Nanodiamonds: Synthesis and Application in Sensing, Catalysis, and the Possible Connection with Some Processes Occurring in Space
Abstract
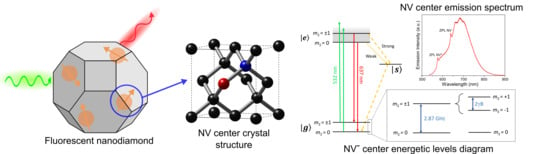
:1. Introduction
2. Nanodiamonds Synthesis Techniques
2.1. Detonation Synthesis
2.2. Chemical Vapor Deposition (CVD)
2.3. Milling of High-Pressure, High-Temperature (HPHT) Microdiamonds
2.4. Pulsed Laser Ablation
- The absorption of the high power laser pulse induces melting of the graphite surface. As the laser energy is deposited in a short timescale, the target surface rapidly becomes a superheated liquid. When the temperature of this liquid reaches a value of about 90% of the critical temperature, TC, a process known as phase explosion occurs [25], leading to the ejection of nanodroplets [42] that eventually turn into nanoparticles after solidification.
- The last step concerns the cooling of the ablation plume. Due to the confinement effect, the ablation plume dissipates excess heat very efficiently through collisional cooling with the liquid molecules, resulting in a short quenching time. The fast cooling rate is the most peculiar characteristic of laser ablation in liquid, which can be in the order of 1010–1011 K s−1 [45]. This strong and fast reduction in the temperature is enough to produce the carbon nanodroplets in an undercooling regime in a few nanoseconds. In this condition of undercooling, NDs form as a metastable phase, starting from the nanodroplets expelled by phase explosion. The transition to the thermodynamic stable allotrope of carbon, namely graphite, is prevented by the rapid quenching; the metastable phase is literally frozen under the undercooling action.
2.5. Nanodiamond Purification Methods
2.6. Synthesis of Nitrogen-Vacancy (NV)Centers-Enriched Nanodiamonds (NDs)
2.7. Fluorescent NDs Synthesis by Pulsed Laser Ablation
3. Optical Sensing with NDs
3.1. Magnetic Sensing
3.2. Electric Field Sensing
3.3. Temperature Sensing
3.4. Other Implementations of NV-NDs
4. Nanodiamonds as Catalysts
5. Possible Connections with Some Processes Occurring in Space
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Bogatyreva, G.P.; Marinich, M.A.; Ishchenko, E.V.; Gvyazdovskaya, V.L.; Bazalii, G.A.; Oleinik, N.A. Application of modified nanodiamonds as catalysts of heterogeneous and electrochemical catalyses. Phys. Solid State 2004, 46, 738–741. [Google Scholar] [CrossRef]
- Vaijayanthimala, V.; Chang, H.-C. Functionalized fluorescent nanodiamonds for biomedical applications. Nanomedicine 2009, 4, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems. Theranostics 2012, 2, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Childress, L.; Hanson, R. Diamond NV centers for quantum computing and quantum networks. MRS Bull. 2013, 38, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Grinolds, M.S.; Pham, L.M.; le Sage, D.; Luan, L.; Walsworth, R.L.; Yacoby, A. Nanoscale magnetometry with NV centers in diamond. MRS Bull. 2013, 38, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.T.; Thomaz, M.F.; Jorge, M.I.B. Luminescence decay time of the 1.945 eV centre in type Ib diamond. J. Phys. C Solid State Phys. 1983, 16, 2177–2181. [Google Scholar] [CrossRef] [Green Version]
- Doherty, M.W.; Manson, N.B.; Delaney, P.; Jelezko, F.; Wrachtrup, J.; Hollenberg, L.C.L. The nitrogen-vacancy colour centre in diamond. Phys. Rep. 2013, 528, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Degen, C.L. Scanning magnetic field microscope with a diamond single-spin sensor. Appl. Phys. Lett. 2008, 92, 243111. [Google Scholar] [CrossRef] [Green Version]
- Dolde, F.; Fedder, H.; Doherty, M.W.; Nöbauer, T.; Rempp, F.; Balasubramanian, G.; Wolf, T.; Reinhard, F.; Hollenberg, L.C.L.; Jelezko, F.; et al. Electric-field sensing using single diamond spins. Nat. Phys. 2011, 7, 459–463. [Google Scholar] [CrossRef]
- Acosta, V.M.; Bauch, E.; Ledbetter, M.P.; Waxman, A.; Bouchard, L.-S.; Budker, D. Temperature Dependence of the Nitrogen-Vacancy Magnetic Resonance in Diamond. Phys. Rev. Lett. 2010, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotoma, S.; Terada, D.; Segawa, T.F.; Igarashi, R.; Harada, Y.; Shirakawa, M. Enrichment of ODMR-active nitrogen-vacancy centres in five-nanometre-sized detonation-synthesized nanodiamonds: Nanoprobes for temperature, angle and position. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maze, J.R.; Stanwix, P.L.; Hodges, J.S.; Hong, S.; Taylor, J.M.; Cappellaro, P.; Jiang, L.; Dutt, M.V.G.; Togan, E.; Zibrov, A.S.; et al. Nanoscale magnetic sensing with an individual electronic spin in diamond. Nature 2008, 455, 644–647. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- McGuinness, L.P.; Yan, Y.; Stacey, A.; Simpson, D.A.; Hall, L.T.; Maclaurin, D.; Prawer, S.; Mulvaney, P.; Wrachtrup, J.; Caruso, F.; et al. Quantum measurement and orientation tracking of fluorescent nanodiamonds inside living cells. Nat. Nanotechnol. 2011, 6, 358–363. [Google Scholar] [CrossRef]
- Schirhagl, R.; Chang, K.; Loretz, M.; Degen, C.L. Nitrogen-Vacancy Centers in Diamond: Nanoscale Sensors for Physics and Biology. Annu. Rev. Phys. Chem. 2014, 65, 83–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, V.; Hemmer, P. Nitrogen-vacancy centers: Physics and applications. MRS Bull. 2013, 38, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, G.; Neumann, P.; Twitchen, D.; Markham, M.; Kolesov, R.; Mizuochi, N.; Isoya, J.; Achard, J.; Beck, J.; Tissler, J.; et al. Ultralong spin coherence time in isotopically engineered diamond. Nat. Mater. 2009, 8, 383–387. [Google Scholar] [CrossRef]
- Álvarez, G.A.; Bretschneider, C.O.; Fischer, R.; London, P.; Kanda, H.; Onoda, S.; Isoya, J.; Gershoni, D.; Frydman, L. Local and bulk 13C hyperpolarization in nitrogen-vacancy-centred diamonds at variable fields and orientations. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- King, J.P.; Jeong, K.; Vassiliou, C.C.; Shin, C.S.; Page, R.H.; Avalos, C.E.; Wang, H.-J.; Pines, A. Room-temperature in situ nuclear spin hyperpolarization from optically pumped nitrogen vacancy centres in diamond. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Boudou, J.-P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, V.Y. Detonation synthesis ultradispersed diamonds: Properties and applications. Russ. Chem. Rev. 2001, 70, 607–626. [Google Scholar] [CrossRef]
- Boudou, J.-P.; Tisler, J.; Reuter, R.; Thorel, A.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J. Fluorescent nanodiamonds derived from HPHT with a size of less than 10nm. Diam. Relat. Mater. 2013, 37, 80–86. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Miotello, A.; Kelly, R. Laser-induced phase explosion: New physical problems when a condensed phase approaches the thermodynamic critical temperature. Appl. Phys. A Mater. Sci. Process. 1999, 69, S67–S73. [Google Scholar] [CrossRef]
- Shirey, S.B.; Cartigny, P.; Frost, D.J.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the geology of mantle carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef] [Green Version]
- Gordon Davies, I. Properties and growth of diamond. INSPEC Publ. Inst. Electr. Eng. 1994. [Google Scholar]
- Shenderova, O.; Gruen, D.M. Preface. In Ultrananocrystalline Diamond; Elsevier: Amsterdam, The Netherlands, 2006; pp. xix–xx. [Google Scholar]
- Dolmatov, V.Y. The Influence of Detonation Synthesis Conditions on the Yield of Condensed Carbon and Detonation Nanodiamond Through the Example of Using TNT-RDX Explosive Mixture. J. Superhard Mater. 2018, 40, 290–294. [Google Scholar] [CrossRef]
- Danilenko, V.V. Synthesis, Properties and Applications of Ultrananocrystalline Diamond; Gruen, D.M., Shenderova, O.A., Vul’, A.Y., Eds.; Springer: Berlin, Germany, 2005. [Google Scholar]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef]
- Ōsawa, E. Recent progress and perspectives in single-digit nanodiamond. Diam. Relat. Mater. 2007, 16, 2018–2022. [Google Scholar] [CrossRef]
- Butler, J.E.; Sumant, A.V. The CVD of Nanodiamond Materials. Chem. Vap. Depos. 2008, 14, 145–160. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, K.-S.; Hwang, N.-M. Gas phase generation of diamond nanoparticles in the hot filament chemical vapor deposition reactor. Carbon 2016, 106, 289–294. [Google Scholar] [CrossRef]
- May, P.W.; Ashfold, M.N.R.; Mankelevich, Y.A. Microcrystalline, nanocrystalline, and ultrananocrystalline diamond chemical vapor deposition: Experiment and modeling of the factors controlling growth rate, nucleation, and crystal size. J. Appl. Phys. 2007, 101, 53115. [Google Scholar] [CrossRef] [Green Version]
- Akaishi, M.; Kanda, H.; Yamaoka, S. Synthesis of diamond from graphite-carbonate system under very high temperature and pressure. J. Cryst. Growth 1990, 104, 578–581. [Google Scholar] [CrossRef]
- Rehor, I.; Cigler, P. Precise estimation of HPHT nanodiamond size distribution based on transmission electron microscopy image analysis. Diam. Relat. Mater. 2014, 46, 21–24. [Google Scholar] [CrossRef]
- Yang, G.-W.; Wang, J.-B.; Liu, Q.-X. Preparation of nano-crystalline diamonds using pulsed laser induced reactive quenching. J. Phys. Condens. Matter 1998, 10, 7923. [Google Scholar] [CrossRef]
- Pearce, S.R.J.; Henley, S.J.; Claeyssens, F.; May, P.W.; Hallam, K.R.; Smith, J.A.; Rosser, K.N. Production of nanocrystalline diamond by laser ablation at the solid/liquid interface. Diam. Relat. Mater. 2004, 13, 661–665. [Google Scholar] [CrossRef]
- Basso, L.; Gorrini, F.; Bazzanella, N.; Cazzanelli, M.; Dorigoni, C.; Bifone, A.; Miotello, A. The modeling and synthesis of nanodiamonds by laser ablation of graphite and diamond-like carbon in liquid-confined ambient. Appl. Phys. A 2018, 124, 72. [Google Scholar] [CrossRef]
- Gorrini, F.; Cazzanelli, M.; Bazzanella, N.; Edla, R.; Gemmi, M.; Cappello, V.; David, J.; Dorigoni, C.; Bifone, A.; Miotello, A. On the thermodynamic path enabling a room-temperature, laser-assisted graphite to nanodiamond transformation. Sci. Rep. 2016, 6, 35244. [Google Scholar] [CrossRef]
- Mazzi, A.; Gorrini, F.; Miotello, A. Liquid nanodroplet formation through phase explosion mechanism in laser-irradiated metal targets. Phys. Rev. E 2015, 92, 031301. [Google Scholar] [CrossRef]
- Saito, K.; Takatani, K.; Sakka, T.; Ogata, Y.H. Observation of the light emitting region produced by pulsed laser irradiation to a solid liquid interface. Appl. Surf. Sci. 2002, 197–198, 56–60. [Google Scholar] [CrossRef]
- Fabbro, R.; Fournier, J.; Ballard, P.; Devaux, D.; Virmont, J. Physical study of laser-produced plasma in confined geometry. J. Appl. Phys. 1990, 68, 775–784. [Google Scholar] [CrossRef]
- Yan, Z.; Chrisey, D.B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Bradac, C.; Osswald, S. Effect of structure and composition of nanodiamond powders on thermal stability and oxidation kinetics. Carbon 2018, 132, 616–622. [Google Scholar] [CrossRef]
- Chiganov, A.S. Selective inhibition of the oxidation of nanodiamonds for their cleaning. Phys. Solid State 2004, 46, 620–621. [Google Scholar] [CrossRef]
- Li, L.; Davidson, J.L.; Lukehart, C.M. Surface functionalization of nanodiamond particles via atom transfer radical polymerization. Carbon 2006, 44, 2308–2315. [Google Scholar] [CrossRef]
- Shenderova, O.; Koscheev, A.; Zaripov, N.; Petrov, I.; Skryabin, Y.; Detkov, P.; Turner, S.; van Tendeloo, G. Surface Chemistry and Properties of Ozone-Purified Detonation Nanodiamonds. J. Phys. Chem. C 2011, 115, 9827–9837. [Google Scholar] [CrossRef]
- Ackermann, J.; Krueger, A. Efficient surface functionalization of detonation nanodiamond using ozone under ambient conditions. Nanoscale 2019, 11, 8012–8019. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Ha, S.; Hong, S.P.; Lee, M.; Lee, S.; Lee, S.W. Chemical purification of detonation-synthesized nanodiamond: Recycling of H2SO4 and optimization of process parameters. Mater. Today Commun. 2019, 21, 100571. [Google Scholar] [CrossRef]
- Hong, S.P.; Ha, S.W.; Lee, S.W. Atmospheric-pressure chemical purification of detonation-synthesized nanodiamond by using perchloric acid: Intensive parametric study to control sp3/sp2carbon ratio. Diam. Relat. Mater. 2018, 81, 27–32. [Google Scholar] [CrossRef]
- Aleksenskii, A. Technology of Preparation of Detonation Nanodiamond. In Detonation Nanodiamonds; Pan Stanford Publishing: Singapore, Singapore, 2014; pp. 37–72. [Google Scholar]
- Reineck, P.; Capelli, M.; Lau, D.W.M.; Jeske, J.; Field, M.R.; Ohshima, T.; Greentree, A.D.; Gibson, B.C. Bright and photostable nitrogen-vacancy fluorescence from unprocessed detonation nanodiamond. Nanoscale 2017, 9, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Teraji, T.; Zaiser, S.; Jakobi, I.; Waldherr, G.; Dolde, F.; Neumann, P.; Doherty, M.W.; Manson, N.B.; Isoya, J.; et al. Perfect alignment and preferential orientation of nitrogen-vacancy centers during chemical vapor deposition diamond growth on (111) surfaces. Appl. Phys. Lett. 2014, 104, 102407. [Google Scholar] [CrossRef] [Green Version]
- Neu, E.; Arend, C.; Gross, E.; Guldner, F.; Hepp, C.; Steinmetz, D.; Zscherpel, E.; Ghodbane, S.; Sternschulte, H.; Steinmüller-Nethl, D.; et al. Narrowband fluorescent nanodiamonds produced from chemical vapor deposition films. Appl. Phys. Lett. 2011, 98, 243107. [Google Scholar] [CrossRef] [Green Version]
- Hui, Y.Y.; Cheng, C.-A.; Chen, O.Y.; Chang, H.-C. Bioimaging and Quantum Sensing Using NV Centers in Diamond Nanoparticles. In Carbon Nanoparticles and Nanostructures; Springer International Publishing: Basel, Switzerland, 2016; pp. 109–137. [Google Scholar]
- Orwa, J.O.; Santori, C.; Fu, K.M.C.; Gibson, B.; Simpson, D.; Aharonovich, I.; Stacey, A.; Cimmino, A.; Balog, P.; Markham, M.; et al. Engineering of nitrogen-vacancy color centers in high purity diamond by ion implantation and annealing. J. Appl. Phys. 2011, 109, 83530. [Google Scholar] [CrossRef]
- Bradac, C.; Gaebel, T.; Naidoo, N.; Rabeau, J.R.; Barnard, A.S. Prediction and Measurement of the Size-Dependent Stability of Fluorescence in Diamond over the Entire Nanoscale. Nano Lett. 2009, 9, 3555–3564. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.R.; Inglis, D.W.; Sandnes, B.; Rabeau, J.R.; Zvyagin, A.V.; Gruber, D.; Noble, C.J.; Vogel, R.; Osawa, E.; Plakhotnik, T. Five-Nanometer Diamond with Luminescent Nitrogen-Vacancy Defect Centers. Small 2009, 5, 1649–1653. [Google Scholar] [CrossRef]
- Abe, E.; Sasaki, K. Tutorial: Magnetic resonance with nitrogen-vacancy centers in diamondmicrowave engineering, materials science, and magnetometry. J. Appl. Phys. 2018, 123, 161101. [Google Scholar] [CrossRef] [Green Version]
- Narayan, J.; Bhaumik, A. Novel synthesis and properties of pure and NV-doped nanodiamonds and other nanostructures. Mater. Res. Lett. 2017, 5, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Basso, L.; Gorrini, F.; Cazzanelli, M.; Bazzanella, N.; Bifone, A.; Miotello, A. An all-optical single-step process for production of nanometric-sized fluorescent diamonds. Nanoscale 2018, 10, 5738–5744. [Google Scholar] [CrossRef]
- Basso, L.; Bazzanella, N.; Cazzanelli, M.; Miotello, A. On the route towards a facile fluorescent nanodiamonds laser-synthesis. Carbon 2019, 153, 148–155. [Google Scholar] [CrossRef]
- Ghiringhelli, L.M.; Los, J.H.; Meijer, E.J.; Fasolino, A.; Frenkel, D. Modeling the Phase Diagram of Carbon. Phys. Rev. Lett. 2005, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basharin, A.Y.; Dozhdikov, V.S.; Kirillin, A.V.; Turchaninov, M.A.; Fokin, L.R. Phase diagram with a region of liquid carbon-diamond metastable states. Tech. Phys. Lett. 2010, 36, 559–562. [Google Scholar] [CrossRef]
- Ionin, A.A.; Kudryashov, S.I.; Seleznev, L.V. Near-critical phase explosion promoting breakdown plasma ignition during laser ablation of graphite. Phys. Rev. E 2010, 82. [Google Scholar] [CrossRef]
- Fu, C.-C.; Lee, H.-Y.; Chen, K.; Lim, T.-S.; Wu, H.-Y.; Lin, P.-K.; Wei, P.-K.; Tsao, P.-H.; Chang, H.-C.; Fann, W. Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc. Natl. Acad. Sci. USA 2007, 104, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.-Y.; Vaijayanthimala, V.; Cheng, C.-A.; Yeh, S.-H.; Chang, C.-F.; Li, C.-L.; Chang, H.-C. The Exocytosis of Fluorescent Nanodiamond and Its Use as a Long-Term Cell Tracker. Small 2011, 7, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.Y.; Hsiao, W.W.-W.; Haziza, S.; Simonneau, M.; Treussart, F.; Chang, H.-C. Single particle tracking of fluorescent nanodiamonds in cells and organisms. Curr. Opin. Solid State Mater. Sci. 2017, 21, 35–42. [Google Scholar] [CrossRef]
- Faklaris, O.; Garrot, D.; Joshi, V.; Druon, F.; Boudou, J.-P.; Sauvage, T.; Georges, P.; Curmi, P.A.; Treussart, F. Detection of Single Photoluminescent Diamond Nanoparticles in Cells and Study of the Internalization Pathway. Small 2008, 4, 2236–2239. [Google Scholar] [CrossRef]
- Tisler, J.; Balasubramanian, G.; Naydenov, B.; Kolesov, R.; Grotz, B.; Reuter, R.; Boudou, J.-P.; Curmi, P.A.; Sennour, M.; Thorel, A.; et al. Fluorescence and Spin Properties of Defects in Single Digit Nanodiamonds. ACS Nano 2009, 3, 1959–1965. [Google Scholar] [CrossRef]
- Mohan, N.; Tzeng, Y.-K.; Yang, L.; Chen, Y.-Y.; Hui, Y.Y.; Fang, C.-Y.; Chang, H.-C. Sub-20-nm Fluorescent Nanodiamonds as Photostable Biolabels and Fluorescence Resonance Energy Transfer Donors. Adv. Mater. 2010, 22, 843–847. [Google Scholar] [CrossRef]
- Tzeng, Y.-K.; Tsai, P.-C.; Liu, H.-Y.; Chen, O.Y.; Hsu, H.; Yee, F.-G.; Chang, M.-S.; Chang, H.-C. Time-Resolved Luminescence Nanothermometry with Nitrogen-Vacancy Centers in Nanodiamonds. Nano Lett. 2015, 15, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Neugart, F.; Zappe, A.; Jelezko, F.; Tietz, C.; Boudou, J.P.; Krueger, A.; Wrachtrup, J. Dynamics of Diamond Nanoparticles in Solution and Cells. Nano Lett. 2007, 7, 3588–3591. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.Y.; Zhang, B.; Chang, Y.-C.; Chang, C.-C.; Chang, H.-C.; Hsu, J.-H.; Chang, K.; Chang, F.-H. Two-photon fluorescence correlation spectroscopy of lipid-encapsulated fluorescent nanodiamonds in living cells. Opt. Express 2010, 18, 5896. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-R.; Lee, H.-Y.; Chen, K.; Chang, C.-C.; Tsai, D.-S.; Fu, C.-C.; Lim, T.-S.; Tzeng, Y.-K.; Fang, C.-Y.; Han, C.-C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. Nanotechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Faklaris, O.; Joshi, V.; Irinopoulou, T.; Tauc, P.; Sennour, M.; Girard, H.; Gesset, C.; Arnault, J.-C.; Thorel, A.; Boudou, J.-P.; et al. Photoluminescent Diamond Nanoparticles for Cell Labeling: Study of the Uptake Mechanism in Mammalian Cells. ACS Nano 2009, 3, 3955–3962. [Google Scholar] [CrossRef] [Green Version]
- Gorrini, F.; Giri, R.; Avalos, C.E.; Tambalo, S.; Mannucci, S.; Basso, L.; Bazzanella, N.; Dorigoni, C.; Cazzanelli, M.; Marzola, P.; et al. Fast and Sensitive Detection of Paramagnetic Species Using Coupled Charge and Spin Dynamics in Strongly Fluorescent Nanodiamonds. ACS Appl. Mater. Interfaces 2019, 11, 24412–24422. [Google Scholar] [CrossRef]
- Lai, N.D.; Zheng, D.; Treussart, F.; Roch, J.-F. Optical determination and magnetic manipulation of a single nitrogen-vacancy color center in diamond nanocrystal. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 15014. [Google Scholar]
- Kianinia, M.; Shimoni, O.; Bendavid, A.; Schell, A.W.; Randolph, S.J.; Toth, M.; Aharonovich, I.; Lobo, C.J. Robust, directed assembly of fluorescent nanodiamonds. Nanoscale 2016, 8, 18032–18037. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-C.; Hsiao, W.W.-W.; Su, M.-C. Fluorescent Nanodiamonds; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2018. [Google Scholar]
- Chapman, R.; Plakhotnik, T. Quantitative luminescence microscopy on Nitrogen-Vacancy Centres in diamond: Saturation effects under pulsed excitation. Chem. Phys. Lett. 2011, 507, 190–194. [Google Scholar] [CrossRef]
- Su, L.-J.; Wu, M.-S.; Hui, Y.Y.; Chang, B.-M.; Pan, L.; Hsu, P.-C.; Chen, Y.-T.; Ho, H.-N.; Huang, Y.-H.; Ling, T.-Y.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Hegyi, A.; Yablonovitch, E. Molecular Imaging by Optically Detected Electron Spin Resonance of Nitrogen-Vacancies in Nanodiamonds. Nano Lett. 2013, 13, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Vaijayanthimala, V.; Cheng, P.-Y.; Yeh, S.-H.; Liu, K.-K.; Hsiao, C.-H.; Chao, J.-I.; Chang, H.-C. The long-term stability and biocompatibility of fluorescent nanodiamond as an in vivo contrast agent. Biomaterials 2012, 33, 7794–7802. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Rondin, L.; Tetienne, J.-P.; Spinicelli, P.; Savio, C.D.; Karrai, K.; Dantelle, G.; Thiaville, A.; Rohart, S.; Roch, J.-F.; Jacques, V. Nanoscale magnetic field mapping with a single spin scanning probe magnetometer. Appl. Phys. Lett. 2012, 100, 153118. [Google Scholar] [CrossRef] [Green Version]
- Rugar, D.; Mamin, H.J.; Sherwood, M.H.; Kim, M.; Rettner, C.T.; Ohno, K.; Awschalom, D.D. Proton magnetic resonance imaging using a nitrogen vacancy spin sensor. Nat. Nanotechnol. 2014, 10, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.; Ropp, C.; Shapiro, B.; Taylor, J.M.; Waks, E. Scanning Localized Magnetic Fields in a Microfluidic Device with a Single Nitrogen Vacancy Center. Nano Lett. 2015, 15, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Aman, H.; Plakhotnik, T. Accuracy in the measurement of magnetic fields using nitrogen-vacancy centers in nanodiamonds. J. Opt. Soc. Am. B 2016, 33, B19. [Google Scholar] [CrossRef]
- Kayci, M.; Chang, H.-C.; Radenovic, A. Electron Spin Resonance of Nitrogen-Vacancy Defects Embedded in Single Nanodiamonds in an ABEL Trap. Nano Lett. 2014, 14, 5335–5341. [Google Scholar] [CrossRef]
- Kumar, P.; Bhattacharya, M. Magnetometry via spin-mechanical coupling in levitated optomechanics. Opt. Express 2017, 25, 19568. [Google Scholar] [CrossRef] [Green Version]
- Ziem, F.C.; Götz, N.S.; Zappe, A.; Steinert, S.; Wrachtrup, J. Highly Sensitive Detection of Physiological Spins in a Microfluidic Device. Nano Lett. 2013, 13, 4093–4098. [Google Scholar] [CrossRef]
- Kaufmann, S.; Simpson, D.A.; Hall, L.T.; Perunicic, V.; Senn, P.; Steinert, S.; McGuinness, L.P.; Johnson, B.C.; Ohshima, T.; Caruso, F.; et al. Detection of atomic spin labels in a lipid bilayer using a single-spin nanodiamond probe. Proc. Natl. Acad. Sci. USA 2013, 110, 10894–10898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinert, S.; Ziem, F.; Hall, L.T.; Zappe, A.; Schweikert, M.; Götz, N.; Aird, A.; Balasubramanian, G.; Hollenberg, L.; Wrachtrup, J. Magnetic spin imaging under ambient conditions with sub-cellular resolution. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendler, T.; Neburkova, J.; Zemek, O.; Kotek, J.; Zappe, A.; Chu, Z.; Cigler, P.; Wrachtrup, J. Optical imaging of localized chemical events using programmable diamond quantum nanosensors. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, V.R.; Aleman, B.J.; Christle, D.J.; Cleland, A.N.; Awschalom, D.D. Electron spin resonance of nitrogen-vacancy centers in optically trapped nanodiamonds. Proc. Natl. Acad. Sci. USA 2012, 109, 13493–13497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamarat, P.; Gaebel, T.; Rabeau, J.R.; Khan, M.; Greentree, A.D.; Wilson, H.; Hollenberg, L.C.L.; Prawer, S.; Hemmer, P.; Jelezko, F.; et al. Stark Shift Control of Single Optical Centers in Diamond. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [Green Version]
- Van Oort, E.; Glasbeek, M. Electric-field-induced modulation of spin echoes of N-V centers in diamond. Chem. Phys. Lett. 1990, 168, 529–532. [Google Scholar] [CrossRef]
- Kucsko, G.; Maurer, P.C.; Yao, N.Y.; Kubo, M.; Noh, H.J.; Lo, P.K.; Park, H.; Lukin, M.D. Nanometre-scale thermometry in a living cell. Nature 2013, 500, 54–58. [Google Scholar] [CrossRef]
- Toyli, D.M.; de las Casas, C.F.; Christle, D.J.; Dobrovitski, V.V.; Awschalom, D.D. Fluorescence thermometry enhanced by the quantum coherence of single spins in diamond. Proc. Natl. Acad. Sci. USA 2013, 110, 8417–8421. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.; Jakobi, I.; Dolde, F.; Burk, C.; Reuter, R.; Waldherr, G.; Honert, J.; Wolf, T.; Brunner, A.; Shim, J.H.; et al. High-Precision Nanoscale Temperature Sensing Using Single Defects in Diamond. Nano Lett. 2013, 13, 2738–2742. [Google Scholar] [CrossRef] [Green Version]
- Plakhotnik, T.; Doherty, M.W.; Cole, J.H.; Chapman, R.; Manson, N.B. All-Optical Thermometry and Thermal Properties of the Optically Detected Spin Resonances of the NV Center in Nanodiamond. Nano Lett. 2014, 14, 4989–4996. [Google Scholar] [CrossRef] [Green Version]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301. [Google Scholar] [CrossRef]
- Piñol, R.; Brites, C.D.S.; Bustamante, R.; Martinez, A.; Silva, N.J.O.; Murillo, J.L.; Cases, R.; Carrey, J.; Estepa, C.; Sosa, C.; et al. Joining Time-Resolved Thermometry and Magnetic-Induced Heating in a Single Nanoparticle Unveils Intriguing Thermal Properties. ACS Nano 2015, 9, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.L.; Verneuil, E.; Gallaire, F.; Baroud, C.N. Time-resolved temperature rise in a thin liquid film due to laser absorption. Phys. Rev. E 2009, 79. [Google Scholar] [CrossRef] [Green Version]
- Doherty, M.W.; Acosta, V.M.; Jarmola, A.; Barson, M.S.J.; Manson, N.B.; Budker, D.; Hollenberg, L.C.L. Temperature shifts of the resonances of the NV-center in diamond. Phys. Rev. B 2014, 90. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, A.; Pramanik, G.; Cai, J.-M.; Algara-Siller, G.; Kaiser, U.; Weil, T.; Tzeng, Y.-K.; Chang, H.C.; McGuinness, L.P.; Plenio, M.B.; et al. Detection of a Few Metallo-Protein Molecules Using Color Centers in Nanodiamonds. Nano Lett. 2013, 13, 3305–3309. [Google Scholar] [CrossRef]
- Sushkov, A.O.; Chisholm, N.; Lovchinsky, I.; Kubo, M.; Lo, P.K.; Bennett, S.D.; Hunger, D.; Akimov, A.; Walsworth, R.L.; Park, H.; et al. All-Optical Sensing of a Single-Molecule Electron Spin. Nano Lett. 2014, 14, 6443–6448. [Google Scholar] [CrossRef] [Green Version]
- Tchernij, S.D.; Lühmann, T.; Herzig, T.; Küpper, J.; Damin, A.; Santonocito, S.; Signorile, M.; Traina, P.; Moreva, E.; Celegato, F.; et al. Single-Photon Emitters in Lead-Implanted Single-Crystal Diamond. ACS Photonics 2018, 5, 4864–4871. [Google Scholar] [CrossRef]
- Campisciano, V.; Gruttadauria, M.; Giacalone, F. Modified Nanocarbons for Catalysis. ChemCatChem 2018, 11, 90–133. [Google Scholar] [CrossRef]
- James, D.H.; Castor, W.M. Styrene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Lee, E.H. Iron Oxide Catalysts for Dehydrogenation of Ethylbenzene in the Presence of Steam. Catal. Rev. 1974, 8, 285–305. [Google Scholar] [CrossRef]
- Zhang, J.; Su, D.S.; Blume, R.; Schlögl, R.; Wang, R.; Yang, X.; Gajović, A. Surface Chemistry and Catalytic Reactivity of a Nanodiamond in the Steam-Free Dehydrogenation of Ethylbenzene. Angew. Chem. Int. Ed. 2010, 49, 8640–8644. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.; Liu, Y.; Mu, X.; Doh, W.-H.; Nhut, J.-M.; Granger, P.; Pham-Huu, C. Macroscopic nanodiamonds/β-SiC composite as metal-free catalysts for steam-free dehydrogenation of ethylbenzene to styrene. Appl. Catal. A Gen. 2015, 499, 217–226. [Google Scholar] [CrossRef]
- Ba, H.; Podila, S.; Liu, Y.; Mu, X.; Nhut, J.-M.; Papaefthimiou, V.; Zafeiratos, S.; Granger, P.; Pham-Huu, C. Nanodiamond decorated few-layer graphene composite as an efficient metal-free dehydrogenation catalyst for styrene production. Catal. Today 2015, 249, 167–175. [Google Scholar] [CrossRef]
- Tveritinova, E.A.; Zhitnev, Y.N.; Kulakova, I.I.; Cherkasov, N.; Maslakov, K.I.; Nesterova, E.A.; Ivanov, A.S.; Savilov, S.V.; Lunin, V.V. The role of structure and surface chemistry of carbon nanomaterials in catalytic conversion of 1,2-dichloroethane. Appl. Surf. Sci. 2015, 355, 74–81. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramirez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.S. Developing a scalable artificial photosynthesis technology through nanomaterials by design. Nat. Nanotechnol. 2016, 11, 1010–1019. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photoconversion: Photocatalytic Conversion of CO2 into Renewable Hydrocarbon Fuels: State-of-the-Art Accomplishment, Challenges, and Prospects. Adv. Mater. 2014, 26, 4598. [Google Scholar] [CrossRef]
- Lum, Y.; Kwon, Y.; Lobaccaro, P.; Chen, L.; Clark, E.L.; Bell, A.T.; Ager, J.W. Trace Levels of Copper in Carbon Materials Show Significant Electrochemical CO2 Reduction Activity. ACS Catal. 2015, 6, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef]
- Nakata, K.; Ozaki, T.; Terashima, C.; Fujishima, A.; Einaga, Y. High-Yield Electrochemical Production of Formaldehyde from CO2 and Seawater. Angew. Chem. Int. Ed. 2013, 53, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Warren-Smith, R.F.; Scarrott, S.M.; Murdin, P. Peculiar optical spectrum of the Red Rectangle. Nature 1981, 292, 317–319. [Google Scholar] [CrossRef]
- Witt, A.N.; Boroson, T.A. Spectroscopy of extended red emission in reflection nebulae. Astrophys. J. 1990, 355, 182. [Google Scholar] [CrossRef]
- Chang, H.-C. Diamonds in space: A brief history and recent laboratory studies. J. Phys. Conf. Ser. 2016, 728, 62004. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://bikanta.com/products (accessed on 21 May 2020).
- Lu, H.-C.; Peng, Y.-C.; Chou, S.-L.; Lo, J.-I.; Cheng, B.-M.; Chang, H.-C. Far UV Excited Luminescence of Nitrogen-Vacancy Centers: Evidence for Diamonds in Space. Angew. Chem. Int. Ed. 2017, 56, 14469–14473. [Google Scholar] [CrossRef]
- Witt, A.N.; Vijh, U.P. Extended Red Emission: Photoluminescence by Interstellar Nanoparticles. Asp. Conf. Ser. 2004, 309, 115–138. [Google Scholar]
- Hull, C.J.; Raj, S.L.; Saykally, R.J. The liquid state of carbon. Chem. Phys. Lett. 2020, 749, 137341. [Google Scholar] [CrossRef]
- Phillips, A.C. The Physics of Stars; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ross, M. The ice layer in Uranus and Neptune—Diamonds in the sky? Nature 1981, 292, 435–436. [Google Scholar] [CrossRef]
- Jones, A.P.; Hendecourt, L.B.; Sheu, S.-Y.; Chang, H.-C.; Cheng, C.-L.; Hill, H.G.M. Surface C-H stretching features on meteoritic nanodiamonds. Astron. Astrophys. 2004, 416, 235–241. [Google Scholar] [CrossRef]
- Chen, C.-F.; Wu, C.-C.; Cheng, C.-L.; Sheu, S.-Y.; Chang, H.-C. The size of interstellar nanodiamonds revealed by infrared spectra of C-H on synthetic diamond nanocrystal surfaces. J. Chem. Phys. 2002, 116, 1211–1214. [Google Scholar] [CrossRef]
- Habart, E.; Testi, L.; Natta, A.; Carbillet, M. Diamonds in HD 97048: A Closer Look. Astrophys. J. 2004, 614, L129–L132. [Google Scholar] [CrossRef]
- Sheu, S.-Y.; Lee, I.-P.; Lee, Y.T.; Chang, H.-C. Laboratory Investigation of Hydrogenated Diamond Surfaces: Implications for the Formation and Size of Interstellar Nanodiamonds. Astrophys. J. 2002, 581, L55–L58. [Google Scholar] [CrossRef]
- Witt, A.N.; Mandel, S.; Sell, P.H.; Dixon, T.; Vijh, U.P. Extended Red Emission in High Galactic Latitude Interstellar Clouds. Astrophys. J. 2008, 679, 497–511. [Google Scholar] [CrossRef]
- Cohen, M.; Anderson, C.M.; Cowley, A.; Coyne, G.V.; Fawley, W.; Gull, T.R.; Harlan, E.A.; Herbig, G.H.; Holden, F.; Hudson, H.S.; et al. The peculiar object HD 44179 (“The red rectangle”). Astrophys. J. 1975, 196, 179. [Google Scholar] [CrossRef]
- Schmidt, G.D.; Cohen, M.; Margon, B. Discovery of optical molecular emission from the bipolar nebula surrounding HD 44179. Astrophys. J. 1980, 239, L133. [Google Scholar] [CrossRef]
- Duley, W.W. Evidence for hydrogenated amorphous carbon in the Red Rectangle. Mon. Not. R. Astron. Soc. 1985, 215, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.; van Winckel, H.; Bond, H.E.; Gull, T.R. Hubble Space TelescopeImaging of HD 44179, The Red Rectangle. Astron. J. 2004, 127, 2362–2377. [Google Scholar] [CrossRef]
- Espacenet—Patent Search. Available online: https://worldwide.espacenet.com/patent/search?q=nanodiamonds (accessed on 4 June 2020).




















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, L.; Cazzanelli, M.; Orlandi, M.; Miotello, A. Nanodiamonds: Synthesis and Application in Sensing, Catalysis, and the Possible Connection with Some Processes Occurring in Space. Appl. Sci. 2020, 10, 4094. https://0-doi-org.brum.beds.ac.uk/10.3390/app10124094
Basso L, Cazzanelli M, Orlandi M, Miotello A. Nanodiamonds: Synthesis and Application in Sensing, Catalysis, and the Possible Connection with Some Processes Occurring in Space. Applied Sciences. 2020; 10(12):4094. https://0-doi-org.brum.beds.ac.uk/10.3390/app10124094
Chicago/Turabian StyleBasso, Luca, Massimo Cazzanelli, Michele Orlandi, and Antonio Miotello. 2020. "Nanodiamonds: Synthesis and Application in Sensing, Catalysis, and the Possible Connection with Some Processes Occurring in Space" Applied Sciences 10, no. 12: 4094. https://0-doi-org.brum.beds.ac.uk/10.3390/app10124094