Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications
Abstract
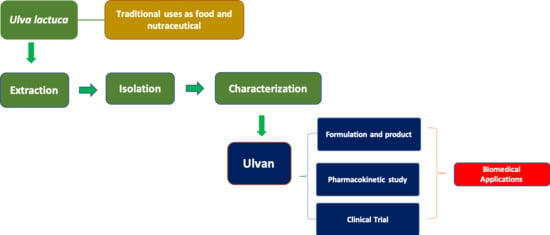
:1. Introduction
2. Methodology
3. Traditional Uses of Ulvan from Ulva sp.
4. Chemical Aspects of Ulvan from Ulva sp.
4.1. Chemistry and Physicochemical Properties
4.2. Extraction
4.3. Isolation, Purification, and Characterization
5. Current Food Applications, Available Formulation Products, and Future Potential as Food Ingredient
6. Biomedical Applications
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Cytotoxic and Anticancer Activity
6.4. Antibacterial Activity
6.5. Antiviral Activity
6.6. Tissue Engineering
6.7. Immunomodulating Activity
6.8. Antihyperlipidemic Activity
6.9. Anticoagulant Activity
7. Pharmacokinetic Studies
8. Studies Related to Toxicity
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morelli, A.; Puppi, D.; Chiellini, F. Perspectives on Biomedical Applications of Ulvan; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128098172. [Google Scholar]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Kammoun, I.; Sellem, I.; Ben Saad, H.; Boudawara, T.; Nasri, M.; Gharsallah, N.; Mallouli, L.; Amara, I. Ben Potential benefits of polysaccharides derived from marine alga Ulva lactuca against hepatotoxicity and nephrotoxicity induced by thiacloprid, an insecticide pollutant. Environ. Toxicol. 2019, 34, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, I.; Bkhairia, I.; Ben Abdallah, F.; Jaballi, I.; Ktari, N.; Boudawara, O.; Nasri, M.; Gharsallah, N.; Hakim, A.; Ben Amara, I. Potential protective effects of polysaccharide extracted from Ulva lactuca against male reprotoxicity induced by thiacloprid. Arch. Physiol. Biochem. 2017, 123, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, F.; Morelli, A. Ulvan: A Versatile Platform of Biomaterials from Renewable Resources. Biomater. Phys. Chem. 2011. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A short review on the valorization of green seaweeds and ulvan: Feedstock for chemicals and biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Guidara, M.; Yaich, H.; Richel, A.; Blecker, C.; Boufi, S.; Attia, H.; Garna, H. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int. J. Biol. Macromol. 2019, 135, 647–658. [Google Scholar] [CrossRef]
- Madhusudan, C.; Manoj, S.; Rahul, K.; Rishi, C.M. Seaweeds: A diet with nutritional, medicinal and industrial value. Res. J. Med. Plant 2011, 5, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of active poly- and oligosaccharidic fractions from Ulva sp. by combining enzyme-assisted extraction (EAE) and depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Ellatef, G.E.F.; Ahmed, O.M.; Abdel-Reheim, E.S.; Abdel-Hamid, A.H.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer Targets Ther. 2017, 9, 67–83. [Google Scholar]
- Ponce, M.; Zuasti, E.; Anguís, V.; Fernández-Díaz, C. Effects of the sulfated polysaccharide ulvan from Ulva ohnoi on the modulation of the immune response in Senegalese sole (Solea senegalensis). Fish Shellfish. Immunol. 2020, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Klongklaew, N.; Praiboon, J.; Tamtin, M.; Srisapoome, P. Antibacterial and antiviral activities of local Thai green macroalgae crude extracts in pacific white shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.; Zhang, M.; Wang, X.; Fu, X.; Guan, H.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Marine Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Carvalho, A.F.U.; Portela, M.C.C.; Sousa, M.B.; Martins, F.S.; Rocha, F.C.; Farias, D.F.; Feitosa, J.P.A. Physiological and physico-chemical characterization of dietary fibre from the green seaweed Ulva fasciata Delile. Braz. J. Biol. 2009, 69, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H.; Guégan, J.P.; Jeftić, J.; Benvegnu, T. Extracted ulvans from green algae Ulva linza of Lebanese origin and amphiphilic derivatives: Evaluation of their physico-chemical and rheological properties. J. Appl. Phycol. 2019, 31, 1931–1946. [Google Scholar] [CrossRef]
- Jmel, M.A.; Anders, N.; Ben Messaoud, G.; Marzouki, M.N.; Spiess, A.; Smaali, I. The stranded macroalga Ulva lactuca as a new alternative source of cellulose: Extraction, physicochemical and rheological characterization. J. Clean. Prod. 2019, 234, 1421–1427. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; Cerezo-Chinarro, O.; Akkerman, R.J.; Olivieri, G.; Wijffels, R.H.; Brandenburg, W.A.; Eppink, M.H.M. Biorefinery of the macroalgae Ulva lactuca: Extraction of proteins and carbohydrates by mild disintegration. J. Appl. Phycol. 2018, 30, 1281–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikionis, S.; Ioannou, E.; Toskas, G.; Roussis, V. Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO. J. Appl. Polym. Sci. 2015, 132, 42153. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of microwave-assisted extraction of polysaccharides from Ulva pertusa and evaluation of their antioxidant activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Dumbrava, A.; Berger, D.; Matei, C.; Radu, M.D.; Gheorghe, E. Characterization and applications of a new composite material obtained by green synthesis, through deposition of zinc oxide onto calcium carbonate precipitated in green seaweeds extract. Ceram. Int. 2018, 44, 4931–4936. [Google Scholar] [CrossRef]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Barthélemy, J.P.; Paquot, M.; Blecker, C.; Attia, H. Impact of extraction procedures on the chemical, rheological and textural properties of ulvan from Ulva lactuca of Tunisia coast. Food Hydrocoll. 2014, 40, 53–63. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Benelhadj, S.; Adjouman, Y.D.; Richel, A.; Blecker, C.; Sindic, M.; Boufi, S.; Attia, H.; Garna, H. Smart ulvan films responsive to stimuli of plasticizer and extraction condition in physico-chemical, optical, barrier and mechanical properties. Int. J. Biol. Macromol. 2020, 150, 714–726. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Bchir, B.; Besbes, S.; Paquot, M.; Richel, A.; Blecker, C.; Attia, H. Chemical composition and functional properties of dietary fibre extracted by Englyst and Prosky methods from the alga Ulva lactuca collected in Tunisia. Algal Res. 2015, 9, 65–73. [Google Scholar] [CrossRef]
- El Azm, N.A.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of bioactive compounds from the sulfated polysaccharides extracts of Ulva lactuca: Post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef] [Green Version]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef] [PubMed]
- Glasson, C.R.K.; Donnet, L.; Angell, A.; Vucko, M.J.; Lorbeer, A.J.; Vamvounis, G.; de Nys, R.; Magnusson, M. Multiple response optimisation of the aqueous extraction of high quality ulvan from Ulva ohnoi. Bioresour. Technol. Rep. 2019, 7, 100262. [Google Scholar] [CrossRef]
- Magnusson, M.; Glasson, C.R.K.; Vucko, M.J.; Angell, A.; Neoh, T.L.; de Nys, R. Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Res. 2019, 41, 101555. [Google Scholar] [CrossRef]
- De Carvalho, M.M.; Noseda, M.D.; Dallagnol, J.C.C.; Ferreira, L.G.; Ducatti, D.R.B.; Gonçalves, A.G.; de Freitas, R.A.; Duarte, M.E.R. Conformational analysis of ulvans from Ulva fasciata and their anticoagulant polycarboxylic derivatives. Int. J. Biol. Macromol. 2020, 162, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yang, X.; Xie, R.; Du, C.; Chi, Y.; Zhang, J.; Wang, P. Efficient production of ulvan lyase from Ulva prolifera by Catenovulum sp. LP based on stage-controlled fermentation strategy. Algal Res. 2020, 46, 101812. [Google Scholar] [CrossRef]
- Fabrowska, J.; Ibañez, E.; Łęska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- Robic, A.; Gaillard, C.; Sassi, J.F.; Leral, Y.; Lahaye, M. Ultrastructure of Ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef] [Green Version]
- Traugott, H.; Zollmann, M.; Cohen, H.; Chemodanov, A.; Liberzon, A.; Golberg, A. Aeration and nitrogen modulated growth rate and chemical composition of green macroalgae Ulva sp. cultured in a photobioreactor. Algal Res. 2020, 47, 101808. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew. Energy 2019, 132, 742–751. [Google Scholar] [CrossRef]
- Mhatre, A.; Navale, M.; Trivedi, N.; Pandit, R.; Lali, A.M. Pilot scale flat panel photobioreactor system for mass production of Ulva lactuca (Chlorophyta). Bioresour. Technol. 2018, 249, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, H.; Wang, P.; Du, C.; Ye, H.; Zuo, S.; Guan, H.; Wang, P. Structural characterization of ulvan extracted from Ulva clathrata assisted by an ulvan lyase. Carbohydr. Polym. 2020, 229, 115497. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Israel, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Integrated biorefinery process for sustainable fractionation of Ulva ohnoi (Chlorophyta): Process optimization and revenue analysis. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Sushytskyi, L.; Lukáč, P.; Synytsya, A.; Bleha, R.; Rajsiglová, L.; Capek, P.; Pohl, R.; Vannucci, L.; Čopíková, J.; Kaštánek, P. Immunoactive polysaccharides produced by heterotrophic mutant of green microalga Chlorella vulgaris G11. Carbohydr. Polym. 2020, 246, 116588. [Google Scholar] [CrossRef]
- Pilatti, F.K.; Ramlov, F.; Schmidt, E.C.; Costa, C.; de Oliveira, E.R.; Bauer, C.M.; Rocha, M.; Bouzon, Z.L.; Maraschin, M. Metabolomics of Ulva lactuca Linnaeus (Chlorophyta) exposed to oil fuels: Fourier transform infrared spectroscopy and multivariate analysis as tools for metabolic fingerprint. Mar. Pollut. Bull. 2017, 114, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Reis, S.E.; Andrade, R.G.C.; Accardo, C.M.; Maia, L.F.; Oliveira, L.F.C.; Nader, H.B.; Aguiar, J.A.K.; Medeiros, V.P. Influence of sulfated polysaccharides from Ulva lactuca L. upon Xa and IIa coagulation factors and on venous blood clot formation. Algal Res. 2020, 45, 101750. [Google Scholar] [CrossRef]
- De Araújo, I.W.; Rodrigues, J.A.; Quinderé, A.L.; Silva, J.D.; de Freitas Maciel, G.; Ribeiro, N.A.; Vanderlei, E.D.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.; et al. Analgesic and anti-inflammatory actions on bradykinin route of a polysulfated fraction from alga Ulva lactuca. Int. J. Biol. Macromol. 2016, 92, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yin, X.; Zeng, Q.; Zhu, L.; Chen, J. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int. J. Biol. Macromol. 2015, 79, 577–582. [Google Scholar] [CrossRef]
- Kientz, B.; Thabard, M.; Cragg, S.M.; Pope, J.; Hellio, C. A new method for removing microflora from macroalgal surfaces: An important step for natural product discovery. Bot. Mar. 2011, 54, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Redouan, E.; Cedric, D.; Emmanuel, P.; Mohamed, E.G.; Bernard, C.; Philippe, M.; Cherkaoui, E.M.; Josiane, C. Improved isolation of glucuronan from algae and the production of glucuronic acid oligosaccharides using a glucuronan lyase. Carbohydr. Res. 2009, 344, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of extraction conditions on the yield and purity of ulvan extracted from Ulva lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Barcellos, P.G.; Rodrigues, J.A.G.; De Queiroz, I.N.L.; De Araújo, I.W.F.; Benevides, N.M.B.; De Souza Mourão, P.A. Structural and physical-chemical analyses of sulfated polysaccharides from the sea lettuce Ulva lactuca and their effects on thrombin generation. Acta Sci. Biol. Sci. 2018, 40, e34916. [Google Scholar] [CrossRef] [Green Version]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Prado-López, S.; Lastra, M.; Grimaldi, M.; Cavazza, A.; Nasi, L.; Salviati, G.; Bigi, F. Macroalgae to nanoparticles: Study of Ulva lactuca L. role in biosynthesis of gold and silver nanoparticles and of their cytotoxicity on colon cancer cell lines. Mater. Sci. Eng. C 2019, 97, 498–509. [Google Scholar] [CrossRef]
- Al-Malki, A.L. In vitro cytotoxicity and pro-apoptotic activity of phycocyanin nanoparticles from Ulva lactuca (Chlorophyta) algae. Saudi J. Biol. Sci. 2020, 27, 894–898. [Google Scholar] [CrossRef]
- Nair, D.; Vanuopadath, M.; Nair, B.G.; Pai, J.G.; Nair, S.S. Identification and characterization of a library of surfactins and fengycins from a marine endophytic Bacillus sp. J. Basic Microbiol. 2016, 56, 1159–1172. [Google Scholar] [CrossRef]
- Buré, C.; Cacas, J.L.; Mongrand, S.; Schmitter, J.M. Characterization of glycosyl inositol phosphoryl ceramides from plants and fungi by mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Mishra, A.; Jha, B. Purification and characterization of cellulase from a marine Bacillus sp. H1666: A potential agent for single step saccharification of seaweed biomass. J. Mol. Catal. B Enzym. 2013, 93, 51–56. [Google Scholar] [CrossRef]
- Moschakis, T.; Biliaderis, C.G. Biopolymer-based coacervates: Structures, functionality and applications in food products. Curr. Opin. Colloid Interface Sci. 2017, 28, 96–109. [Google Scholar] [CrossRef]
- Costa, C.; Alves, A.; Pinto, P.R.; Sousa, R.A.; Borges Da Silva, E.A.; Reis, R.L.; Rodrigues, A.E. Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohydr. Polym. 2012, 88, 537–546. [Google Scholar] [CrossRef]
- Jmel, M.A.; Anders, N.; Ben Yahmed, N.; Marzouki, M.N.; Spiess, A.; Smaali, I. Efficient enzymatic saccharification of macroalgal biomass using a specific thermostable GH 12 endoglucanase from Aspergillus terreus JL1. World J. Microbiol. Biotechnol. 2020, 36, 5. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Guedes, É.A.C.; da Silva, T.G.; Aguiar, J.S.; de Barros, L.D.; Pinotti, L.M.; Sant’Ana, A.E.G. Cytotoxic activity of marine algae against cancerous cells. Braz. J. Pharmacogn. 2013, 23, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Kolsi, R.B.A.; Fakhfakh, J.; Krichen, F.; Jribi, I.; Chiarore, A.; Patti, F.P.; Blecker, C.; Allouche, N.; Belghith, H.; Belghith, K. Structural characterization and functional properties of antihypertensive Cymodocea nodosa sulfated polysaccharide. Carbohydr. Polym. 2016, 151, 511–522. [Google Scholar] [CrossRef]
- Ramu Ganesan, A.; Shanmugam, M.; Bhat, R. Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef]
- Morelli, A.; Massironi, A.; Puppi, D.; Creti, D.; Domingo Martinez, E.; Bonistalli, C.; Fabroni, C.; Morgenni, F.; Chiellini, F. Development of ulvan-based emulsions containing flavour and fragrances for food and cosmetic applications. Flavour Fragr. J. 2019, 34, 411–425. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Amin, H.H. Potential Using of Ulvan Polysaccharide from Ulva lactuca as a Prebiotic in Synbiotic Yogurt Production. J. Probiotics Health 2019, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. A-Mangostin Hydrogel Film Based Chitosan-Alginate for Recurrent Aphthous Stomatitis. Appl. Sci. 2019, 9, 5235. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.A.; Reis, R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Kellogg, J.; Lila, M.A. Chemical and in vitro assessment of Alaskan coastal vegetation antioxidant capacity. J. Agric. Food Chem. 2013, 61, 11025–11032. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, É. Assessment of the antioxidant and antibacterial activities of three species of edible seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Bondu, S.; Bonnet, C.; Gaubert, J.; Deslandes, É.; Turgeon, S.L.; Beaulieu, L. Bioassay-guided fractionation approach for determination of protein precursors of proteolytic bioactive metabolites from macroalgae. J. Appl. Phycol. 2015, 27, 2059–2074. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Arunkumar, K.; Carvalho, I.S. Antioxidant activity and lipid profile of three seaweeds of Faro, Portugal. Rev. Bras. Bot. 2016, 39, 9–17. [Google Scholar] [CrossRef]
- Botta, A.; Martínez, V.; Mitjans, M.; Balboa, E.; Conde, E.; Vinardell, M.P. Erythrocytes and cell line-based assays to evaluate the cytoprotective activity of antioxidant components obtained from natural sources. Toxicol. In Vitro 2014, 28, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.; El-Twab, S.A.; Hetta, M.; Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Sathivel, A.; Raghavendran, H.R.B.; Srinivasan, P.; Devaki, T. Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on d-Galactosamine induced hepatitis in rats. Food Chem. Toxicol. 2008, 46, 3262–3267. [Google Scholar] [CrossRef] [PubMed]
- Hussein, U.K.; Mahmoud, H.M.; Farrag, A.G.; Bishayee, A. Chemoprevention of Diethylnitrosamine-Initiated and Phenobarbital-Promoted Hepatocarcinogenesis in Rats by Sulfated Polysaccharides and Aqueous Extract of Ulva lactuca. Integr. Cancer Ther. 2015, 14, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Liu, D.; Lin, G.P.; Wu, Y.J.; Gao, L.Y.; Ai, C.; Huang, Y.-F.; Wang, M.F.; El-Seedi, H.R.; Chen, X.H.; et al. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.E. Biologically active steroid from the green alga Ulva lactuca. Phyther. Res. 2000, 14, 641–643. [Google Scholar] [CrossRef]
- Lin, H.C.; Tsai, W.S.; Chiu, T.H. Antioxidant properties of seven cultivated and natural edible seaweed extracts from Taiwan. J. Aquat. Food Prod. Technol. 2012, 21, 248–264. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Arsianti, A.A.; Fadilah, F.; Fatmawaty, Y.; Wibisono, L.K.; Kusmardi, S.; Azizah, N.N.; Putrianingsih, R.; Murniasih, T.; Rasyid, A.; Pangestuti, R. Phytochemical composition and anticancer activity of seaweeds Ulva lactuca and Eucheuma cottonii against breast MCF-7 and colon HCT-116 cells. Asian J. Pharm. Clin. Res. 2016, 9, 115–119. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In vitro cytotoxicity assessment of ulvan, a polysaccharide extracted from green algae. Phyther. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]
- Vonthron-Sénécheau, C.; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.M. Antiprotozoal activities of organic extracts from french marine seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef] [Green Version]
- El Amrani Zerrifi, S.; Tazart, Z.; El Khalloufi, F.; Oudra, B.; Campos, A.; Vasconcelos, V. Potential control of toxic cyanobacteria blooms with Moroccan seaweed extracts. Environ. Sci. Pollut. Res. 2019, 26, 15218–15228. [Google Scholar] [CrossRef]
- Anjali, K.P.; Sangeetha, B.M.; Devi, G.; Raghunathan, R.; Dutta, S. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): The compound characterization and functional applications in medicine—A comparative study. J. Photochem. Photobiol. B Biol. 2019, 200, 111622. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.M.; Miller-Hope, Z.; Lloyd, E.; Williams, B.S.; Bolduc, C.; Meader, J.M.; Weiss, F.; Burkholder, K.M. Antimicrobial activity of extracts from macroalgae Ulva lactuca against clinically important Staphylococci is impacted by lunar phase of macroalgae harvest. Lett. Appl. Microbiol. 2016, 62, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.P.; O’Sullivan, L.; Prieto, M.L.; Gardiner, G.E.; Lawlor, P.G.; Leonard, F.; Duggan, P.; McLoughlin, P.; Hughes, H. Extraction and bioautographic-guided separation of antibacterial compounds from Ulva lactuca. J. Appl. Phycol. 2012, 24, 513–523. [Google Scholar] [CrossRef]
- Sasikala, C.; Geetha Ramani, D. Comparative study on antimicrobial activity of seaweeds. Asian J. Pharm. Clin. Res. 2017, 10, 384–386. [Google Scholar]
- Mishra, A.K. Sargassum, Gracilaria and Ulva Exhibit Positive Antimicrobial Activity against Human Pathogens. OALib 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Habbu, P.; Warad, V.; Shastri, R.; Savant, C.; Madagundi, S.; Kekare, P. In vitro and in vivo antimicrobial activity of Ulva lactuca Linn. (green algae) associated endophytic bacterial Strains. J. Appl. Pharm. Sci. 2016, 6, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Chan, Y.L.; Li, T.L.; Wu, C.J. Inhibition of Japanese Encephalitis Virus Infection by the Sulfated Polysaccharide Extracts from Ulva lactuca. Mar. Biotechnol. 2012, 14, 468–478. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Wang, W.; Zhao, X.; Zhang, J.; Ewart, S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean Univ. China 2012, 11, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Sanina, N.; Davydova, L.; Chopenko, N.; Kostetsky, E.; Shnyrov, V. Modulation of immunogenicity and conformation of HA1 subunit of influenza a virus H1/N1 hemagglutinin in tubular immunostimulating complexes. Int. J. Mol. Sci. 2017, 18, 1895. [Google Scholar] [CrossRef] [Green Version]
- Chopenko, N.; Mazeika, A.; Davydova, L.; Stenkova, A.; Leonova, G.; Kostetsky, E.; Sanina, N. Effectivity of nanovaccine against tick-borne encephalitis. J. Phys. Conf. Ser. 2018, 1092, 012020. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Glasson, C.R.K.; Magnusson, M.; Vamvounis, G.; Sims, I.M.; Carnachan, S.M.; Hinkley, S.F.R.; Lopata, A.L.; de Nys, R.; Taki, A.C. The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. Int. J. Biol. Macromol. 2020, 150, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.A.; Alves, A.; Nunes, C.; Coimbra, M.A.; Pires, R.A.; Reis, R.L. Carboxymethylation of ulvan and chitosan and their use as polymeric components of bone cements. Acta Biomater. 2013, 9, 9086–9097. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, R.A.; Reis, R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. Part A 2013, 101, 998–1006. [Google Scholar] [CrossRef]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Della Pina, C.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef]
- Trentin, R.; Custódio, L.; Rodrigues, M.J.; Moschin, E.; Sciuto, K.; da Silva, J.P.; Moro, I. Exploring Ulva australis Areschoug for possible biotechnological applications: In vitro antioxidant and enzymatic inhibitory properties, and fatty acids contents. Algal Res. 2020, 50, 101980. [Google Scholar] [CrossRef]
- Fernandes, H.; Salgado, J.M.; Martins, N.; Peres, H.; Oliva-Teles, A.; Belo, I. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019, 281, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Del Olmo, A.; Picon, A.; Nuñez, M. The microbiota of eight species of dehydrated edible seaweeds from North West Spain. Food Microbiol. 2018, 70, 224–231. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an innovative green technology for the effective enrichment of galician algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [Green Version]
- Fumanal, M.; Di Zeo, D.E.; Anguís, V.; Fernández-Diaz, C.; Alarcón, F.J.; Piñera, R.; Albaladejo-Riad, N.; Esteban, M.A.; Moriñigo, M.A.; Balebona, M.C. Inclusion of dietary Ulva ohnoi 5% modulates Solea senegalensis immune response during Photobacterium damselae subsp. piscicida infection. Fish Shellfish Immunol. 2020, 100, 186–197. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baz, F.K.; El-Baroty, G.S. Natural preservative ingredient from marine alga Ulva lactuca L. Int. J. Food Sci. Technol. 2009, 44, 1688–1695. [Google Scholar] [CrossRef]
- Tariq, A.; Athar, M.; Ara, J.; Sultana, V.; Ehteshamul-Haque, S.; Ahmad, M. Biochemical evaluation of antioxidant activity in extracts and polysaccharide fractions of seaweeds. Glob. J. Environ. Sci. Manag. 2015, 1, 47–62. [Google Scholar]
- Wathoni, N.; Yuan Shan, C.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef] [PubMed]
- Al-Amoudi, O.A.; Mutawie, H.H.; Patel, A.V.; Blunden, G. Chemical composition and antioxidant activities of Jeddah corniche algae, Saudi Arabia. Saudi J. Biol. Sci. 2009, 16, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Vane, J.; Botting, R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987, 1, 89–96. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-ΚB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sanjivkumar, M.; Chandran, M.N.; Suganya, A.M.; Immanuel, G. Investigation on bio-properties and in-vivo antioxidant potential of carrageenans against alloxan induced oxidative stress in Wistar albino rats. Int. J. Biol. Macromol. 2020, 151, 650–662. [Google Scholar] [CrossRef]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Itoe, M.A.; Blunden, G.; Mota, M.M.; Tasdemir, D. Assessment of dual life stage antiplasmodial activity of British seaweeds. Mar. Drugs 2013, 11, 4019–4034. [Google Scholar] [CrossRef] [Green Version]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Huy, B.T.; Truong, H.B.; Bui, M.L.; Thanh, T.T.T.; Dao, D.Q. Structure analysis of sulfated polysaccharides extracted from green seaweed Ulva lactuca: Experimental and density functional theory studies. Mon. Chem. Chem. Mon. 2018, 149, 197–205. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G. Medicinal Plants for the Prevention and Treatment of Bacterial Infections. Curr. Pharm. Des. 2005, 11, 2405–2427. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.H.; Kim, S.K. Seaweed polysaccharides and their potential biomedical applications. Starch Staerke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Popa, E.G.; Reis, R.L.; Gomes, M.E. Seaweed polysaccharide-based hydrogels used for the regeneration of articular cartilage. Crit. Rev. Biotechnol. 2015, 35, 410–424. [Google Scholar] [CrossRef] [Green Version]
- Nader, N.D. Immunomodulation Mechanisms in Disease and in the Surgical Patient. Immunol. Investig. 2017, 46, 765–768. [Google Scholar] [CrossRef] [Green Version]
- Tabarsa, M.; You, S.G.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Hucker, W.; Mehran, R.; Messé, S.R.; et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants. J. Am. Coll. Cardiol. 2017, 70, 3042–3067. [Google Scholar] [CrossRef]
- Taboada, C.; Millán, R.; Míguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, G.R.; Déjean, G.; Davies, G.J.; Brumer, H. Learning from microbial strategies for polysaccharide degradation. Biochem. Soc. Trans. 2016, 44, 94–108. [Google Scholar] [CrossRef] [PubMed]



| Biomedical Applications | Test Object | Test Type | Ref |
|---|---|---|---|
| Antioxidant | 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging | in vitro | [68,75,76] |
| Oxygen radical absorbance capacity (ORAC) | in vitro | [77,78] | |
| Ferric reducing antioxidant power assays; β-carotene linoleic acid bleaching methods | in vitro | [75,79] | |
| Erythrocyte-based assays | in vitro | [80,81] | |
| AAPH assays | in vitro | ||
| Nitric oxide | in vitro | [75] | |
| Cytoprotection against H2O2-induced damage in yeast cells and zebrafish embryos | in vitro | [76] | |
| Anti-inflammatory | Mice injected with radicals such as D-Gal (500 mg/kg) | in vivo | [82,83,84] |
| Topical mice ear edema test | in vivo | [85] | |
| Use of Vero cells (anti-inflammatory effect due to infection) | in vitro | [86] | |
| Anticancer | HepG2 cells | in vitro | [31,60,87] |
| MCF-7 cells | in vitro | [31,60,88] | |
| Non-cancerous baby hamster kidney (BHK) cells | in vitro | [60] | |
| Caco-2 cells (human colon cancer) | in vitro | [88] | |
| LS174 cells (human colon carcinoma) | in vitro | [76] | |
| A549 cells (human lung carcinoma) | in vitro | ||
| Fem-x cells (malignant melanoma) | in vitro | ||
| K562 cells (chronic myelogenous leukemia) | in vitro | ||
| NCI-H292 cells (human lung mucoepidermoid carcinoma) | in vitro | [68] | |
| HeLa cells | in vitro | [87] | |
| L929 cells (mouse lung) | in vitro | [89] | |
| Mammalian L6 cells | in vivo | [90] | |
| Keratinocytes | in vivo | [80] | |
| 3T3 fibroblasts | |||
| Antibacterial | Microcystis aeruginosa (cyanobacterium) | in vitro | [91] |
| Escherichia coli, followed by Klebsiella pneumonia, and Salmonella typhi | in vivo | [92] | |
| Methicillin-resistant Staphylococcus aureus (MRSA) | in vitro | [93] | |
| Micrococcus luteus | in vitro | [77] | |
| Brochothrix thermosphacta | in vitro | ||
| E. coli | in vitro | ||
| Bacillus subtilis | in vitro | [94] | |
| Streptococcus lactis | in vitro | [95] | |
| Pseudomonas putida | in vitro | [96] | |
| Mycobacterium tuberculosis H37 RV strain | in vitro | [97] | |
| Antiviral | Japanese encephalitis virus | in vivo | [98] |
| Influenza virus | in vivo | [99] | |
| HA1 subunit of influenza A H1/N1virus | in vivo | [100] | |
| the matrix of the encephalitis antigen | in vitro | [101] | |
| Immunomodulating | makrofag murine RAW264.7 | in vitro | [102] |
| Senegalese sole | in vivo | [14] | |
| sel makrofag J774A.1 | in vitro | [103] | |
| Antihyperlipidemic | Mice induced malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) | in vitro | [18,37] |
| Anticoagulant | Activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT) | in vitro | [51] |
| Mice | in vivo | [36] | |
| Tissue engineering | ulvan can be used in cartilage tissue engineering | in vitro and in vivo | [74,104,105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications. Appl. Sci. 2020, 10, 5488. https://0-doi-org.brum.beds.ac.uk/10.3390/app10165488
Cindana Mo’o FR, Wilar G, Devkota HP, Wathoni N. Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications. Applied Sciences. 2020; 10(16):5488. https://0-doi-org.brum.beds.ac.uk/10.3390/app10165488
Chicago/Turabian StyleCindana Mo’o, Faradila Ratu, Gofarana Wilar, Hari Prasad Devkota, and Nasrul Wathoni. 2020. "Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications" Applied Sciences 10, no. 16: 5488. https://0-doi-org.brum.beds.ac.uk/10.3390/app10165488