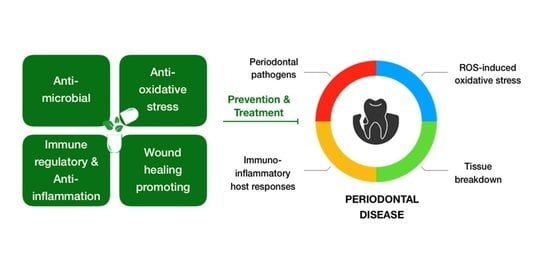
The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights
Abstract
:1. Introduction
2. Immuno-Inflammatory Pathogenesis of Periodontal Disease
3. The Role of Oxidative Stress in the Immuno-Inflammatory Paradigm of Periodontal Disease
3.1. Oxidants in Periodontal Tissues
3.2. Innate Antioxidant Defense Systems in Periodontal Tissues
3.3. Oxidative Stress and Its Involvement in the Development of Periodontal Disease via the Immuno-Inflammatory Pathway
4. Antioxidant Phytochemicals and Periodontal Disease
4.1. Overview of the Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease
4.2. Classification of Antioxidant Phytochemicals
4.3. Pharmacological Activities of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways
4.4. Challenges and Future Prospects
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kumar, S. Evidence-based update on diagnosis and management of gingivitis and periodontitis. Dent. Clin. N. Am. 2019, 63, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.G.; Petersen, P.E. Periodontal health through public health--the case for oral health promotion. Periodontology 2000 2012, 60, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Arigbede, A.O.; Babatope, B.O.; Bamidele, M.K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Falcao, A.; Bullón, P. A review of the influence of periodontal treatment in systemic diseases. Periodontology 2000 2019, 79, 117–128. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK554590/ (accessed on 27 August 2020).
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. Periodontal disease: The portrait of an epidemic. J. Public Health Emerg. 2020, 4, 10. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Vincent, R.R.; Appukuttan, D.; Prakash, P.S.G.; Victor, D.J. Oxidative stress: Role in pathogenesis of periodontal disease. Int. J. Pharm. Biol. Sci. 2017, 8, 1033–1041. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative stress and antioxidant system in periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [Green Version]
- Gumus, P.; Huseyinalemdaroglu, B.; Buduneli, N. The role of oxidative stress in the interaction of periodontal disease with systemic diseases or conditions. Oxid. Antioxid. Med. Sci. 2016, 5, 33–38. [Google Scholar] [CrossRef]
- World Health Organization. The world medicines situation 2011. In Traditional Medicines: Global Situation, Issues and Challenges; WHO: Geneva, Switzerland, 2011; pp. 1–14. [Google Scholar]
- Paur, I.; Carlsen, M.H.; Halvorsen, B.L.; Blomhoff, R. Antioxidants in Herbs and Spices: Roles in Oxidative Stress and Redox Signaling. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK92763/ (accessed on 15 September 2020).
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- AlJehani, Y.A. Risk factors of periodontal disease: Review of the literature. Int. J. Dent. 2014, 2014, 182513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, A.M.; Alencar, J.B.; Visentainer, J.E.L.; Silva, C.O. Immunopathogenesis of Chronic Periodontitis. IntechOpen 2017. Available online: https://www.intechopen.com/books/periodontitis-a-useful-reference/immunopathogenesis-of-chronic-periodontitis (accessed on 17 September 2020).
- Huang, N.; Gibson, F.C. Immuno-pathogenesis of periodontal disease: Current and emerging aradigms. Curr. Oral Health Rep. 2014, 1, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Jornvall, H.; Furchgott, R.F. Endothelium-derived relaxing factor: Discovery, early studies, and identification as nitric oxide. In Nobel Lectures in Physiology or Medicine 1996–2000; Jornvall, H., Ed.; World Scientific Publishing Company: Singapore, 2003; pp. 152–169. [Google Scholar]
- Martina, A.; Plevkova, J.; Strapkova, A.; Buday, T. Nitric oxide—Important messenger in human body. Open J. Mol. Integr. Physiol. 2012, 2. [Google Scholar] [CrossRef]
- Anggård, E. Nitric oxide: Mediator, murderer, and medicine. Lancet 1994, 343, 1199–1206. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Villanueva, C.; Giulivi, C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic. Biol. Med. 2010, 49, 307–316. [Google Scholar] [CrossRef] [Green Version]
- McNeill, E.; Crabtree, M.J.; Sahgal, N.; Patel, J.; Chuaiphichai, S.; Iqbal, A.J.; Hale, A.B.; Greaves, D.R.; Channon, K.M. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 2015, 79, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Druhan, L.J.; Zweier, J.L. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch. Biochem. Biophys. 2010, 494, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Rudrakshi, C.; Prabhuji, M.L.V.; Parween, S.; Jyothsna, S. Relationship between antioxidants and the development of the periodontal disease. J. Cytol. Tissue Biol. 2017, 4, 16. [Google Scholar]
- Halliwell, B. Antioxidants: The basics—What they are and how to evaluate them. Adv. Pharmacol. 1997, 38, 3–20. [Google Scholar]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Patlevič, P.; Vašková, J.; Švorc, P., Jr.; Vaško, L.; Švorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants, Free Radical Medicine and Biology. IntechOpen 2019. Available online: https://www.intechopen.com/books/free-radical-medicine-and-biology/nonenzymatic-exogenous-and-endogenous-antioxidants (accessed on 23 September 2020).
- Lima, D.P.; Diniz, D.G.; Moimaz, S.A.; Sumida, D.H.; Okamoto, A.C. Saliva: Reflection of the body. Int. J. Infect. Dis. 2010, 14, e184–e188. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, P.D.V.; Grégio, A.M.; Machado, M.A.; de Lima, A.A.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlorke, D.; Flemmig, J.; Gau, J.; Furtmüller, P.G.; Obinger, C.; Arnhold, J. New insights into thiocyanate oxidation by human myeloperoxidase. J. Inorg. Biochem. 2016, 162, 117–126. [Google Scholar] [CrossRef]
- Carlsson, J. Salivary peroxidase: An important part of our defense against oxygen toxicity. J. Oral Pathol. 1987, 16, 412–416. [Google Scholar] [CrossRef]
- Chandler, J.D.; Day, B.J. Thiocyanate: A potentially useful therapeutic agent with host defense and antioxidant properties. Biochem. Pharmacol. 2012, 84, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- San Gabriel, P.T.; Liu, Y.; Schroder, A.L.; Zoellner, H.; Chami, B. The role of thiocyanate in modulating myeloperoxidase activity during disease. Int. J. Mol. Sci. 2020, 21, 6450. [Google Scholar] [CrossRef]
- Ashby, M.T. Hypothiocyanite. Adv. Inorg. Chem. 2012, 64, 263–303. [Google Scholar]
- Minic, I. Antioxidant role of saliva. J. Otolaryngol. Res. 2019, 2, 124. [Google Scholar]
- Tóthová, L.; Celec, P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef] [Green Version]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012, 17, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Ito, H.; Asman, B.; Bergström, K. Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J. Clin. Periodontol. 2006, 33, 126–129. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2007, 147, 255–264. [Google Scholar] [CrossRef]
- Ling, M.R.; Chapple, I.L.; Matthews, J.B. Neutrophil superoxide release and plasma C-reactive protein levels pre- and post-periodontal therapy. J. Clin. Periodontol. 2016, 43, 652–658. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Su, H.; Gornitsky, M.; Velly, A.M.; Yu, H.; Benarroch, M.; Schipper, H.M. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic. Biol. Med. 2009, 46, 914–921. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Ramchandraprasad, M.V.; Bajaj, P.; Rao, N.S.; Agarwal, E. Protein carbonyl: An oxidative stress marker in gingival crevicular fluid in healthy, gingivitis, and chronic periodontitis subjects. Contemp. Clin. Dent. 2013, 4, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Baltacioğlu, E.; Akalin, F.A.; Alver, A.; Değer, O.; Karabulut, E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch. Oral Biol. 2008, 53, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Ekuni, D.; Tomofuji, T.; Tamaki, N.; Sanbe, T.; Azuma, T.; Yamanaka, R.; Yamamoto, T.; Watanabe, T. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch. Oral Biol. 2008, 53, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Petrone, W.F.; English, D.K.; Wong, K.; McCord, J.M. Free radicals and inflammation: Superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc. Natl. Acad. Sci. USA 1980, 77, 1159–1163. [Google Scholar] [CrossRef] [Green Version]
- Grill, H.P.; Zweier, J.L.; Kuppusamy, P.; Weisfeldt, M.L.; Flaherty, J.T. Direct measurement of myocardial free radical generation in an in vivo model: Effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. J. Am. Coll. Cardiol. 1992, 20, 1604–1611. [Google Scholar] [CrossRef] [Green Version]
- Laine, M.L.; Crielaard, W.; Loos, B.G. Genetic susceptibility to periodontitis. Periodontology 2000 2012, 58, 37–68. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Andric, M.; Miletic, M.; Beljic-Ivanovic, K.; Knezevic, A.; Mojsilovic, S.; Milasin, J. Epstein-Barr virus infection induces bone resorption in apical periodontitis via increased production of reactive oxygen species. Med. Hypotheses 2016, 94, 40–42. [Google Scholar] [CrossRef]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front. Physiol. 2017, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Moseley, R.; Waddington, R.J.; Embery, G.; Rees, S.G. The modification of alveolar bone proteoglycans by reactive oxygen species in vitro. Connect Tissue Res. 1998, 37, 13–28. [Google Scholar] [CrossRef]
- Misaki, H.; Suzuki, M.; Yoshie, H.; Hara, K. The effect of superoxide dismutase on the inflammation induced by periodontal pathogenic bacteria and wound healing of gingival incisions. J. Jpn. Assoc. Periodontol. 1990, 32, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Petelin, M.; Pavlica, Z.; Ivanusa, T.; Sentjurc, M.; Skaleric, U. Local delivery of liposome-encapsulated superoxide dismutase and catalase suppress periodontal inflammation in beagles. J. Clin. Periodontol. 2000, 27, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.; Rodríguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Rev. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, D.P.; Gotto, A.M., Jr. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [Green Version]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [Green Version]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Fearon, I.M.; Phillips, G.; Carr, T.; Taylor, M.; Breheny, D.; Faux, S.P. The role of oxidative stress in smoking-related diseases. Mini Rev. Org. Chem. 2011, 8, 360–371. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care (New Rochelle) 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Stanisic, D.; Obradovic, R.; Vujovic, S.; Jovanovic, M.; Zivkovic, V. The connection of periodontal disease and diabetes mellitus: The role of matrix metalloproteinases and oxidative stress. Serbian J. Exp. Clin. Res. 2019. [Google Scholar] [CrossRef]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [Green Version]
- Cook-Mills, J.M. Hydrogen peroxide activation of endothelial cell-associated MMPs during VCAM-1-dependent leukocyte migration. Cell. Mol. Biol. (Noisy-le-Grand) 2006, 52, 8–16. [Google Scholar]
- Osorio, C.; Cavalla, F.; Paula-Lima, A.; Díaz-Araya, G.; Vernal, R.; Ahumada, P.; Gamonal, J.; Hernández, M. H2O2 activates matrix metalloproteinases through the nuclear factor kappa B pathway and Ca(2+) signals in human periodontal fibroblasts. J. Periodontal. Res. 2015, 50, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ríos, P.; Pussinen, P.J.; Vernal, R.; Hernández, M. Oxidative stress in the local and systemic events of apical periodontitis. Front. Physiol. 2017, 8, 869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desarda, H.; Gaikwad, S. Matrix metalloproteinases & Implication in periodontitis-A short review. J. Dent. Allied Sci. 2013, 2, 66. [Google Scholar]
- Sam, C.H.; Lu, H.K. The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. J. Dent. Sci. 2009, 4, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.A.; Rossa, C.J.; Garlet, G.P.; Nogueira, A.V.; Cirelli, J.A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J. Appl. Oral. Sci. 2012, 20, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.H.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-kappa B and JNK. Cell Death Differ. 2006, 13, 730–737. [Google Scholar] [CrossRef]
- Kang, S.W.; Park, H.J.; Ban, J.Y.; Chung, J.H.; Chun, G.S.; Cho, J.O. Effects of nicotine on apoptosis in human gingival fibroblasts. Arch. Oral Biol. 2011, 56, 1091–1097. [Google Scholar] [CrossRef]
- Oben, K.Z.; Alhakeem, S.S.; McKenna, M.K.; Brandon, J.A.; Mani, R.; Noothi, S.K.; Jinpeng, L.; Akunuru, S.; Dhar, S.K.; Singh, I.P.; et al. Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget 2017, 8, 77436–77452. [Google Scholar] [CrossRef] [Green Version]
- Morse, D.; Pischke, S.E.; Zhou, Z.; Davis, R.J.; Flavell, R.A.; Loop, T.; Otterbein, S.L.; Otterbein, L.E.; Choi, A.M. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J. Biol. Chem. 2003, 278, 36993–36998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melino, M.; Hii, C.S.; McColl, S.R.; Ferrante, A. The effect of the JNK inhibitor, JIP peptide, on human T lymphocyte proliferation and cytokine production. J. Immunol. 2008, 181, 7300–7306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, P.H.; Yang, M.X.; Wang, N.N.; Jin, L.J.; Dong, Y.; Cai, X.; Chen, L.L. Porphyromonas gingivalis-induced NLRP3 inflammasome activation and its downstream interleukin-1β release depend on caspase-4. Front. Microbiol. 2020, 11, 1881. [Google Scholar] [CrossRef]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2020, 82, 93–114. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kurita-Ochiai, T.; Kobayashi, R.; Suzuki, T.; Ando, T. Regulation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated periodontal disease. Inflamm. Res. 2017, 66, 59–65. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Lian, D.; Dai, L.; Xie, Z.; Zhou, X.; Liu, X.; Zhang, Y.; Huang, Y.; Chen, Y. Periodontal ligament fibroblasts migration injury via ROS/TXNIP/Nlrp3 inflammasome pathway with Porphyromonas gingivalis lipopolysaccharide. Mol. Immunol. 2018, 103, 209–219. [Google Scholar] [CrossRef]
- Chiu, A.V.; Saigh, M.A.; McCulloch, C.A.; Glogauer, M. The role of NrF2 in the regulation of periodontal health and disease. J. Dent. Res. 2017, 96, 975–983. [Google Scholar] [CrossRef]
- Hale, A.N.; Ledbetter, D.J.; Gawriluk, T.R.; Rucker, E.B., 3rd. Autophagy: Regulation and role in development. Autophagy 2013, 9, 951–972. [Google Scholar] [CrossRef]
- Greabu, M.; Giampieri, F.; Imre, M.M.; Mohora, M.; Totan, A.; Pituru, S.M.; Ionescu, E. Autophagy, one of the main steps in periodontitis pathogenesis and evolution. Molecules 2020, 25, 4338. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Gershovich, E. The prevention of periodontal disease-An overview. Periodontology 2000 2020, 84, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; You, H.J.; Lian, H.J.; Huang, C.H. Patients receiving comprehensive periodontal treatment have better clinical outcomes than patients receiving conventional periodontal treatment. J. Formos. Med. Assoc. 2016, 115, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.; Orabueze, I.; Okorie, N. Antioxidants properties of natural and synthetic chemical compounds: Therapeutic effects on biological system. Acta Sci. Pharm. Sci. 2019, 3, 28–42. [Google Scholar] [CrossRef]
- Varela-López, A.; Bullón, P.; Giampieri, F.; Quiles, J.L. Non-nutrient, naturally occurring phenolic compounds with antioxidant activity for the prevention and treatment of periodontal diseases. Antioxidants 2015, 4, 447–481. [Google Scholar] [CrossRef]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Blaggana, V.; Upadhyay, P.; Jindal, M.; Gupta, S.; Nishat, S. Antioxidant therapy (lycopene and green tea extract) in periodontal disease: A promising paradigm. J. Indian Soc. Periodontol. 2019, 23, 25–30. [Google Scholar]
- Sharopov, F.; Braun, M.S.; Gulmurodov, I.; Khalifaev, D.; Isupov, S.; Wink, M. Antimicrobial, antioxidant, and anti-Inflammatory activities of essential oils of selected aromatic plants from Tajikistan. Foods 2015, 4, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, A.; Varghese, S.S.; Doraiswamy, J.N.; Malaiappan, S. Herbs as an antioxidant arsenal for periodontal diseases. J. Intercult. Ethnopharmacol. 2016, 5, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.; Bahna, S.L. Hypersensitivity reactions to food additives. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, B.Y.; Kang, H.G.; Ku, H.O.; Cho, J.H. Effects of butylated hydroxyanisole on the development and functions of reproductive system in rats. Toxicology 2005, 208, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Botterweck, A.; Verhagen, H.; Goldbohm, R.; Kleinjans, J.; Brandt, P.V.D.; Brandt, P.V.D. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Koche, D.; Shirsat, R.; Kawale, M. An overerview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 9, 1–11. [Google Scholar]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant. Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Delgado-Adámez, J. Agrifood By-products as a Source of Phytochemical Compounds. IntechOpen 2018. Available online: https://www.intechopen.com/books/descriptive-food-science/agrifood-by-products-as-a-source-of-phytochemical-compounds (accessed on 5 November 2018).
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Palozza, P. Prooxidant actions of carotenoids in biologic systems. Nutr. Rev. 1998, 56, 257–265. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst.) 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.R.S.; Muthukumar, N.M.S.A.; Selvakumar, P.M. Phytochemicals as a potential source for anti-microbial, anti-oxidant and wound healing-a review. MOJ Biorg. Org. Chem. 2018, 2, 61–70. [Google Scholar]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [Green Version]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Batchu, S.N.; Chaudhary, K.R.; Wiebe, G.J.; Seubert, J.M. Chapter 28—Bioactive Compounds in Heart Disease. In Bioactive Food as Dietary Interventions for Cardiovascular Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 431–442. [Google Scholar]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Chan, J.Y.; Yuen, A.C.; Chan, R.Y.; Chan, S.W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Provinciali, M.; Pierpaoli, E.; Piacenza, F.; Giacconi, R.; Costarelli, L.; Basso, A.; Recchioni, R.; Marcheselli, F.; Bray, D.; Benlhassan, K.; et al. Chapter 22–nutritional modulators of cellular senescence in vitro. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 293–312. [Google Scholar]
- Chung, H.S.; Shin, J.C. Characterization of antioxidant alkaloids and phenolic acids from anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem. 2007, 104, 1670–1677. [Google Scholar] [CrossRef]
- Dalimunthe, A.; Hasibuan, P.A.; Silalahi, J.; Sinaga, S.; Satria, D. Antioxidant activity of alkaloid compounds from Litsea Cubeba Lour. Orient J. Chem. 2018, 34, 1149–1152. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Lee, K.E.; Bajpai, V.K.; Gajurel, P.R.; Gu, K.S.; Kumar, P. Ethnopharmacological properties and medicinal uses of Litsea cubeba. Plants 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Lagha, A.; Dudonné, S.; Desjardins, Y.; Grenier, D. Wild blueberry (Vaccinium angustifolium Ait.) polyphenols target Fusobacterium nucleatum and the host inflammatory response: Potential innovative molecules for treating periodontal diseases. J. Agric. Food Chem. 2015, 63, 6999–7008. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, M.; Urolagin, S.S.; Pentyala, K.B.; Urolagin, S.B.; Menaka, K.B.; Bhoi, S. Novel therapeutic approach for the treatment of periodontitis by curcumin. J. Clin. Diagn. Res. 2014, 8, ZC65–ZC69. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Nugala, B.; Namasi, A.; Emmadi, P.; Krishna, P.M. Role of green tea as an antioxidant in periodontal disease: The Asian paradox. J. Indian Soc. Periodontol. 2012, 16, 313–316. [Google Scholar] [CrossRef]
- Rizzo, A.; Bevilacqua, N.; Guida, L.; Annunziata, M.; Carratelli, C.R.; Paolillo, R. Effect of resveratrol and modulation of cytokine production on human periodontal ligament cells. Cytokine 2012, 60, 197–204. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Kawasaki-Cárdenas, P.; Arroyo-Cruz, S.R.; Maldonado-Frías, S. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. Eur. J. Pharmacol. 2006, 541, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hadley, C.W.; Clinton, S.K.; Schwartz, S.J. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J. Nutr. 2003, 133, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, K.; Kaufmann, K.; Bitsch, R.; Böhm, V. Effects of ingestion of tomatoes, tomato juice and tomato purée on contents of lycopene isomers, tocopherols and ascorbic acid in human plasma as well as on lycopene isomer pattern. Br. J. Nutr. 2006, 95, 734–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hininger, I.A.; Meyer-Wenger, A.; Moser, U.; Wright, A.; Southon, S.; Thurnham, D.; Chopra, M.; Van Den Berg, H.; Olmedilla, B.; Favier, A.E.; et al. No significant effects of lutein, lycopene or beta-carotene supplementation on biological markers of oxidative stress and LDL oxidizability in healthy adult subjects. J. Am. Coll. Nutr. 2001, 20, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hrishi, T.S.; Kundapur, P.P.; Naha, A.; Thomas, B.S.; Kamath, S.; Bhat, G.S. Effect of adjunctive use of green tea dentifrice in periodontitis patients—A randomized controlled pilot study. Int. J. Dent. Hyg. 2016, 14, 178–183. [Google Scholar] [CrossRef]
- Roodgaryan, R.; Jenabian, N.; Moghadamnia, A.A.; Pouramir, M.; Khadir, F. Clinical and biochemical effects of dark chocolate in moderate chronic periodontitis. Caspian J. Dent. Res. 2015, 4, 43–49. [Google Scholar]
- Kajiura, Y.; Nishikawa, Y.; Lew, J.H.; Kido, J.I.; Nagata, T.; Naruishi, K. β-carotene suppresses Porphyromonas gingivalis lipopolysaccharide-mediated cytokine production in THP-1 monocytes cultured with high glucose condition. Cell Biol. Int. 2018, 42, 105–111. [Google Scholar] [CrossRef]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher intakes of fruits and vegetables, β-carotene, vitamin C, α-tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Lombardo Bedran, T.B.; Palomari Spolidorio, D.; Grenier, D. Green tea polyphenol epigallocatechin-3-gallate and cranberry proanthocyanidins act in synergy with cathelicidin (LL-37) to reduce the LPS-induced inflammatory response in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. Arch. Oral Biol. 2015, 60, 845–853. [Google Scholar] [CrossRef]
- Paterniti, I.; Briguglio, E.; Mazzon, E.; Galuppo, M.; Oteri, G.; Cordasco, G.; Cuzzocrea, S. Effects of Hypericum Perforatum, in a rodent model of periodontitis. BMC Complement. Altern. Med. 2010, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Chen, Z.; Liu, H.; Xuan, Y.; Wang, X.; Luan, Q. Green tea epigallocatechin-3-gallate alleviates Porphyromonas gingivalis-induced periodontitis in mice. Int. Immunopharmacol. 2015, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, M.; Yamamoto, T.; Ichioka, H.; Honjo, K.; Yamamoto, K.; Oseko, F.; Kita, M.; Mazda, O.; Kanamura, N. β-cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch. Oral Biol. 2013, 58, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Santos, J.; La, V.D.; Howell, A.B.; Grenier, D. A-type cranberry proanthocyanidins inhibit the RANKL-dependent differentiation and function of human osteoclasts. Molecules 2011, 16, 2365–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balci Yuce, H.; Lektemur Alpan, A.; Gevrek, F.; Toker, H. Investigation of the effect of astaxanthin on alveolar bone loss in experimental periodontitis. J. Periodontal. Res. 2018, 53, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, C.; Tian, B.; Zhou, X.; Ma, N.; Qian, Y. Myricetin prevents alveolar bone loss in an experimental ovariectomized mouse model of periodontitis. Int. J. Mol. Sci. 2016, 17, 422. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Ding, Y.; Bao, C.; Li, W. Mangiferin ameliorates Porphyromonas gingivalis-induced experimental periodontitis by inhibiting phosphorylation of nuclear factor-κB and Janus kinase 1-signal transducer and activator of transcription signaling pathways. J. Periodontal. Res. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.Z.; Casarin, R.C.; Cirano, F.R.; Tenenbaum, H.T.; Casati, M.Z. Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal. Res. 2017, 52, 201–209. [Google Scholar] [CrossRef]
- Chapple, I.L.; Milward, M.R.; Ling-Mountford, N.; Weston, P.; Carter, K.; Askey, K.; Dallal, G.E.; De Spirt, S.; Sies, H.; Patel, D.; et al. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: A double-blind RCT. J. Clin. Periodontol. 2012, 39, 62–72. [Google Scholar] [CrossRef]
- Chava, V.K.; Vedula, B.D. Thermo-reversible green tea catechin gel for local application in chronic periodontitis: A 4-week clinical trial. J. Periodontol. 2013, 84, 1290–1296. [Google Scholar] [CrossRef]
- Grover, S.; Tewari, S.; Sharma, R.K.; Singh, G.; Yadav, A.; Naula, S.C. Effect of subgingivally delivered 10% emblica officinalis gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis—A randomized placebo-controlled clinical trial. Phytother. Res. 2016, 30, 956–962. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epriliati, I.; Ginjom, R. Bioavailability of Phytochemicals, Phytochemicals—A Global Perspective of Their Role in Nutrition and Health. IntechOpen 2012. Available online: https://www.intechopen.com/books/phytochemicals-a-global-perspective-of-their-role-in-nutrition-and-health/bioavailability-of-phytochemicals (accessed on 30 September 2020).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, T.T.T.; Chu, P.-M.; Tuan, V.P.; Te, J.S.-L.; Lee, I.-T. The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights. Antioxidants 2020, 9, 1211. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9121211
Vo TTT, Chu P-M, Tuan VP, Te JS-L, Lee I-T. The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights. Antioxidants. 2020; 9(12):1211. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9121211
Chicago/Turabian StyleVo, Thi Thuy Tien, Pei-Ming Chu, Vo Phuoc Tuan, Joyce Si-Liang Te, and I-Ta Lee. 2020. "The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights" Antioxidants 9, no. 12: 1211. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9121211