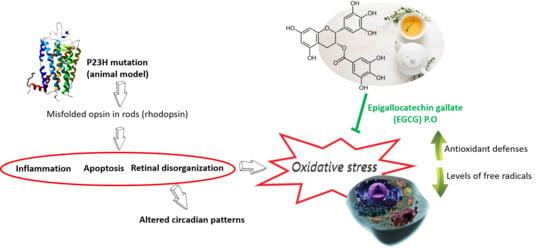
Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Visual Assessment: Visual Acuity and Contrast Sensitivity
2.3. ERG Recordings
2.4. Mixed Scotopic ERG Response
2.5. Isolation of the Cone Response Using a Double-Flash Protocol
2.6. Activity and Body Temperature Recording
2.7. Sample Collection and Biochemical Evaluation
2.8. Total Protein Quantification
2.9. Lipid Peroxidation
2.10. Protein Carbonyl Groups
2.11. Nitrosative Damage
2.12. GSH/GSSG Ratio
2.13. Total Antioxidant Capacity (TAC)
2.14. Antioxidant Enzymes
2.15. Statistical Analysis
3. Results
3.1. Visual Parameters
3.2. ERG Recordings
3.3. Temperature and Locomotor Rhythm
3.4. Oxidative Stress Parameters
3.5. Antioxidant Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coussa, R.G.; Chakarova, C.; Ajlan, R.; Taha, M.; Kavalec, C.; Gomolin, J.; Khan, A.; Lopez, I.; Ren, H.; Waseem, N.; et al. Genotype and Phenotype Studies in Autosomal Dominant Retinitis Pigmentosa (adRP) of the French Canadian Founder Population. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8297–8305. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Xu, K.; Zhang, X.; Xie, Y.; Li, Y. Sector Retinitis Pigmentosa caused by mutations of the RHO gene. Eye 2019, 33, 592–599. [Google Scholar] [CrossRef]
- Mitchell, J.; Balem, F.; Tirupula, K.; Man, D.; Dhiman, H.K.; Yanamala, N.; Ollesch, J.; Planas-Iglesias, J.; Jennings, B.J.; Gerwert, K.; et al. Comparison of the molecular properties of retinitis pigmentosa P23H and N15S amino acid replacements in rhodopsin. PLoS ONE 2019, 14, e0214639. [Google Scholar] [CrossRef]
- Machida, S.; Kondo, M.; Jamison, J.A.; Khan, N.W.; Kononen, L.T.; Sugawara, T.; Bush, R.A.; Sieving, P.A. P23H rhodopsin transgenic rat: Correlation of retinal function with histopathology. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3200–3209. [Google Scholar] [PubMed]
- LaVail, M.M.; Nishikawa, S.; Steinberg, R.H.; Naash, M.I.; Duncan, J.L.; Trautmann, N.; Matthes, M.T.; Yasumura, D.; Lau-Villacorta, C.; Chen, J.; et al. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp. Eye Res. 2018, 167, 56–90. [Google Scholar] [CrossRef] [PubMed]
- Monai, N.; Yamauchi, K.; Tanabu, R.; Gonome, T.; Ishiguro, S.I.; Nakazawa, M. Characterization of photoreceptor degeneration in the rhodopsin P23H transgenic rat line 2 using optical coherence tomography. PLoS ONE 2018, 13, e0193778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besharse, J.C.; McMahon, D.G. The Retina and Other Light-sensitive Ocular Clocks. J. Biol. Rhythm. 2016, 31, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.; Driver, H.S.; Heon, E.; Flanagan, J.; Shapiro, C.M. Sleep and daytime sleepiness in retinitis pigmentosa patients. J. Sleep Res. 2001, 10, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Chen, T.; Yang, G.Q.; Peng, G.H.; Yan, Z.J.; Huang, Y.F. Anthocyanin can arrest the cone photoreceptor degeneration and act as a novel treatment for retinitis pigmentosa. Int. J. Ophthalmol. 2016, 9, 153–158. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.W.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; McGinnis, J.F. Oxidative stress: The achilles’ heel of neurodegenerative diseases of the retina. Front. Biosci. 2012, 17, 1976–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sizova, O.S.; Shinde, V.M.; Lenox, A.R.; Gorbatyuk, M.S. Modulation of cellular signaling pathways in P23H rhodopsin photoreceptors. Cell Signal. 2014, 26, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmody, R.J.; McGowan, A.J.; Cotter, T.G. Reactive oxygen species as mediators of photoreceptor apoptosis in vitro. Exp. Cell Res. 1999, 248, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.J.; Cotter, T.G. Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ. 2000, 7, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Tsubura, A.; Yoshizawa, K.; Kuwata, M.; Uehara, N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol. Histopathol. 2010, 25, 933–944. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Wong, L.L.; Pye, Q.N.; Chen, L.; Seal, S.; McGinnis, J.F. Defining the catalytic activity of nanoceria in the P23H-1 rat, a photoreceptor degeneration model. PLoS ONE 2015, 10, e0121977. [Google Scholar] [CrossRef] [Green Version]
- Maleki, S.; Gopalakrishnan, S.; Ghanian, Z.; Sepehr, R.; Schmitt, H.; Eells, J.; Ranji, M. Optical imaging of mitochondrial redox state in rodent model of retinitis pigmentosa. J. Biomed. Opt. 2013, 18, 16004. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Trachsel-Moncho, L.; Benlloch-Navarro, S.; Grima, M.; Almansa, I.; Araiz, J.; Poch, E. Oxidative stress and autophagy markers in rd10 mice retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2262. [Google Scholar]
- Alavi, M.V.; Chiang, W.-C.; Kroeger, H.; Yasumura, D.; Matthes, M.T.; Iwawaki, T.; LaVail, M.M.; Gould, D.B.; Lin, J.H. In Vivo Visualization of Endoplasmic Reticulum Stress in the Retina Using the ERAI Reporter MouseEndoplasmic Reticulum Stress in the Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6961–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Ben Gdara, N.; Sanchez-Cano, A.I.; Insa, G.; Orduna, E.; Pinilla, I. Hepatic oxidative stress in pigmented P23H rhodopsin transgenic rats with progressive retinal degeneration. Free Radic. Biol. Med. 2018, 124, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Esquiva, G.; Fuentes-Broto, L.; Segura, F.; Sanchez-Cano, A.; Cuenca, N.; Pinilla, I. Age-related changes in photosensitive melanopsin-expressing retinal ganglion cells correlate with circadian rhythm impairments in sighted and blind rats. Chronobiol. Int. 2016, 33, 374–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.O.; Chan, K.P.; Yang, Y.P.; Qin, Y.J.; Li, W.Y.; Chan, S.O.; Wang, C.C.; Pang, C.P. Effects of EGCG content in green tea extract on pharmacokinetics, oxidative status and expression of inflammatory and apoptotic genes in the rat ocular tissues. J. Nutr. Biochem. 2015, 26, 1357–1367. [Google Scholar] [CrossRef]
- Gupta, S.K.; Halder, N.; Srivastava, S.; Trivedi, D.; Joshi, S.; Varma, S.D. Green tea (Camellia sinensis) protects against selenite-induced oxidative stress in experimental cataractogenesis. Ophthalmic Res. 2002, 34, 258–263. [Google Scholar] [CrossRef]
- Lee, H.S.; Chauhan, S.K.; Okanobo, A.; Nallasamy, N.; Dana, R. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea 2011, 30, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Chen, L.; Jiang, L.; Lai, T.Y. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci. Lett. 2015, 600, 132–136. [Google Scholar] [CrossRef]
- Zhang, B.; Osborne, N.N. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006, 1124, 176–187. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Safa, R.; Rusciano, D.; Osborne, N.N. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007, 1159, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.H.; Ko, M.L.; Chen, C.F. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp. Eye Res. 2008, 86, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Emoto, Y.; Yoshizawa, K.; Kinoshita, Y.; Yuki, M.; Yuri, T.; Tsubura, A. Green tea extract attenuates MNU-induced photoreceptor cell apoptosis via suppression of heme oxygenase-1. J. Toxicol. Pathol. 2016, 29, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xu, C.; Chen, Y.; Liang, J.J.; Xu, Y.; Chen, S.L.; Huang, S.; Yang, Q.; Cen, L.P.; Pang, C.P.; et al. Green Tea Extract Ameliorates Ischemia-Induced Retinal Ganglion Cell Degeneration in Rats. Oxid. Med. Cell Longev. 2019, 2019, 8407206. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; Wang, C.; Song, D.; Song, Y.; Dunaief, J.L. Intraperitoneal injection of (-)-Epigallocatechin-3-gallate protects against light-induced photoreceptor degeneration in the mouse retina. Mol. Vis. 2017, 23, 171–178. [Google Scholar]
- Zhang, B.; Rusciano, D.; Osborne, N.N. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 2008, 1198, 141–152. [Google Scholar] [CrossRef]
- Li, C.P.; Yao, J.; Tao, Z.F.; Li, X.M.; Jiang, Q.; Yan, B. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: A potential role for reducing UVB light-induced retinal damage. Biochem. Biophys. Res. Commun. 2013, 438, 739–745. [Google Scholar] [CrossRef]
- Orhan, E.; Dalkara, D.; Neuille, M.; Lechauve, C.; Michiels, C.; Picaud, S.; Leveillard, T.; Sahel, J.A.; Naash, M.I.; Lavail, M.M.; et al. Genotypic and phenotypic characterization of P23H line 1 rat model. PLoS ONE 2015, 10, e0127319. [Google Scholar] [CrossRef] [Green Version]
- Segura, F.; Arines, J.; Sanchez-Cano, A.; Perdices, L.; Orduna-Hospital, E.; Fuentes-Broto, L.; Pinilla, I. Development of optokinetic tracking software for objective evaluation of visual function in rodents. Sci. Rep. 2018, 8, 10009. [Google Scholar] [CrossRef] [Green Version]
- Segura, F.; Sanchez-Cano, A.; Jarabo, S.; Lopez de la Fuente, C.; Cuenca, N.; Villegas-Perez, M.P.; Pinilla, I. Assessment of Visual and Chromatic Functions in a Rodent Model of Retinal Degeneration. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6275–6283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choh, V.; Gurdita, A.; Tan, B.; Feng, Y.; Bizheva, K.; McCulloch, D.L.; Joos, K.M. Isoflurane and ketamine:xylazine differentially affect intraocular pressure-associated scotopic threshold responses in Sprague-Dawley rats. Doc. Ophthalmol. 2017, 135, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nixon, P.J.; Bui, B.V.; Armitage, J.A.; Vingrys, A.J. The contribution of cone responses to rat electroretinograms. Clin. Exp. Ophthalmol. 2001, 29, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, I.; Lund, R.D.; Sauve, Y. Contribution of rod and cone pathways to the dark-adapted electroretinogram (ERG) b-wave following retinal degeneration in RCS rats. Vis. Res. 2004, 44, 2467–2474. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.A.; Madrid, J.A. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Green, L.C.; de Luzuriaga, K.R.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef] [Green Version]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Miller, N.J.; Paganga, G.; Wiseman, S.; Van Nielen, W.; Tijburg, L.; Chowienczyk, P.; Rice-Evans, C.A. Total antioxidant activity of low density lipoproteins and the relationship with alpha-tocopherol status. FEBS Lett. 1995, 365, 164–166. [Google Scholar] [CrossRef] [Green Version]
- Nagai, K.; Jiang, M.H.; Hada, J.; Nagata, T.; Yajima, Y.; Yamamoto, S.; Nishizaki, T. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002, 956, 319–322. [Google Scholar] [CrossRef]
- Yu, N.H.; Pei, H.; Huang, Y.P.; Li, Y.F. (-)-Epigallocatechin-3-Gallate Inhibits Arsenic-Induced Inflammation and Apoptosis through Suppression of Oxidative Stress in Mice. Cell Physiol. Biochem. 2017, 41, 1788–1800. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yip, Y.W.Y.; Ren, J.; Hui, W.K.; He, J.N.; Yu, Q.X.; Chu, K.O.; Ng, T.K.; Chan, S.O.; Pang, C.P.; et al. Green tea catechins alleviate autoimmune symptoms and visual impairment in a murine model for human chronic intraocular inflammation by inhibiting Th17-associated pro-inflammatory gene expression. Sci. Rep. 2019, 9, 2301. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Milam, A.H.; Li, Z.Y.; Fariss, R.N. Histopathology of the human retina in retinitis pigmentosa. Prog. Retinal Eye Res. 1998, 17, 175–205. [Google Scholar]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Beal, M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505. [Google Scholar] [CrossRef]
- Williams, D.L. Oxidative stress and the eye. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 179–192. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Gallego-Pinazo, R.; Garcia-Medina, J.J.; Zanon-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramirez, C.; Lopez-Galvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Butterfield, D.A. Oxidative stress and neurodegeneration. Brain Res. Bull. 2017, 133, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, J.H.; Chiang, H.S.; Wu, W.B.; Lin, H.H.; Hong, J.Y.; Hung, C.F. Effects of (-)-epigallocatechin gallate on RPE cell migration and adhesion. Mol. Vis. 2010, 16, 586–595. [Google Scholar] [PubMed]
- Chen, F.; Jiang, L.; Shen, C.; Wan, H.; Xu, L.; Wang, N.; Jonas, J.B. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-D-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012, 90, e609–e615. [Google Scholar] [CrossRef] [PubMed]
- Thichanpiang, P.; Wongprasert, K. Green tea polyphenol epigallocatechin-3-gallate attenuates TNF-alpha-induced intercellular adhesion molecule-1 expression and monocyte adhesion to retinal pigment epithelial cells. Am. J. Chin. Med. 2015, 43, 103–119. [Google Scholar] [CrossRef]
- Sampath, C.; Zhu, Y.; Sang, S.; Ahmedna, M. Bioactive compounds isolated from apple, tea, and ginger protect against dicarbonyl induced stress in cultured human retinal epithelial cells. Phytomedicine 2016, 23, 200–213. [Google Scholar] [CrossRef]
- Ren, J.L.; Yu, Q.X.; Liang, W.C.; Leung, P.Y.; Ng, T.K.; Chu, W.K.; Pang, C.P.; Chan, S.O. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci. Rep. 2018, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Aubin, S.; Gacon, C.; Jennum, P.; Ptito, M.; Kupers, R. Altered sleep–wake patterns in blindness: A combined actigraphy and psychometric study. Sleep Med. 2016, 24, 100–108. [Google Scholar] [CrossRef]
- Park, K.-S.; Han, J.-Y.; Moon, D.-C.; Hong, J.T.; Oh, K.-W. (–)-Epigallocatechin-3-O-Gallate Augments Pentobarbital-Induced Sleeping Behaviors Through Cl-Channel Activation. J. Med. Food 2011, 14, 1456–1462. [Google Scholar] [CrossRef]
- Hu, Z.; Oh, S.; Ha, T.W.; Hong, J.T.; Oh, K.W. Sleep-Aids Derived from Natural Products. Biomol. Ther. 2018, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ueda, T.; Armstrong, D. Preventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eye. Ophthalmic Res. 1996, 28, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Yonemitsu, K.; Koreeda, A.; Tsunenari, S. Inhibitory activity of epigallocatechin gallate (EGCg) in paraquat-induced microsomal lipid peroxidation—A mechanism of protective effects of EGCg against paraquat toxicity. Toxicology 2003, 183, 143–149. [Google Scholar] [CrossRef]
- Orsolic, N.; Sirovina, D.; Gajski, G.; Garaj-Vrhovac, V.; Jazvinscak Jembrek, M.; Kosalec, I. Assessment of DNA damage and lipid peroxidation in diabetic mice: Effects of propolis and epigallocatechin gallate (EGCG). Mutat. Res. 2013, 757, 36–44. [Google Scholar] [CrossRef]
- Koonyosying, P.; Kongkarnka, S.; Uthaipibull, C.; Svasti, S.; Fucharoen, S.; Srichairatanakool, S. Green tea extract modulates oxidative tissue injury in beta-thalassemic mice by chelation of redox iron and inhibition of lipid peroxidation. Biomed. Pharmacother. 2018, 108, 1694–1702. [Google Scholar] [CrossRef]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (-)-Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells Against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef]
- James, K.D.; Forester, S.C.; Lambert, J.D. Dietary pretreatment with green tea polyphenol, (−)-epigallocatechin-3-gallate reduces the bioavailability and hepatotoxicity of subsequent oral bolus doses of (−)-epigallocatechin-3-gallate. Food Chem. Toxicol. 2015, 76, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef]
- James, K.D.; Kennett, M.J.; Lambert, J.D. Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2018, 111, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Huggins, T.; Staton, M.; Dyer, D.; Detorie, N.; Walla, M.; Baynes, J.; Thorpe, S. o-Tyrosine and Dityrosine Concentrations in Oxidized Proteins and Lens Proteins with Age a. Ann. N. Y. Acad. Sci. 1992, 663, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.; Leeuwenburgh, C. Aging and the role of reactive nitrogen species. Ann. N. Y. Acad. Sci. 2002, 959, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Chakravarti, D.N. Protein Tyrosine Nitration: Role in Aging. Curr. Aging Sci. 2017, 10, 246–262. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparlo, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging (Albany NY) 2018, 10, 868–901. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [Green Version]
- El-Missiry, M.A.; Othman, A.I.; El-Sawy, M.R.; Lebede, M.F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. Int. J. Radiat. Biol. 2018, 94, 798–808. [Google Scholar] [CrossRef]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T.; et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-D.; Zhang, Y.-Y.; Wang, K.-L.; Huang, Y.-P.; Yang, Z.-B.; Liu, Z. The involvement of Nrf2 in the protective effects of (-)-Epigallocatechin-3-gallate (EGCG) on NaAsO2-induced hepatotoxicity. Oncotarget 2017, 8, 65302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.M.; Chan, H.Y.E.; Huang, Y.; Chen, Z.Y. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol. Nutr. Food Res. 2007, 51, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Fang, Y.; Wei, S.M. Effect and mechanism of epigallocatechin-3-gallate (EGCG). against the hydrogen peroxide-induced oxidative damage in human dermal fibroblasts. J. Cosmet. Sci. 2013, 64, 35–44. [Google Scholar] [PubMed]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 30233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef] [Green Version]
- Church, R.J.; Gatti, D.M.; Urban, T.J.; Long, N.; Yang, X.; Shi, Q.; Eaddy, J.S.; Mosedale, M.; Ballard, S.; Churchill, G.A.; et al. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef]
- Dong, R.; Wang, D.; Wang, X.; Zhang, K.; Chen, P.; Yang, C.S.; Zhang, J. Epigallocatechin-3-gallate enhances key enzymatic activities of hepatic thioredoxin and glutathione systems in selenium-optimal mice but activates hepatic Nrf2 responses in selenium-deficient mice. Redox Biol. 2016, 10, 221–232. [Google Scholar] [CrossRef] [Green Version]








| SD × LE | P23H × LE | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | Locomotor Activity | Temperature | Locomotor Activity | |||||
| Vehicle (n = 5) | EGCG (n = 5) | Vehicle (n = 5) | EGCG (n = 5) | Vehicle (n = 5) | EGCG (n = 5) | Vehicle (n = 5) | EGCG (n = 5) | |
| Rhythm parameters | ||||||||
| Mesor (°C) | 36.96 ± 0.04 | 36.99 ± 0.04 | 4.47 ± 0.42 * | 3.14 ± 0.21 # | 37.16 ± 0.13 | 36.96 ± 0.03 | 2.69 ± 0.28 # | 2.67 ± 0.21 |
| Amplitude (°C) | 0.37 ± 0.01 * | 0.46 ± 0.10 | 2.67 ± 0.35 | 2.24 ± 0.24 | 0.51 ± 0.06 # | 0.56 ± 0.05 | 1.90 ± 0.17 | 1.60 ± 0.10 |
| Acrophase (min) | 1118.26 ± 23.66 | 1088.46 ± 11.86 | 1145.71 ± 35.07 | 1175.15 ± 30.04 | 1022.90 ± 30.69 | 1005.43 ± 14.16 | 1056.88 ± 21.28 | 982.23 ± 7.71 * |
| Acrophase (hh:mm) | 2:43 ± 0:23 | 2:13 ± 0:11 | 3:10 ± 0:35 | 3:40 ± 0:30 | 1:07 ± 0:30 | 0:50 ± 0:14 | 1:41 ± 0:21 | 0:27 ± 0:07 * |
| Variance (%) | 26.08 ± 1.076 * | 30.49 ± 7.936 | 11.77 ± 1.59 | 14.04 ± 2.18 | 38.03 ± 4.806 # | 42.05 ± 4.473 | 13.24 ± 1.39 | 8.40 ± 0.49 * |
| Period (min) | 1445.00 ± 20.00 | 1440.00 ± 5.00 | 1441.67 ± 3.33 | 1445.00 ± 4.08 | 1442.50 ± 2.50 | 1445.00 ± 4.08 | 1442.50 ± 2.50 | 1460.00 ± 8.66 * |
| Non-parametric variables | ||||||||
| IS | 0.63 ± 0.03 | 0.61 ± 0.03 | 0.33 ± 0.02 | 0.35 ± 0.03 | 0.69 ± 0.04 | 0.68 ± 0.02 | 0.32 ± 0.03 | 0.23 ± 0.01 * |
| IV | 0.25 ± 0.03 | 0.21 ± 0.02 | 0.96 ± 0.01 | 0.91 ± 0.09 | 0.18 ± 0.03 | 0.18 ± 0.02 | 1.08 ± 0.07 | 1.08 ± 0.04 |
| RA | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.50 ± 0.02 | 0.64 ± 0.03 # | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.60 ± 0.04 | 0.51 ± 0.04 |
| L2 (hh:mm) | 5:05 ± 2:30 * | 6:35 ± 0:21 | 6:31 ± 0:24 * | 6:27 ± 0:13 | 13:55 ± 1:24 # | 12:42 ± 0:56 | 6:45 ± 0:20 # | 6:30: ± 0:33 |
| VL2 (°C) | 37.53 ± 0.08 | 37.57 ± 0.07 | 4.67 ± 0.89 * | 3.06 ± 0.25 | 37.43 ± 0.07 | 37.29 ± 0.04 * | 2.82 ± 0.13 # | 2.27 ± 0.16 |
| Media (°C) | 37.43 ± 0.04 | 37.45 ± 0.04 | 4.87 ± 0.43 * | 3.51 ± 0.21 # | 37.48 ± 0.03 | 37.45 ± 0.02 | 3.07 ± 0.29 # | 3.06 ± 0.21 |
| CFI | 0.30 ± 0.00 | 0.28 ± 0.01 # | 0.60 ± 0.01 | 0.63 ± 0.02 | 0.29 ± 0.01 | 0.29 ± 0.00 | 0.66 ± 0.03 | 0.61 ± 0.02 |
| DesynchroInd L2 | - | - | 0.19 ± 0.14 * | 0.02 ± 0.01 | -- | - | 0.60 ± 0.11 # | 0.52 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdices, L.; Fuentes-Broto, L.; Segura, F.; Cuenca, N.; Orduna-Hospital, E.; Pinilla, I. Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats. Antioxidants 2020, 9, 718. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080718
Perdices L, Fuentes-Broto L, Segura F, Cuenca N, Orduna-Hospital E, Pinilla I. Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats. Antioxidants. 2020; 9(8):718. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080718
Chicago/Turabian StylePerdices, Lorena, Lorena Fuentes-Broto, Francisco Segura, Nicolás Cuenca, Elvira Orduna-Hospital, and Isabel Pinilla. 2020. "Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats" Antioxidants 9, no. 8: 718. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080718