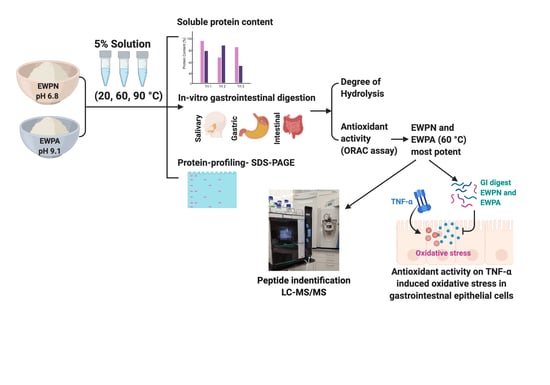
Effect of pH and Heat Treatment on the Antioxidant Activity of Egg White Protein-Derived Peptides after Simulated In-Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Temperature Treatment of Egg White Powder (EWP)
2.3. Protein Content and Solubility
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Simulated In-Vitro Digestion of Egg White Powder
2.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. Cell Culture
2.8. Reactive Oxygen Species (ROS) Measurement in Gastrointestinal Epithelial Cells
2.9. Peptide Profile of In Vitro Digested EWP through LC-MS/MS
2.10. Statistical Analysis
3. Result and Discussion
3.1. Effect of Heat Treatment on Protein Content and Solubility of Egg White Protein(EWP)
3.2. Degree of Hydrolysis (DH) of Simulated Digested EWP
3.3. Antioxidant Properties of In Vitro Digested EWp by ORAC Assay
3.4. Effect of EWP Hydrolysates on TNF Alpha-Induced Superoxide Generation
3.5. Identification and Characterization of Peptides in the EWP Hydrolysates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA AMS. Egg Markets Overview. Available online: https://www.ams.usda.gov/ (accessed on 1 May 2020).
- Kovacs-Nolan, J.; Phillips, M.; Mine, Y. Advances in the value of eggs and egg components for human health. J. Agric. Food Chem. 2005, 53, 8421–8431. [Google Scholar] [CrossRef]
- Evenepoel, P.; Geypens, B.; Luypaerts, A.; Hiele, M.; Ghoos, Y.; Rutgeerts, P. Digestibility of Cooked and Raw Egg Protein in Humans as Assessed by Stable Isotope Techniques. J Nutr. 1998, 128, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Xu, Y.; Shi, M.; Su, Y.; Li, X.; Li, J.; Yang, Y. Effect of dry-heat and guar gum on properties of egg white powder: Analysis of forming capacity and baking performance. Food Hydrocoll. 2020, 99, 105333. [Google Scholar] [CrossRef]
- Gao, X.; Yao, Y.; Wu, N.; Xu, M.; Zhao, Y.; Tu, Y. The sol-gel-sol transformation behavior of egg white proteins induced by alkali. Int. J. Biol. Macromol. 2020, 155, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Noutomi, T.; Haga, N. Thermally Induced Changes in Egg White Proteins. J. Agric. Food Chem. 1990, 38, 2122–2125. [Google Scholar] [CrossRef]
- Van Der Plancken, I.; Van Loey, A.; Hendrickx, M.E. Effect of heat-treatment on the physico-chemical properties of egg white proteins: A kinetic study. J. Food Eng. 2006, 75, 316–326. [Google Scholar] [CrossRef]
- Hoppe, A.; Jung, S.; Patnaik, A.; Zeece, M.G. Effect of high pressure treatment on egg white protein digestibility and peptide products. Innov. Food Sci. Emerg. Technol. 2013, 17, 54–62. [Google Scholar] [CrossRef]
- Nyemb, K.; Guérin-Dubiard, C.; Pézennec, S.; Jardin, J.; Briard-Bion, V.; Cauty, C.; Rutherfurd, S.M.; Dupont, D.; Nau, F. The structural properties of egg white gels impact the extent of in vitro protein digestion and the nature of peptides generated. Food Hydrocoll. 2016, 54, 315–327. [Google Scholar] [CrossRef]
- Matsuda, T.; Watanabe, K.; Sato, Y. Heat-Induced Aggregation of Egg White Proteins as Studied by Vertical Flat-Sheet Polyacrylamide Gel Electrophoresis. J. Food Sci. 1981, 46, 1829–1834. [Google Scholar] [CrossRef]
- Matsudomi, N.; Oka, H.; Sonoda, M. Inhibition against heat coagulation of ovotransferrin by ovalbumin in relation to its molecular structure. Food Res. Int. 2002, 35, 821–827. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Effects of pH, temperature and pulsed electric fields on the turbidity and protein aggregation of ovomucin-depleted egg white. Food Res. Int. 2017, 91, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, N.; Liu, Y. Effect of Different Heat Treatments on In Vitro Digestion of Egg White Proteins and Identification of Bioactive Peptides in Digested Products. J. Food Sci. 2018, 83, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Leeb, E.; Götz, A.; Letzel, T.; Cheison, S.C.; Kulozik, U. Influence of denaturation and aggregation of β-lactoglobulin on its tryptic hydrolysis and the release of functional peptides. Food Chem. 2015, 187, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Lassé, M.; Deb-Choudhury, S.; Haines, S.; Larsen, N.; Gerrard, J.A.; Dyer, J.M. The impact of pH, salt concentration and heat on digestibility and amino acid modification in egg white protein. J. Food Compos. Anal. 2015. [Google Scholar] [CrossRef]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, W.; He, R.; Xiong, F.; Ma, H. Protein breakdown and release of antioxidant peptides during simulated gastrointestinal digestion and the absorption by everted intestinal sac of rapeseed proteins. LWT 2017, 86, 424–429. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chi, Y.; Cheng, Y.; Zhao, Y. Physicochemical properties, in vitro digestibility and antioxidant activity of dry-heated egg white protein. Food Chem. 2018, 246, 18–25. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers Barking: Essex, UK, 1986; ISBN 0853343861. [Google Scholar]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen Radical Absorbance Capacity of Peptides from Egg White Protein Ovotransferrin and their Interaction with Phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Zhu, Y.; Vanga, S.K.; Wang, J.; Raghavan, V. Impact of food processing on the structural and allergenic properties of egg white. Trends Food Sci. Technol. 2018, 78, 188–196. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, W.; Yang, R.; Yan, W.; Sun, Q. Aggregation of egg white proteins with pulsed electric fields and thermal processes. J. Sci. Food Agric. 2016, 96, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Zhang, J.W. Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J. Agric. Food Chem. 2002, 50, 2679–2683. [Google Scholar] [CrossRef]
- Shin, M.; Han, Y.; Ahn, K. The influence of the time and temperature of heat treatment on the allergenicity of egg white proteins. Allergy, Asthma Immunol. Res. 2013, 5, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Van der Plancken, I.; Van Remoortere, M.; Indrawati; Van Loey, A.; Hendrickx, M.E. Heat-induced changes in the susceptibility of egg white proteins to enzymatic hydrolysis: A kinetic study. J. Agric. Food Chem. 2003, 51, 3819–3823. [Google Scholar] [CrossRef]
- Wang, J.; Liao, W.; Nimalaratne, C.; Chakrabarti, S.; Wu, J. Purification and characterization of antioxidant peptides from cooked eggs using a dynamic in vitro gastrointestinal model in vascular smooth muscle A7r5 cells. npj Sci. Food 2018, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Antioxidant Peptides Identified from Ovotransferrin by the ORAC Method Did Not Show Anti-Inflammatory and Antioxidant Activities in Endothelial Cells. J. Agric. Food Chem. 2016, 64, 113–119. [Google Scholar] [CrossRef]
- Pohanka, M. Analytical Tools for the Determination of Antioxidants and Antioxidant Capacity in Biological Samples, Principles and Applications. Curr. Org. Chem. 2017, 21. [Google Scholar] [CrossRef]
- Li, J.-M.; Fan, L.M.; Christie, M.R.; Shah, A.M. Acute Tumor Necrosis Factor Alpha Signaling via NADPH Oxidase in Microvascular Endothelial Cells: Role of p47phox Phosphorylation and Binding to TRAF4. Mol. Cell. Biol. 2005, 25, 2320–2330. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Sun, J.; Liu, Y.; Zeng, H.; Su, Y.; Yang, Y. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem. 2012, 135, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Vassallo, D.V.; Wiggers, G.A. bioactive peptides and hydrolysates from egg proteins as a new tool for protection against cardiovascular problems. Curr. Pharm. Des. 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and Activity Study of Egg Protein Ovotransferrin derived Peptides (IRW and IQW) on Endothelial Inflammatory Response and Oxidative Stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, Y.L.; Chen, J.; Zhou, L. Synergistic inhibition of lipid oxidation by pea protein hydrolysate coupled with licorice extract in a liposomal model system. J. Agric. Food Chem. 2013, 61, 8452–8461. [Google Scholar] [CrossRef]
- Dávalos, A.; Miguel, M.; Bartolomé, B.; López-Fandiño, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Aluko, R.E. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Ltd.: Cambridge, MA, USA, 2015; pp. 105–140. ISBN 9781782420972. [Google Scholar]
- Nyemb, K.; Jardin, J.; Causeur, D.; Guérin-Dubiard, C.; Dupont, D.; Rutherfurd, S.M.; Nau, F. Investigating the impact of ovalbumin aggregate morphology on in vitro ovalbumin digestion using label-free quantitative peptidomics and multivariate data analysis. Food Res. Int. 2014, 63, 192–202. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, P.S.; Nolasco, E.; Handa, A.; Naldrett, M.J.; Alvarez, S.; Majumder, K. Effect of pH and Heat Treatment on the Antioxidant Activity of Egg White Protein-Derived Peptides after Simulated In-Vitro Gastrointestinal Digestion. Antioxidants 2020, 9, 1114. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111114
Rao PS, Nolasco E, Handa A, Naldrett MJ, Alvarez S, Majumder K. Effect of pH and Heat Treatment on the Antioxidant Activity of Egg White Protein-Derived Peptides after Simulated In-Vitro Gastrointestinal Digestion. Antioxidants. 2020; 9(11):1114. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111114
Chicago/Turabian StyleRao, Priyanka Singh, Emerson Nolasco, Akihiro Handa, Michael J. Naldrett, Sophie Alvarez, and Kaustav Majumder. 2020. "Effect of pH and Heat Treatment on the Antioxidant Activity of Egg White Protein-Derived Peptides after Simulated In-Vitro Gastrointestinal Digestion" Antioxidants 9, no. 11: 1114. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111114