry (Rubus idaeus L.) Cultivars and Their In Vitro Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants Origin
2.2. Plant Material Preparation
2.3. Dry Matter Content
2.4. Phenolic Acid and Flavonol Separation and Identification
2.5. Carotenoid and Chlorophyll Separation and Identification
2.6. ABTS·+ Radical Cation Scavenging Activity Assay
2.6.1. ABTS Reagent Preparation
2.6.2. Antioxidant Activity Measurement
2.7. Statistical Analysis
3. Results
3.1. Polyphenol Content
3.2. Carotenoid and Chlorophyll Contents
3.3. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oszmiański, J.; Wojdyl̷o, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Costea, T.; Lupu, A.R.; Vlase, L.; Nencu, I.; Gîrd, C.E. Phenolic content and antioxidant activity of a raspberry leaf dry extract. Rom. Biotechnol. Lett. 2016, 21, 11345–11356. [Google Scholar]
- Grochowski, D.M.; Paduch, R.; Wiater, A.; Dudek, A.; Pleszczyńska, M.; Tomczykowa, M.; Granica, S.; Polak, P.; Tomczyk, M. In vitro antiproliferative and antioxidant effects of extracts from Rubus caesius leaves and their quality evaluation. Evid. Based Complement Alternat Med. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Veljković, B.; Đorđević, N.; Dolićanin, Z.; Ličina, B.; Topuzović, M.; Stanković, M.; Zlatić, N.; Dajić-Stevanović, Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not. Bot. Hortic. Agrobot. 2019, 47, 359–367. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Gu, Y.; Ye, C.; Cao, Y.; Liu, Z.; Yin, J. Antithrombotic activity of fractions and components obtained from raspberry leaves (Rubus chingii). Food Chem. 2012, 132, 181–185. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Papetti, A.; Dabić Zagorac, D.Č.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crop. Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Antioxidant capacity, phenolic compounds and minerals content of blackcurrant (Ribes nigrum L.) leaves as influenced by harvesting date and extraction method. Ind. Crop. Prod. 2014, 53, 133–139. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Rishton, G.M. Natural products as a robust source of new drugs and drug leads: Past successes and present day issues. Am. J. Cardiol. 2008, 101, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Moschona, A.; Kyriakidis, K.D.; Kleontas, A.D.; Liakopoulou-Kyriakides, M. Comparative study of natural phenolic acids and flavonols as antiplatelet and anti-inflammatory agents. Grant Med. J. 2017, 2, 54–66. [Google Scholar]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Skupień, K.; Ochniam, I.; Grajkowski, J.; Krzywy-Gawrońska, E. Nutrients, antioxidants, and antioxidant activity of organically and conventionally grown raspberries. J. Appl Bot. Food Qual. 2011, 84, 85–89. [Google Scholar]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria x ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Tassoni, A.; Tango, N.; Ferri, M. Comparison of biogenic amine and polyphenol profiles of grape berries and wines obtained following conventional, organic and biodynamic agricultural and oenological practices. Food Chem. 2013, 139, 405–413. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301. [Google Scholar] [CrossRef]
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakmak, I.; Ozturk, L.; Cooper, J.M.; Volakakis, N.; et al. Effects of agronomic management and climate on leaf phenolic profiles, disease severity and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef]
- Vagiri, M.; Johansson, E.; Rumpunen, K. Phenolic compounds in black currant leaves—An interaction between the plant and foliar diseases? J. Plant Interact. 2017, 12, 193–199. [Google Scholar] [CrossRef]
- Ohene, I.; Maalekuu, B.K. Effect of some postharvest treatments on the quality and shelf life of three cultivars of carrot (Daucus carota L.) during storage at room temperature. Am. J. Clin Nutr. 2013, 3, 64–72. [Google Scholar]
- Hallmann, E.; Rozpara, E.; Słowianek, M.; Leszczyńska, J. The effect of organic and conventional farm management on the allergenic potency and bioactive compounds status of apricots (Prunus armeniaca L). Food Chem. 2019, 279, 171–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Nala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gutierrez-Albanchez, E.; Kirakosyan, A.; Bolling, S.F.; García-Villaraco, A.; Gutierrez-Mañero, J.; Ramos-Solano, B. Biotic elicitation as a tool to improve berry (Strawberry and Raspberry) extract potential on metabolic syndrome related enzymes in vitro. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Guo, X.; Zhang, M.; Liu, R.; Sui, W. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food Funct. 2018, 9, 2112–2120. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Liu, Q.; Quiles, J.L.; Filosa, R.; Kamal, M.A.; Wang, F.; Kai, G.; Xiao, J. Protective effects of raspberry on the oxidative damage in HepG2 cells through Keap1/Nrf2-dependent signaling pathway. Food Chem. Toxicol. 2019, 133, 110781. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Milenkovic, D. Anthocyanins: From sources and bioavailability to cardiovascular health benefits and molecular mechanisms of action. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Sayyari, M.; Castillo, S.; Valero, D.; Díaz-Mula, H.M.; Serrano, M. Acetyl salicylic acid alleviates chilling injury and maintains nutritive and bioactive compounds and antioxidant activity during postharvest storage of pomegranates. Postharvest Biol. Technol. 2011, 60, 136–142. [Google Scholar] [CrossRef]
- Gudej, J. Kaemferol and quercetin glycosides from Rubus idaeus L. leaves. Acta Pol. Pharm. 2003, 60, 313–316. [Google Scholar] [PubMed]
- Buricova, L.; Andjelkovic, M.; Cermakova, A.; Reblova, Z.; Jurcek, O.; Kolehmainen, E.; Verhe, R.; Kvasnicka, F. Antioxidant capacities and antioxidants of strawberry, blackberry and raspberry leaves. Czech. J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenyl propanoid pathway. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Behn, H.; Schurr, U.; Ulbrich, A.; Noga, G. Development-dependent UV-B responses in red oak leaf lettuce (Lactuca sativa L.): Physiological mechanisms and significance for hardening. Eur. J. Hortic. Sci. 2011, 76, 33. [Google Scholar]
- Dudzinska, D.; Luzak, B.; Boncler, M.; Rywaniak, J.; Sosnowska, D.; Podsedek, A.; Watala, C. CD39/NTPDase-1 expression and activity in human umbilical vein endothelial cells are differentially regulated by leaf extracts from Rubus caesius and Rubus idaeus. Cell Mol. Biol. Lett. 2014, 19, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J. Agric. Sci. Technol. 2012, 14, 1497–1509. [Google Scholar]
- Shen, J.; Jiang, C.; Yan, Y.; Liu, B.; Zu, C. Effect of increased UV-B radiation on carotenoid accumulation and total antioxidant capacity in tobacco (Nicotiana tabacum L.) leaves. Genet. Mol. Res. 2017, 16, 1–11. [Google Scholar] [CrossRef]






| Cultivation System | Localization | Type of Soil | Kind of Fertilizer | Dose of Fertilizers and Time of Given | Plant Protection System |
|---|---|---|---|---|---|
| organic farm no. 1 | Zakroczym | sandy middle soil IVa and IVb category (15% floatable particles) pH 5.5 | cow manure | 35 t ha−1 one year before raspberry planting | Grevit 200 SL |
| (52°26′′ N 20°36′′ E) | |||||
| organic farm no. 2 | Załuski | sandy middle soil, sandy-clay IV category (20% floatable particles), pH 5.5 | cow manure | 30 t ha−1 one year before raspberry planting | no protection |
| (52°37′′ N 20°22′′ E) | |||||
| organic farm no. 3 | Radzanów | sandy middle soil IVa and III category (10% floatable particles), pH 6.0 | sheep manure, green manure | 10 t ha−1 and 15 t ha−1 one year before raspberry planting, | Bioczos 33 SL, Grevit 200 SL |
| (51°33′′ N 20°51′′ E) | |||||
| conventional farm no. 1 | Czerwińsk nad Wisłą | sandy-loamy middle soil IV and III category (20% floatable particles), pH 5.5 | Hydrocomplex 12-11-18; Superba 8-11-36 | (200 kg ha−1, 150 kg ha−1) in autumn a year before raspberry planting; 3 doses in time of cultivation | Signum 33 WG, Miros 20 SP, |
| (52°23′′ N 20°20′′ E) | |||||
| conventional farm no. 2 | Czerwińsk nad Wisłą | sandy-loamy middle soil IV and III category (25% floatable particles), pH 5.5 | amonium nitrate, polyphosphate, magnesium sulphate | in autumn a year before raspberry planting; 3 doses in time of cultivation | Calypso 480 SC, Miros 20 SP, Zato 50 WG |
| (52°23′′ N 20°20′′ E) | |||||
| conventional farm no. 3 | Czerwińsk nad Wisłą | sandy-clay middle soil II and III category (20% floatable particles) pH 6.0 | Rosafert 5-12-24-3 | 250 kg ha−1 in autumn a year before raspberry planting; 4 doses in time of cultivation | Calypso 480 SC, Miros 20 SP, Zato 50 WG |
| (52°25′′ N 20°23′′ E) |
| Examined Compounds | Organic Raspberry (n = 20) | Conventional Raspberry (n = 24) | p-Value |
|---|---|---|---|
| dry matter | 29.81 ± 1.26a 1 | 25.64 ± 0.73b | 0.0055 |
| total polyphenols | 136.10 ± 6.86a | 119.95 ± 14.19b | 0.0001 |
| total phenolic acids | 64.09 ± 3.80a | 52.94 ± 6.42b | < 0.0001 |
| chlorogenic | 5.66 ± 0.57a | 3.81 ± 0.50b | 0.0188 |
| caffeic | 24.98 ± 3.32a | 4.64 ± 0.82b | < 0.0001 |
| p-coumaric | 14.77 ± 0.83b | 25.02 ± 3.89a | 0.0243 |
| ellagic | 15.18 ± 2.41a | 16.96 ± 2.46a | N.S. 2 |
| salicylic | 3.51 ± 1.12a | 2.51 ± 0.08b | < 0.0001 |
| total flavonoids | 72.01 ± 3.77a | 67.01 ± 7.47a | N.S. |
| quercetin-3-O-rutinoside | 5.40 ± 1.12a | 1.42 ± 0.34b | 0.0009 |
| quercetin-3-O-glucoside | 31.31 ± 4.13a | 21.49 ± 3.55a | N.S. |
| luteolin | 8.87 ± 1.43b | 20.94 ± 3.90a | 0.0117 |
| myrycetin | 7.84 ± 1.10a | 6.33 ± 2.78a | N.S. |
| quercetin | 18.59 ± 1.12a | 16.84 ± 2.78a | N.S. |
| Examined Compounds | ‘Polana’ cv. (n = 8) | ‘Polka’ cv. (n = 12) | ‘Tulameen’ cv. (n = 8) | ‘Laszka’ cv. (n = 8) | ‘Glen Ample’ cv. (n = 8) | p-Value |
|---|---|---|---|---|---|---|
| dry matter | 26.06 ± 0.73a 1 | 27.77 ± 1.06a | 29.72 ± 2.88a | 28.85 ± 1.86a | 25.14 ± 0.81a | N.S. 2 |
| total polyphenols | 128.51 ± 2.78a | 151.75 ± 20.67a | 136.95 ± 19.10a | 88.08 ± 13.17a | 118.95 ± 7.48 | N.S. |
| total phenolic acids | 62.81 ± 1.83a | 66.55 ± 8.594a | 55.55 ± 11.30a | 37.35 ± 7.16a | 63.52 ± 4.36a | N.S. |
| chlorogenic | 4.46 ± 0.59a | 5.13 ± 0.83a | 4.96 ± 0.17a | 6.04 ± 1.40a | 2.44 ± 0.12a | N.S. |
| caffeic | 6.56 ± 1.59a | 12.60 ± 1.40ab | 27.00 ± 8.70b | 8.61 ± 3.59ab | 15.27 ± 4.99ab | 0.0401 |
| p-coumaric | 28.81 ± 6.15a | 22.67 ± 5.92a | 13.92 ± 1.17a | 10.10 ± 2.43a | 25.14 ± 2.24a | N.S. |
| ellagic | 20.04 ± 5.10ab | 23.22 ± 3.89b | 6.46 ± 1.01a | 9.96 ± 1.50a | 17.54 ± 0.87ab | 0.0046 |
| salicylic | 2.93 ± 0.11a | 2.92 ± 0.36a | 3.20 ± 0.36a | 2.65 ± 0.27a | 3.13 ± 0.23a | N.S. |
| total flavonoids | 65.71 ± 2.62a | 85.19 ± 12.43a | 81.40 ± 7.84a | 50.73 ± 6.37a | 55.43 ± 3.14a | N.S. |
| quercetin-3-O-rutinoside | 2.12 ± 0.17ab | 4.95 ± 1.01ab | 7.05 ± 2.22b | 0.53 ± 0.18a | 0.63 ± 0.18a | 0.0010 |
| quercetin-3-O-glucoside | 14.80 ± 1.10a | 36.43 ± 4.95b | 46.88 ± 6.02b | 13.34 ± 1.51a | 13.09 ± 3.79a | < 0.0001 |
| luteolin | 3.74 ± 0.49a | 23.07 ± 6.61b | 16.14 ± 1.23ab | 5.64 ± 1.22ab | 24.86 ± 4.76b | 0.0078 |
| myrycetin | 4.46 ± 0.42a | 9.52 ± 1.79a | 3.09 ± 0.23a | 8.48 ± 1.96a | 8.29 ± 1.91a | N.S. |
| quercetin | 40.59 ± 2.21c | 11.23 ± 0.34a | 8.25 ± 1.90a | 22.74 ± 3.00b | 8.56 ± 1.38a | < 0.0001 |
| Examined Compounds | Organic Raspberry (n = 20) | Conventional Raspberry (n = 24) | p-Value |
|---|---|---|---|
| total carotenoids | 2.61 ± 0.12b 1 | 3.14 ± 0.10a | 0.0014 |
| neoxanthin | 0.045 ± 0.01a | 0.025 ± 0.00b | 0.0013 |
| lutein | 1.23 ± 0.05a | 1.06 ± 0.03b | 0.0069 |
| zeaxanthin | 0.80 ± 0.03a | 0.70 ± 0.02b | 0.0118 |
| violaxanthin | 0.017 ± 0.001b | 0.026 ± 0.002a | 0.0004 |
| alpha-carotene | 0.060 ± 0.01b | 0.109 ± 0.01a | 0.0001 |
| beta-carotene | 0.46 ± 0.03b | 1.22 ± 0.06a | < 0.0001 |
| total chlorophylls | 5.75 ± 0.30b | 10.52 ± 0.60a | < 0.0001 |
| chlorophyll b | 1.79 ± 0.08b | 2.43 ± 0.12a | 0.0001 |
| chlorophyll a | 3.96 ± 0.23b | 8.09 ± 0.48a | < 0.0001 |
| chlorophyll a/b | 2.19 ± 0.06b | 3.29 ± 0.05a | < 0.0001 |
| Examined Compounds | ‘Polana’ cv. (n = 8) | ‘Polka’ cv. (n = 12) | ‘Tulameen’ cv. (n = 8) | ‘Laszka’ cv. (n = 8) | ‘Glen Ample’ cv. (n = 8) | p-Value |
|---|---|---|---|---|---|---|
| total carotenoids | 2.70 1 ± 0.07a | 3.00 ± 0.22a | 2.88 ± 0.19a | 3.17 ± 0.12a | 2.72 ± 0.22a | N.S. 2 |
| neoxanthin | 0.033 ± 0.00a | 0.024 ± 0.00a | 0.037 ± 0.01a | 0.062 ± 0.01b | 0.021 ± 0.00a | < 0.0001 |
| lutein | 1.08 ± 0.03a | 1.15 ± 0.04a | 1.23 ± 0.12a | 1.19 ± 0.08a | 1.05 ± 0.03a | N.S. |
| zeaxanthin | 0.70 ± 0.02a | 0.75 ± 0.03a | 0.80 ± 0.08a | 0.79 ± 0.05a | 0.68 ± 0.02a | N.S. |
| violaxanthin | 0.024 ± 0.002a | 0.024 ± 0.002a | 0.018 ± 0.001a | 0.017 ± 0.001a | 0.025 ± 0.004a | N.S. |
| alpha-carotene | 0.070 ± 0.01a | 0.088 ± 0.01a | 0.100 ± 0.01a | 0.095 ± 0.02a | 0.080 ± 0.02a | N.S. |
| beta-carotene | 0.79 ± 0.09a | 0.96 ± 0.14a | 0.70 ± 0.04a | 1.01 ± 0.18a | 0.87 ± 0.20a | N.S. |
| total chlorophylls | 7.64 ± 0.35a | 9.13 ± 1.06a | 6.73 ± 0.30a | 9.62 ± 1.44a | 8.25 ± 1.49a | N.S. |
| chlorophyll b | 1.95 ± 0.04a | 2.23 ± 0.19a | 1.93 ± 0.14a | 2.45 ± 0.21a | 2.10 ± 0.26a | N.S. |
| chlorophyll a | 5.69 ± 0.31a | 6.90 ± 0.87a | 4.81 ± 0.17a | 7.17 ± 1.23a | 6.15 ± 1.24a | N.S. |
| chlorophyll a/b | 2.91 ± 0.11a | 2.93 ± 0.17a | 2.56 ± 0.11a | 2.75 ± 0.27a | 2.71 ± 0.27a | N.S. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponder, A.; Hallmann, E. Phenolics and Carotenoid Contents in the Leaves of Different Organic and Conventional Raspberry (Rubus idaeus L.) Cultivars and Their In Vitro Activity. Antioxidants 2019, 8, 458. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8100458
Ponder A, Hallmann E. Phenolics and Carotenoid Contents in the Leaves of Different Organic and Conventional Raspberry (Rubus idaeus L.) Cultivars and Their In Vitro Activity. Antioxidants. 2019; 8(10):458. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8100458
Chicago/Turabian StylePonder, Alicja, and Ewelina Hallmann. 2019. "Phenolics and Carotenoid Contents in the Leaves of Different Organic and Conventional Raspberry (Rubus idaeus L.) Cultivars and Their In Vitro Activity" Antioxidants 8, no. 10: 458. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8100458