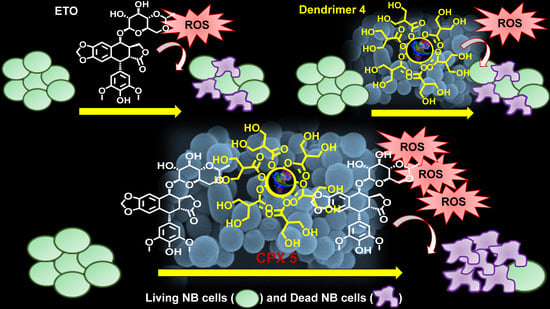
Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Entrapment Reaction of ETO in Dendrimer 4
2.3. Principal Components Analysis (PCA) on FTIR Spectral Data
2.4. Quantitative Investigations by UV Spectrophotometric Analysis: ETO Loading (DL%) Determination
2.4.1. Preparation of Calibration Curve
2.4.2. Estimation of ETO Amount (DL%) Loaded in CPX 5
2.5. Morphology, Size and Z-potential of CPX 5
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Dynamic Light Scattering (DLS)
2.6. Evaluation of CPX Solubility
2.7. Cell Culture Conditions and Treatments
2.8. Cell Viability Assay
2.9. Detection of Hydrogen Peroxide (H2O2) Production
2.10. Statistical Analyses
3. Results and Discussion
3.1. Entrapment of ETO in Dendrimer 4
3.1.1. Chemistry
3.1.2. FTIR Characterization
3.1.3. NMR Characterization
3.1.4. Principal Components Analysis (PCA) on FTIR Spectral Data
3.1.5. Quantitative Investigations by UV Spectrophotometric Analysis: ETO Loading (DL%) Determination
Calibration Curve
Estimation of ETO Amount in CPX 5 (DL%) and of Entrapment Efficiency (EE%)
3.1.6. Morphology, Size, and Z-potential of CPX 5
3.1.7. Evaluation of CPX 5 Solubility
3.2. Biological Activity of CPX 5 on Human NB Cells
3.2.1. CPX 5 Revealed a Synergistic Cytotoxic Effect Exerted by Dendrimer 4 per se and by the Complexed ETO Slowly Released over Time
3.2.2. CPX 5 Potentiates the Cytotoxic Action of ETO by Increasing Reactive Oxygen Species (ROS) Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.; Boggia, R. Pectin microdispersion vs non-polyamidoamine dendrimer nanodispersions: Two biocompatible approaches to increase Ellagic Acid water solubility and allow its more ways therapeutic administration. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Frechet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Hourani, R.; Kakkar, A. Advances in the Elegance of Chemistry in Designing Dendrimers. Macromol. Rapid Commun. 2010, 31, 947–974. [Google Scholar] [CrossRef]
- De Jesus, O.L.P.; Ihre, H.R.; Gagne, L.; Frechet, J.M.J.; Szoka, F.C. Polyester dendritic systems for drug delivery applications: In Vitro and in vivo evaluation. Bioconjugate Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Alfei, S.; Taptue, G.B.; Catena, S.; Bisio, A. Synthesis of Water-soluble, Polyester-based Dendrimer Prodrugs for Exploiting Therapeutic Properties of Two Triterpenoid Acids. Chin. J. Polym. Sci. 2018, 36, 999–1010. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Ponassi, M.; Rosano, C.; Zoppi, V.; Spallarossa, A. Hydrophilic and amphiphilic water-soluble dendrimer prodrugs suitable for parenteral administration of a non-soluble non-nucleoside HIV-1 reverse transcriptase inhibitor thiocarbamate derivative. Eur. J. Pharm. Sci. 2018, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P. Biosynthesis of lignans. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 5, pp. 459–503. [Google Scholar]
- Mounia, G.; Zhen-Zhou, J.; Lu-Yong, Z. Podophyllotoxin, a medicinal agent of plant origin: Past, present and future. Chin. J. Nat. Med. 2012, 10, 161–169. [Google Scholar] [CrossRef]
- Bromberg, K.D.; Burgin, A.B.; Osheroff, N. A two-drug model for etoposide action against human topoisomerase IIα. J. Biol. Chem. 2003, 278, 7406–7412. [Google Scholar] [CrossRef] [Green Version]
- Drewinko, B.; Barlogie, B. Survival and cycle-progression delay of human lymphoma cells in vitro exposed to VP-16-213. Cancer Treat. Rep. 1976, 60, 1295–1306. [Google Scholar]
- Jardin, I. Podophyllotoxins. In Anticancer Agents Based on Natural Product Models, 1st ed.; Cassady, J.M., Douros, J.D., Eds.; Academic Press: New York, NY, USA, 1980; pp. 319–351. [Google Scholar]
- Issell, B.F.; Muggia, F.M.; Carter, S.K. Etoposide [VP-16] Current Status and New Developments, 1st ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–353. [Google Scholar]
- Thomson, P.D.R. Drug Information for Health Care Professional, 24th ed.; PDR Network, LLC: Bedminster, NJ, USA, 2004; Volume 1, pp. 1–256. [Google Scholar]
- Van Maanen, J.M.; Retèl, J.; de Vries, J.; Pinedo, H.M. Mechanism of action of antitumor drug etoposide: A review. J. Natl. Cancer Inst. 1988, 80, 1526–1533. [Google Scholar] [CrossRef]
- Shah, J.C.; Chen, J.R.; Chow, D. Preformulation study of etoposide: Identification of physicochemical characteristics responsible for the low and erratic oral bioavailability of etoposide. Pharm. Res. 1989, 6, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Williams, S.D.; Einhorn, L.H.; Birch, R.; Greco, F.A. Successful treatment of resistant germinal neoplasms with VP-16 and cisplatin: Results of a Southeastern Cancer Study Group trial. J. Clin. Oncol. 1985, 3, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Greco, F.A. Etoposide: Twenty years later. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1995, 6, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.L. Low-dose oral etoposide: A new role for an old drug? J. Clin. Oncol. 1990, 8, 1607–1609. [Google Scholar] [CrossRef]
- Lowis, S.P.; Newell, D.R. Etoposide for the treatment of paediatric tumours: What is the best way to give it? Eur. J. Cancer 1996, 32, 2291–2297. [Google Scholar] [CrossRef]
- You, B.; Tranchand, B.; Girard, P.; Falandry, C.; Ribba, B.; Chabaud, S.; Souquet, P.J.; Court-Fortune, I.; Trillet-Lenoir, V.; Fournel, C.; et al. Etoposide pharmacokinetics and survival in patients with small cell lung cancer: A multicentre study. Lung Cancer 2008, 62, 261–272. [Google Scholar] [CrossRef]
- Dow, L.; Sinkule, J.A.; Look, A.T.; Horvath, A.; Evans, W.E. Comparative Cytotoxic and Cytokinetic Effects of the Epipodophyllotoxins, 4’-Demethylepipodophyllotoxin-9-(4,6-0-2-ethylidened-d-glucopyranoside) and 4’-Demethylepipodophyllotoxin-9-(4,6-0-2-thenylidene-β-d-glucopyranoside) and Their Metabolites on Human Leukemic Lymphoblasts. Cancer Res. 1983, 43, 5699–5706. [Google Scholar]
- Ho, D.H.W.; Kanellopoulos, K.A.; Yap, H.Y.; Casimir, M.; Savaraj, N.; Issell, B.; Bodey, G.P. Clinical Pharmacology of Etoposide by Radioimmunoassay. Proc. Am. Assoc. Cancer Res. 1983, 24, 131. [Google Scholar]
- Ayres, D.C.; Lim, C.D. Modification of the Pendant Ring of Podophyllotoxin. Cancer Chemother. Pharmacol. 1982, 7, 99–101. [Google Scholar] [CrossRef]
- Yoshioka, S. Stability of Drugs and Dosage Forms, 1st ed.; Kluwer Academic Publishers: New York, NY, USA, 2002; pp. 1–225. [Google Scholar]
- Zhou, X.M.; Lee, K.J.H.; Cheng, J.; Wu, S.S.; Chen, H.X.; Guo, X.; Cheng, Y.C.; Lee, K.H. New 7-Lactone Ring-Modified Ary lamino Etoposide Analogs as Inhibitors of Human DNA Topoisomerase II. J. Med. Chem. 1994, 37, 287–292. [Google Scholar] [CrossRef]
- Nahak, P.; Karmakar, G.; Chettri, P.; Roy, B.; Guha, P.; Besra, S.E.; Soren, A.; Bykov, A.G.; Akentiev, A.V.; Noskov, B.A.; et al. Influence of Lipid Core Material on Physicochemical Characteristics of an Ursolic Acid-Loaded Nanostructured Lipid Carrier: An Attempt to Enhance Anticancer Activity. Langmuir 2016, 32, 9816–9825. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, G.; Li, Y.; Wang, X.; Liu, S. Cell-Penetrating Hyperbranched Polyprodrug Amphiphiles for Synergistic Reductive Milieu-Triggered Drug Release and Enhanced Magnetic Resonance Signals. J. Am. Chem. Soc. 2015, 137, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Y.; Liu, T.; Hu, X.; Zhang, G.; You, Y.; Liu, S. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 2011, 32, 6595–6605. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Xie, X.; Wang, C.; You, J.; Mo, F.; Jin, B.; Chen, J.; Shao, J.; Chen, H.; et al. Dendrimeric anticancer prodrugs for targeted delivery of ursolic acid to folate receptor-expressing cancer cells: Synthesis and biological evaluation. Eur. J. Pharm. Sci. 2015, 70, 55–63. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar]
- Xu, J.; Luo, S.; Shi, W.; Liu, S. Two-Stage Collapse of Unimolecular Micelles with Double Thermoresponsive Coronas. Langmuir 2006, 22, 989–997. [Google Scholar] [CrossRef]
- Luo, S.; Xu, J.; Zhu, Z.; Wu, C.; Liu, S. Phase Transition Behavior of Unimolecular Micelles with Thermoresponsive Poly (N-isopropylacrylamide) Coronas. J. Phys. Chem. 2006, 110, 9132–9139. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Jiang, X.; Zhu, Z.; Rao, J.; Yin, J.; Wu, T.; Liu, H.; Liu, S. Thermosensitive Unimolecular Micelles Surface-Decorated with Gold Nanoparticles of Tunable Spatial Distribution. Chem. Mater. 2007, 19, 2489–2495. [Google Scholar] [CrossRef]
- Luo, S.; Hu, X.; Ling, C.; Liu, X.; Chen, S.; Han, M. Multiarm star-like unimolecular micelles with a dendritic core and a dual thermosensitive shell. Polym. Int. 2011, 60, 717–724. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Satija, J.; Sai, V.V.R.; Mukherji, S. Dendrimers in biosensors: Concept and applications. J. Mater. Chem. 2011, 21, 14367–14386. [Google Scholar] [CrossRef]
- Caminade, A.M. Dendrimers as biological sersors. In Dendrimers: Towards Catalytic, Material and Biomedical Uses, 1st ed.; Caminade, A.M., Turrin, C.O., Laurent, R., Ouali, A., Delavaux-Nicot, B., Eds.; John Wiley and Sons Ltd. Inc.: Chichester, UK, 2011; pp. 375–392. [Google Scholar]
- Kim, J.-H.; Park, K.; Nam, H.Y.; Lee, S.; Kim, K.; Kwon, I.C. Polymers for bioimaging. Prog. Polym. Sci. 2007, 32, 1031–1053. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, G.; Chen, X. Polymeric materials for theranostic applications. Pharm. Res. 2014, 31, 1358–1376. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Drechsler, M.; Puglia, C.; Cortesi, R. New Strategies for the Delivery of Some Natural Anti-oxidants with Therapeutic Properties. Mini Rev. Med. Chem. 2019, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Larsen, S.W.; Yaghmur, A. Citrem-phosphatidylcholine nano-self-assemblies: Solubilization of bupivacaine and its role in triggering a colloidal transition from vesicles to cubosomes and hexosomes. Phys. Chem. Chem. Phys. 2019, 21, 15142–15150. [Google Scholar] [CrossRef]
- Liu, D.; Angelova, A.; Liu, J.; Garamus, V.M.; Angelov, B.; Zhang, X.; Li, Y.; Feger, G.; Li, N.; Zou, A. Self-assembly of mitochondria-specific peptide amphiphiles amplifying lung cancer cell death through targeting the VDAC1-hexokinase-II complex. J. Mater. Chem. 2019, 7, 4706–4716. [Google Scholar] [CrossRef]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Shao, X.; Bor, G.; Al-Hosayni, S.; Salentinig, S.; Yaghmur, A. Structural characterization of self-assemblies of new omega-3 lipids: Docosahexaenoic acid and docosapentaenoic acid monoglycerides. Phys. Chem. Chem. Phys. 2018, 20, 23928–23941. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Esposito, E.; Sticozzi, C.; Ravani, L.; Drechsler, M.; Muresan, X.M.; Cervellati, F.; Cortesi, R.; Valacchi, G. Effect of new curcumin-containing nanostructured lipid dispersions on human keratinocytes proliferative responses. Exp. Dermatol. 2015, 24, 449–454. [Google Scholar] [CrossRef]
- Angelov, B.; Garamus, V.M.; Drechsler, M.; Angelova, A. Structural analysis of nanoparticulate carriers for encapsulation of macromolecular drugs. J. Mol. Liq. 2017, 235, 83–89. [Google Scholar] [CrossRef]
- Esposito, E.; Fantin, M.; Marti, M.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Sivieri, E.; Lain, F.; Menegatti, E.; Morari, M.; et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm. Res. 2008, 25, 1521–1530. [Google Scholar] [CrossRef]
- Qin, L.; Wang, M.; Zhu, R.; You, S.; Zhou, P.; Wang, S. The in vitro sustained release profile and antitumor effect of etoposide-layered double hydroxide nanohybrids. Int. J. Nanomed. 2013, 8, 2053–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy, 1st ed.; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–111. [Google Scholar]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Kulkarni, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Shao, X.-R.; Wei, X.-Q.; Song, Z.; Hao, L.-Y.; Cai, X.-X.; Zhang, Z.-R.; Peng, Q.; Lin, Y.-F. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, Z.; Jin, E.; Sun, Q.; Zhang, B.; Tang, J.; Shen, Y. Facile Synthesis of Polyester Dendrimers as Drug Delivery Carriers. Macromolecules 2013, 46, 37–42. [Google Scholar] [CrossRef]
- Ma, X.; Tang, J.; Shen, Y.; Fan, M.; Tang, H.; Radosz, M. Facile Synthesis of Polyester Dendrimers from Sequential Click Coupling of Asymmetrical Monomers. J. Am. Chem. Soc. 2009, 131, 14795–14803. [Google Scholar] [CrossRef] [PubMed]
- Ihre, H.; Hult, A.; Fréchet, J.M.J.; Gitsov, I. Double-stage convergent approach for the synthesis of functionalized dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid. Macromolecules 1998, 31, 4061–4068. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR characterization of dendrimers based on 2,2-bis-(hydroxymethyl)-propanoic acid (bis-HMPA) containing peripheral amino acid residues for gene transfection. Org. Commun. 2017, 10, 144–177. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyesterbased dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. ChemistrySelect 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Alfei, S.; Signorello, M.G.; Schito, A.M.; Catena, S.; Turrini, F. Reshaped as polyester-based nanoparticles, gallic acid inhibits platelet aggregation, reactive oxygen species production and multi-resistant Gram positive bacteria with an efficiency never obtained. Nanoscale Adv. 2019, 1, 4148–4157. [Google Scholar] [CrossRef] [Green Version]
- Tavares de Sousa, C.; da Silva, G.R.; Pianetti, G.A.; Solano, A.G.R. Spectrophotometric determination of etoposide from polymeric implant and application in the study of in vitro release profile. Rev. Bras. Farm. 2013, 94, 295–301. [Google Scholar]
- Negre-Salvayre, A.; Augé, N.; Duval, C.; Robbesyn, F.; Thiers, J.C.; Nazzal, D.; Benoist, H.; Salvayre, R. Detection of intracellular reactive oxygen species in cultured cells using fluorescent probes. Methods Enzymol. 2002, 352, 62–71. [Google Scholar]
- Medina, S.H.; El-Sayed, M.E.H. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009, 109, 3141–3157. [Google Scholar] [CrossRef]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 16, 261. [Google Scholar] [CrossRef] [Green Version]
- Kaminskas, L.M.; Boyd, B.J.; Porter, C.J.H. Dendrimer pharmacokinetics: The effect of size, structure and surface characteristics on ADME properties. Nanomedicine 2011, 6, 1063–1084. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Beijnen, J.H.; Holthuis, J.J.M.; Kerkdijk, H.G.; van der Houwen, O.A.G.J.; Paalman, A.C.A.; Bult, A.; Underberg, W.J.M. Degradation kinetics of etoposide in aqueous solution. Int. J. Pharm. 1988, 41, 169–183. [Google Scholar] [CrossRef]
- Strife, R.J.; Jardine, L.; Colvin, M. Analysis of the anticancer drugs VP 16-213 and VM 26 and their metabolites by high-performance liquid chromatography. J. Chromatogr. Biomed. Sci. Appl. 1980, 182, 211–220. [Google Scholar] [CrossRef]
- Strife, R.J.; Jardine, L.; Colvin, M. Analysis of the anticancer drugs etoposide (VP 16-213) and teneposide (VM 26) by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. Biomed. Sci. Appl. 1981, 224, 168–174. [Google Scholar] [CrossRef]
- Mross, K.; Bewermeier, P.; Kruger, W.; Stockschlader, M.; Zander, A.; Hossfeld, D.K. Pharmacokinetics of undiluted or diluted high-dose etoposide with or without busulfan administered to patients with hematologic malignancies. J. Clin. Oncol. 1994, 12, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Sinkule, J.A.; Evans, W.E. High performance liquid chromatographic analysis of the semisynthetic epipodophyllotoxins teneposide and etoposide using electrochemical detection. J. Pharm. Sci. 1984, 73, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Igwemezie, L.N.; Kaul, S.; Barbhaiya, R.H. Assessment of toxicokinetics and toxicodynamics following intravenous administration of etoposide phosphate in beagle dogs. Pharm. Res. 1995, 12, 117–123. [Google Scholar] [CrossRef]
- Van Opstal, M.A.J.; van der Horst, F.A.L.; Holthuis, J.J.M.; Van Bennekom, W.P.; Bult, A. Automated reversed phase chromatographic analysis of etoposide and teneposide in plasma using on-line surfactant-mediated sample clean-up and column-switching. J. Chromatogr. Biomed. Sci. Appl. 1989, 495, 139–151. [Google Scholar] [CrossRef]
- Mehrabi, M.; Esmaeilpour, P.; Akbarzadeh, A.; Saffari, Z.; Farahnak, M.; Farhangi, A.; Chiani, M. Efficacy of pegylated liposomal etoposide nanoparticles on breast cancer cell lines. Turk. J. Med. Sci. 2016, 46, 567–571. [Google Scholar] [CrossRef]
- Callewaert, M.; Dukic, S.; Van Gulick, L.; Vittier, M.; Gafa, V.; Andry, M.-C.; Molinari, M.; Roullin, V.G. Etoposide encapsulation in surface-modified poly (lactide-co-glycolide) nanoparticles strongly enhances glioma antitumor efficiency. J. Biomed. Mater. Res. Part A 2013, 101, 1319–1327. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharm. Dev. Technol. 2014, 19, 651–661. [Google Scholar] [CrossRef]
- Thiyagarajan, A.; Saravanabhavan, S.; Thangarasu, V. Preparation and Biopharmaceutical Evaluation of Novel Polymeric Nanoparticles Containing Etoposide for Targeting Cancer Cells. Turk. J. Pharm. Sci. 2019, 16, 132–140. [Google Scholar] [CrossRef]
- Rizvi, A.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Prokop, A.; Davidson, J.M. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 2008, 97, 3518–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, M.P.; Labhasetwar, V. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbieet, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [Green Version]
- McMillan, J.; Batrakova, E.; Gendelman, H.E. Cell delivery of therapeutic nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 563–601. [Google Scholar]
- Tocris, A. Bio-Techne Brand. Bio-Techne SRL. Available online: https://www.tocris.com/products/etoposide_1226 (accessed on 3 January 2020).
- McEvoy, G.K. American Hospital Formulary Service—Drug Information 92; Bethesda, M.D., Ed.; American Society of Hospital Pharmacists, Inc.: Bethesda, MD, USA, 1992; p. 524. [Google Scholar]
- Cell Signaling Technology. For Research Use Only. Not for Use in Diagnostic Procedures. 2014. Available online: https://media.cellsignal.com/pdf/2200.pdf (accessed on 15 March 2018).
- Yadav, K.S.; Sawant, K.K. Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation. Curr. Drug Deliv. 2010, 7, 51–64. [Google Scholar] [CrossRef]
- Zare Kazemabadi, F.; Heydarinasab, A.; Akbarzadeh, A.; Ardjmand, M. Preparation, characterization and in vitro evaluation of PEGylated nanoliposomal containing etoposide on lung cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3222–3230. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.; Øra, I.; Pörn-Ares, I.; Påhlman, S. Arsenic trioxide-induced death of neuroblastoma cells involves activation of Bax and does not require p53. Clin. Cancer Res. 2004, 10, 3179–3188. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, S.; Bellincampi, L.; Ballerini, S.; Ranalli, M.; Pastore, A.; Cortese, C.; Federici, G. Role of GST P1-1 in mediating the effect of etoposide on human neuroblastoma cell line Sh-Sy5y. J. Cell. Biochem. 2002, 86, 340–347. [Google Scholar] [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-mediated antioxidant response and aerobic metabolism: Two crucial factors involved in determining the multi-drug resistance of high-risk neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naha, P.C.; Byrne, H.J. Generation of intracellular reactive oxygen species and genotoxicity effect to exposure of nanosized polyamidoamine (PAMAM) dendrimers in PLHC-1 cells in vitro. Aquat. Toxicol. 2013, 132–133, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| A | CETO (µg/mL) | εETOC (M−1 L cm−1) |
|---|---|---|
| 0.149 | 18.7 | 4690 |
| 0.152 | 19.0 | 4709 |
| 0.146 | 18.3 | 4696 |
| 0.149 | 18.7 | 4690 |
| 0.148 | 18.6 | 4684 |
| 0.151 | 18.9 | 4703 |
| Dendrimer 4 (µg, µmol) | ETO Content (µg, µmol) | DL% (w/w) | EE% (w/w) | ETO Content (molesETO/mole4) | Molecular Weight (MW) of CPX 5 |
|---|---|---|---|---|---|
| 31.3, 0.0043 | 18.7, 0.032 | 37.4 | 89.3 | 7.4 | 11,631 |
| Z-AVE Size (nm) 1 | PdI 2 | Average Particles Size (nm) by SEM Analysis | Z-potential (mV) 3 |
|---|---|---|---|
| 69.2 ± 1.08 | 0.536 ± 0.002 | 76.25 ± 17.3 | −45 ± 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9010050
Alfei S, Marengo B, Domenicotti C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants. 2020; 9(1):50. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9010050
Chicago/Turabian StyleAlfei, Silvana, Barbara Marengo, and Cinzia Domenicotti. 2020. "Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells" Antioxidants 9, no. 1: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9010050