Thermal Analysis of Photoelectron Emission (PE) and X-ray Photoelectron Spectroscopy (XPS) Data for Iron Surfaces Scratched in Air, Water, and Liquid Organics
Abstract
:Featured Application
Abstract
1. Introduction
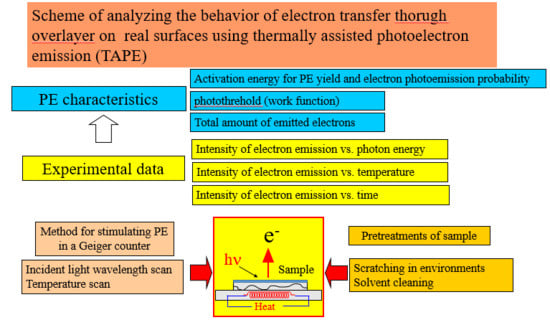
2. Materials and Methods
2.1. Materials
2.2. Thermally Assisted PE
2.3. XPS(X-ray Photoelectron Spectroscopy)
3. Results and Discussion
3.1. XPS
3.1.1. Dependence of XPS Spectra on Scratching Environments and Temperature
3.1.2. Plots of XPS Intensity of Core Spectra Against Scratching Environments
3.1.3. Curve Resolution of O 1s Spectrum and Temperature Dependence of XPS O 1s Component Intensity on Scratching Environments
3.2. Activation Energies Obtained by Arrhenius Plots of the XPS Characetristics
3.3. The PE Characteristics during Temperature Scan Against Scratching Environments
3.4. The PE Characteristics during Wavelength (Photon Energy) Scan Against Scratching Environments
3.5. PE Quantum Yields at Different Photon Energies during Wavelength Scan for Scratching Environments
3.6. Effects of FexO Layer and Hydroxyl Groups at the Surface Overlayer on PE
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ciniero, A.; Le Rouzic, J.; Baikie, I.; Reddyhoff, T. The origins of triboemission—Correlating wear damage with electron emission. Wear 2017, 374–375, 113–119. [Google Scholar] [CrossRef]
- Kraljic, M.; Mandic, Z.; Duic, L. Inhibition of steel corrosion by polyaniline coatings. Corros. Sci. 2003, 45, 181–198. [Google Scholar] [CrossRef]
- Spicer, W.E. Surface analysis by means of photoemission and other photon-stimulated processes. In Chemistry and Physics of Solid Surfaces; Vanselow, R., Tong, S.Y., Eds.; CRC Press: Boca Raton, FL, USA, 1977; pp. 235–254. [Google Scholar]
- Spicer, W.E.; Herrera-Gomez, A. Modern theory and applications of photocathodes. In Proceedings of the SPIE’s 1993 International Symposium on Optics, Imaging, and Instrumentation, San Diego, CA, USA, 11–16 July 1993; Volume 2022, pp. 18–35. [Google Scholar]
- Momose, Y.; Suzuki, D.; Tsuruya, K.; Sakurai, T.; Nakayama, K. Transfer of electrons on scratched iron surfaces: Photoelectron emission and X-ray photoelectron spectroscopy studies. Friction 2018, 6, 98–115. [Google Scholar] [CrossRef] [Green Version]
- Momose, Y.; Tsuruya, K.; Sakurai, T.; Nakayama, K. Photoelectron emission and XPS studies of real iron surfaces subjected to scratching in air, water, and organic liquids. Sur. Interface Anal. 2016, 48, 202–211. [Google Scholar] [CrossRef]
- Momose, Y.; Sakurai, T.; Nakayama, K. Photoelectron emission characteristics of iron surfaces scratched in different environments: Dependence on photon energy irradiation methods. Sur. Interface Anal. 2018, 50, 1319–1335. [Google Scholar] [CrossRef]
- Fowler, R.H. The analysis of photoelectric sensitivity curves for clean metals at various temperatures. Phys. Rev. 1931, 38, 45–56. [Google Scholar] [CrossRef]
- DuBridge, L.A. A further experimental test of Fowler’s theory of photoelectric emission. Phys. Rev. 1932, 39, 108–118. [Google Scholar] [CrossRef]
- DuBridge, L.A. New Theories of the Photoelectric Effect; Hermann and Cie: Paris, France, 1935. [Google Scholar]
- Ames, I.; Christensen, R.L. Anomalous photoelectric emission from nickel. IBM J. Res. Dev. 1963, 7, 34–39. [Google Scholar] [CrossRef]
- Conder, K. Electronic and Ionic Conductivity in Metal Oxides; Paul Scherrer Institute: Villigen, Switzerland, 2015; pp. 1–44. [Google Scholar]
- Momose, Y.; Suzuki, D.; Sakurai, T.; Nakayama, K. Influence of temperature and photon energy on quantum yield of photoemission from real iron surfaces. Appl. Phys. A 2014, 117, 1525–1534. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; McIntyre, N.S. Studies of the oxidation of iron by water vapour using X-ray photoelectron spectroscopy and QUASES. Surf. Sci. 2004, 572, 217–227. [Google Scholar] [CrossRef]
- Wagner, C.D.; Rigs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin = Elmer: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Graat, P.C.J.; Somers, M.A.J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe2p spectra. Appl. Surf. Sci. 1996, 100–101, 36–40. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Muhler, M.; Schlögl, R.; Ertl, G. The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene. J. Catal. 1992, 138, 413–444. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Ogawa, Y.; Ando, D.; Sutou, Y.; Koike, J. Effects of O2 and N2 flow rate on the electrical properties of Fe-O-N thin films. Mater. Trans. 2014, 55, 1606–1610. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Merchant, P.; Collins, R.; Kershaw, R.; Dwight, K.; Wold, A. The electrical, optical and photoconducting properties of Fe2−xCrxO3 (0 ≤ x ≤ 0.47). J. Solid State Chem. 1979, 27, 307–315. [Google Scholar] [CrossRef]
- Spicer, W.E. Negative affinity 3-5 photocathodes: Their physics and technology. Appl. Phys. 1977, 12, 115–130. [Google Scholar] [CrossRef]
- Zhuravlev, A.G.; Alperovich, V.L. Relaxational kinetics of photoemission and photon-enhanced thermionic emission from p-GaAs surface with nonequilibrium Cs overlayers. Appl. Surf. Sci. 2018, 461, 10–16. [Google Scholar] [CrossRef]




















| Environments | Peak I | Peak II* | Peak III | |||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Peak Intensity (103 counts/s) | Binding Energy (eV) | Peak Intensity (103 counts/s) | Binding Energy (eV) | Binding Energy (eV) | Peak Intensity (103 counts/s) | Binding Energy (eV) | |
| Air | 25 | 0.80 | 720.9 | 8.24 | 712.0 | 2.40 | 708.0 | |
| 200 | 0.98 | 720.0 | 11.99 | 712.0 | ||||
| 339 | 1.82 | 720.8 | 24.00 | 712.0 | ||||
| Benzene | 25 | 1.53 | 720.8 | 12.01 | 712.0 | 5.37 | 708.1 | |
| 200 | 1.08 | 720.5 | 12.99 | 711.9 | ||||
| 339 | 2.03 | 720.6 | 24.00 | 711.9 | ||||
| Cyclohexane | 25 | 1.18 | 720.5 | 12.01 | 711.9 | 4.90 | 708.0 | |
| 200 | 1.85 | 720.5 | 20.10 | 712.0 | ||||
| 339 | 1.66 | 720.1 | 22.19 | 711.8 | ||||
| Water | 25 | 2.01 | 721.0 | 18.08 | 712.9 | 1.48 | 708.0 | |
| 200 | 2.06 | 720.6 | 18.00 | 712.4 | ||||
| 339 | 2.45 | 720.8 | 24.02 | 712.5 | ||||
| Methanol | 25 | 2.49 | 721.6 | 18.09 | 712.3 | 7.46 | 708.6 | |
| 200 | 2.77 | 720.3 | 28.50 | 712.3 | ||||
| 339 | 3.34 | 720.5 | 25.90 | 713.1 | ||||
| Ethanol | 25 | 2.80 | 721.4 | 18.01 | 712.1 | 10.77 | 708.6 | |
| 200 | 1.67 | 720.9 | 26.02 | 712.4 | ||||
| 339 | 2.31 | 721.3 | 29.00 | 712.5 | ||||
| Acetone | 25 | 1.92 | 720.6 | 17.85 | 712.1 | 6.88 | 708.1 | |
| 200 | 1.67 | 720.8 | 22.18 | 712.1 | ||||
| 339 | 2.62 | 720.8 | 27.92 | 712.4 | ||||
| Average | 720.7 | 712.3 | 708.1 | |||||
| * Peak II at 25 °C is assigned to FeOOH, and Peak II at 200 and 339 °C is assigned to Fe3O4. | ||||||||
| Environments | Peak 1 | Peak 2 | |||
|---|---|---|---|---|---|
| Temperature (°C) | Peak Intensity (103 counts/s) | Binding Energy (eV) | Peak Intensity (103 counts/s) | Binding Energy (eV) | |
| Air | 25 | 2.18 | 55.1 | 1.50 | 51.7 |
| 200 | 3.01 | 54.9 | |||
| 339 | 4.80 | 54.8 | |||
| Benzene | 25 | 2.63 | 53.9 | 2.44 | 52.2 |
| 200 | 3.60 | 54.6 | |||
| 339 | 3.60 | 54.9 | |||
| Cyclohexane | 25 | 3.00 | 54.5 | 2.63 | 52.3 |
| 200 | 3.83 | 54.8 | |||
| 339 | 5.00 | 54.8 | |||
| Water | 25 | 4.00 | 55.2 | 1.55 | 52.1 |
| 200 | 3.83 | 55.2 | |||
| 339 | 5.00 | 55.1 | |||
| Methanol | 25 | 4.25 | 54.7 | 3.63 | 52.6 |
| 200 | 5.82 | 55.0 | |||
| 339 | 5.40 | 55.0 | |||
| Ethanol | 25 | 4.25 | 54.4 | 4.42 | 52.5 |
| 200 | 5.40 | 54.9 | |||
| 339 | 6.21 | 55.1 | |||
| Acetone | 25 | 4.19 | 54.8 | 3.34 | 52.5 |
| 200 | 4.80 | 54.9 | |||
| 339 | 6.01 | 55.1 | |||
| Average | 54.8 | 52.5 | |||
| Environments | Temperature | Binding Energy of the Components (eV) | ||
|---|---|---|---|---|
| (°C) | O2- | OH− and OHδ− | H2O | |
| Air | 25 | 530.6 | 532.3 | 533.4 |
| 200 | 530.7 | 532.5 | 533.9 | |
| 339 | 530.6 | 531.9 | 533.8 | |
| Benzene | 25 | 530.5 | 532.0 | 533.2 |
| 200 | 530.6 | 531.8 | 533.0 | |
| 339 | 530.6 | 531.8 | 533.1 | |
| Cyclohexane | 25 | 530.5 | 532.3 | 534.2 |
| 200 | 530.5 | 531.7 | 533.2 | |
| 339 | 530.7 | 532.0 | 533.4 | |
| Water | 25 | 530.6 | 531.9 | 533.4 |
| 200 | 530.7 | 532.2 | 533.6 | |
| 339 | 530.9 | 531.9 | 533.5 | |
| Methanol | 25 | 530.8 | 532.2 | 533.5 |
| 200 | 530.7 | 531.9 | 533.3 | |
| 339 | 531.1 | 532.2 | 533.6 | |
| Ethanol | 25 | 530.7 | 532.2 | 533.8 |
| 200 | 530.8 | 532.2 | 533.3 | |
| 339 | 530.9 | 532.1 | 533.3 | |
| Acetone | 25 | 530.7 | 532.3 | 533.5 |
| 200 | 530.7 | 532.0 | 533.3 | |
| 339 | 530.7 | 531.6 | 533.3 | |
| Average | 530.7 | 532.1 | 533.5 | |
| Scratching Environment | Activation Energies for the Fe 2p, Fe 3p, O 1s, and C 1s Intensities (unit: counts) | Activation Energies for Intensity of O2-, OH-, and H2O Components in the O1s Spectra (unit: counts) | Activation Energies for the Ratio of O2- Intensity/Fe 2p Intensity | Activation Energies for the Ratio of O2- Intensity/Fe 3p Intensity | Activation Energies for the Maximum Peak Intensity in the Fe 2p, Fe 3p, and O 1s Spectra (unit: counts·s−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔEFe 2p (eV) | ΔEFe 3p (eV) | ΔEO 1s (eV) | ΔEC 1s (eV) | ΔEO2- (eV) | ΔEOH- (eV) | ΔEH2O (eV) | ΔEO2-/Fe 2p (eV) | ΔEO2-/Fe 3p (eV) | ΔEFe 2pPEAK (eV) | ΔEFe 3pPEAK (eV) | ΔEO 1sPEAK (eV) | |
| Air | 0.028 | 0.031 | 0.049 | 0.072 | 0.034 | 0.044 | 0.040 | 0.048 | 0.036 | 0.053 | ||
| Benzene | 0.011 | 0.012 | 0.026 | 0.027 | 0.029 | 0.016 | 0.016 | 0.017 | 0.026 | |||
| Cyclohexane | 0.013 | 0.019 | 0.033 | 0.057 | 0.032 | 0.044 | 0.038 | 0.032 | 0.024 | 0.036 | ||
| Water | 0.023 | |||||||||||
| Methanol | 0.027 | 0.007 | 0.046 | 0.031 | 0.036 | 0.035 | 0.031 | |||||
| Ethanol | 0.011 | 0.029 | 0.009 | 0.052 | 0.005 | 0.047 | 0.041 | 0.024 | 0.019 | 0.029 | ||
| Acetone | 0.026 | 0.021 | 0.032 | 0.032 | 0.015 | 0.021 | 0.021 | 0.021 | 0.016 | 0.027 | ||
| Average | 0.017 | 0.018 | 0.032 | 0.012 | 0.048 | 0.025 | 0.016 | 0.035 | 0.031 | 0.031 | 0.022 | 0.034 |
| a) The values were obtained from the slope of Arrhenius plots of approximately straight lines with R2 ≥ 0.82. | ||||||||||||
| b) The XPS data were as follows: (1) the XPS intensity of Fe 2p, Fe 3p, O 1s, and C 1s; (2) XPS intensity of the O2-, OH-, and H2O components of the O 1s spectrum; (3) the ratio of O2- intensity/Fe 2p intensity; (4) the ratio of O2- intensity/Fe 3p intensity; (5) the maximum peak intensity in the Fe 2p, Fe 3p, and O 1s spectra. | ||||||||||||
| Scratching Environment | Acceptor Number a) | Activation Energies for the O 1s Intensity in the XPS Measurement | Activation Energies for Intensity of O2- Component in the O 1s Spectra (unit: counts) | Activation Energies for the Peak Intensity in the O 1s Spectra (unit: counts·s−1) | Activation Energies for the PE Quantum Yields in the Up1 Scan | Activation Energies for the PE Quantum Yields in the Up2 Scan | Activation Energies for the Electron Photoemission Probability, αA, in the Wavelength Scan | Activation Energies for the Total Count of Emitted Electrons, NT, in the Wavelength Scan | Activation Energies for Photothresholds, ϕ |
|---|---|---|---|---|---|---|---|---|---|
| ΔEO 1s b) (eV) | ΔEO2-b) (eV) | ΔEO 1sPEAKb) (eV) | ΔEaUp1 c) (eV) | ΔEaUp2 c) (eV) | ΔEaA d) (eV) | ΔENT d) (eV) | ΔEϕ e) (eV) | ||
| Air | 0.049 | 0.072 | 0.053 | 0.212 | 0.028 | 0.074 | 0.054 | 0.002 | |
| Water | 54.8 | 0.145 | 0.038 | 0.084 | 0.064 | 0.002 | |||
| Methanol | 41.3 | 0.027 | 0.046 | 0.031 | 0.159 | 0.020 | 0.113 | 0.088 | 0.003 |
| Ethanol | 37.1 | 0.029 | 0.052 | 0.029 | 0.079 | 0.026 | 0.081 | 0.053 | 0.003 |
| Acetone | 12.5 | 0.026 | 0.032 | 0.027 | 0.035 | 0.021 | 0.105 | 0.069 | 0.002 |
| Benzene | 8.2 | 0.026 | 0.027 | 0.026 | 0.039 | 0.035 | 0.092 | 0.079 | 0.002 |
| Cyclohexane | 0.033 | 0.057 | 0.036 | 0.111 | 0.033 | 0.081 | 0.059 | 0.002 | |
| a) Gutmann, V. Ion pairing and outer sphere effect. Chimia 1977, 31, 1-7. | |||||||||
| b) These values come from Table 4. | |||||||||
| c) These values come from Table 2 in Friction 2018, 6(1): 98–115. | |||||||||
| d) These values come from Table 2 in Sur. Interface Anal. 2018, 50, 1319–1336. | |||||||||
| e) These ϕ values are in Table 1 in Sur. Interface Anal. 2018, 50, 1319–1336. | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momose, Y.; Sakurai, T.; Nakayama, K. Thermal Analysis of Photoelectron Emission (PE) and X-ray Photoelectron Spectroscopy (XPS) Data for Iron Surfaces Scratched in Air, Water, and Liquid Organics. Appl. Sci. 2020, 10, 2111. https://0-doi-org.brum.beds.ac.uk/10.3390/app10062111
Momose Y, Sakurai T, Nakayama K. Thermal Analysis of Photoelectron Emission (PE) and X-ray Photoelectron Spectroscopy (XPS) Data for Iron Surfaces Scratched in Air, Water, and Liquid Organics. Applied Sciences. 2020; 10(6):2111. https://0-doi-org.brum.beds.ac.uk/10.3390/app10062111
Chicago/Turabian StyleMomose, Yoshihiro, Takao Sakurai, and Keiji Nakayama. 2020. "Thermal Analysis of Photoelectron Emission (PE) and X-ray Photoelectron Spectroscopy (XPS) Data for Iron Surfaces Scratched in Air, Water, and Liquid Organics" Applied Sciences 10, no. 6: 2111. https://0-doi-org.brum.beds.ac.uk/10.3390/app10062111