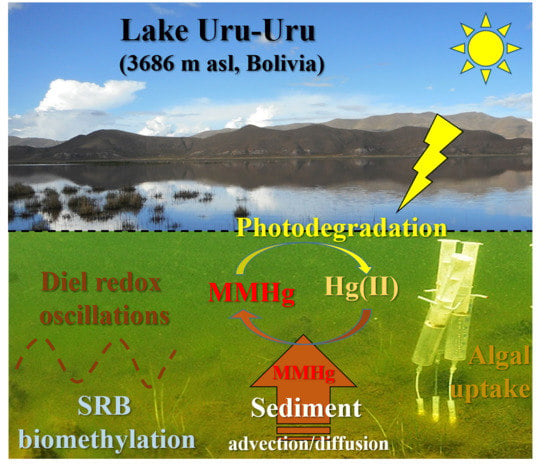
Accumulation of Methylmercury in the High-Altitude Lake Uru Uru (3686 m a.s.l, Bolivia) Controlled by Sediment Efflux and Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lake Uru Uru General Settings
2.2. Sampling Location, Strategy, and Elemental Analysis
2.3. Sediment–Water Flux Measurement and Calculation
3. Results
3.1. Diel and Seasonal Variations of Mercury Species in Surface Water
3.2. Diel and Seasonal Variations of Mercury Species in Surface Sediment Porewater
4. Discussions
4.1. DOC and Sulfides Drive MMHg Accumulation in Sediment PW and Effluxes Towards the SW
4.2. Diel and Seasonal Changes in the Hg Partitioning between Dissolved and Particulate Phases in SW
4.3. Solar Radiation Influence on MMHg Photodegradation and DGM Production in Surface Water
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, W.F.; Lamborg, C.H.; Turekian, H.D.; Holland, K.K. 11.4 - Geochemistry of Mercury in the Environment. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 91–129. [Google Scholar] [CrossRef]
- Guédron, S.; Audry, S.; Achá, D.; Bouchet, S.; Point, D.; Condom, T.; Heredia, C.; Campillo, S.; Baya, P.A.; Groleau, A.; et al. Diagenetic production, accumulation and sediment-water exchanges of methylmercury in contrasted sediment facies of Lake Titicaca (Bolivia). Sci. Total Environ. 2020, 723, 138088. [Google Scholar] [CrossRef] [PubMed]
- Guédron, S.; Huguet, L.; Vignati, D.A.L.; Liu, B.; Gimbert, F.; Ferrari, B.J.D.; Zonta, R.; Dominik, J. Tidal cycling of mercury and methylmercury between sediments and water column in the Venice Lagoon (Italy). Mar. Chem. 2012, 130–131, 1–11. [Google Scholar]
- Bouchet, S.; Amouroux, D.; Rodriguez-Gonzalez, P.; Tessier, E.; Monperrus, M.; Thouzeau, G.; Clavier, J.; Amice, E.; Deborde, J.; Bujan, S. MMHg production and export from intertidal sediments to the water column of a tidal lagoon (Arcachon Bay, France). Biogeochemistry 2013, 114, 341–358. [Google Scholar]
- Benoit, J.M.; Shull, D.H.; Harvey, R.M.; Beal, S.A. Effect of bioirrigation on sediment - water exchange of methylmercury in Boston Harbor, Massachusetts. Environ. Sci. Technol. 2009, 43, 3669–3674. [Google Scholar] [PubMed]
- Hammerschmidt, C.R.; Fitzgerald, W.F. Sediment—Water exchange of methylmercury determined from shipboard benthic flux chambers. Mar. Chem. 2008, 109, 86–97. [Google Scholar] [CrossRef]
- Hamelin, S.; Amyot, M.; Barkay, T.; Wang, Y.; Planas, D. Methanogens: Principal methylators of mercury in lake periphyton. Environ. Sci. Technol. 2014, 45, 7693–7700. [Google Scholar]
- Achá, D.; Iniguez, V.; Roulet, M.; Guimarães, J.R.D.; Luna, R.; Alanoca, L.; Sanchez, S. Sulfate-Reducing Bacteria in Floating Macrophyte Rhizospheres from an Amazonian Floodplain Lake in Bolivia and Their Association with Hg Methylation. Appl. Environ. Microbiol. 2005, 71, 7531–7535. [Google Scholar] [CrossRef] [Green Version]
- Garcia Bravo, A.; Bouchet, S.; Guédron, S.; Amouroux, D.; Dominik, J.; Zopfi, J. High methylmercury production under iron-reducing conditions in sediments impacted by sewage treatment plant discharges. Water Res. 2015, 80, 245–255. [Google Scholar]
- Kerin, E.J.; Gilmour, C.C.; Roden, E.; Suzuki, M.T.; Coates, J.D.; Mason, R.P. Mercury methylation by dissimilatory iron-reducing bacteria. Appl. Environ. Microbiol. 2006, 72, 7919–7921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Point, D.; Monperrus, M.; Tessier, E.; Amouroux, D.; Chauvaud, L.; Thouzeau, G.; Jean, F.; Amice, E.; Grall, J.; Leynaert, A.; et al. Biological control of trace metal and organometal benthic fluxes in a eutrophic lagoon (Thau Lagoon, Mediterranean Sea, France). Estuar. Coast. Shelf Sci. 2007, 72, 457–471. [Google Scholar] [CrossRef]
- Guédron, S.; Cossa, D.; Grimaldi, M.; Charlet, L. Methylmercury in tailings ponds of Amazonian gold mines (French Guiana): Field observations and an experimental flocculation method for in situ remediation. Appl. Geochem. 2011, 26, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Choe, K.Y.; Gill, G.A.; Lehman, R.D.; Han, S.; Heim, W.A.; Coale, K.H. Sediment—Water exchange of total mercury and monomethyl mercury in the San Francisco Bay - Delta. Limnol. Oceanogr. 2004, 49, 1512–1527. [Google Scholar] [CrossRef]
- Skyllberg, U. Competition among thiols and inorganic sulfides and polysulfides for Hg and MeHg in wetland soils and sediments under suboxic conditions: Illumination of controversies and implications for MeHg net production. J. Geophys. Res. Biogeosci. (2005–2012) 2008, 113, 1–14. [Google Scholar] [CrossRef]
- Bouchet, S.; Goni-Urriza, M.; Monperrus, M.; Guyoneaud, R.; Fernandez, P.; Heredia, C.; Tessier, E.; Gassie, C.; Point, D.; Guédron, S.; et al. Linking microbial activities and low molecular weight thiols to Hg methylation in biofilms and periphyton from high altitude tropical lakes (Bolivian altiplano). Environ. Sci. Technol. 2018, 52, 9758–9767. [Google Scholar] [CrossRef] [PubMed]
- Guédron, S.; Devin, S.; Vignati, D.A.L. Total and methylmercury partitioning between colloids and true solution: From case studies in sediment overlying and porewaters to a generalized model. Environ. Toxicol. Chem. 2016, 35, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Helbling, E.W.; Villafañe, V.; Buma, A.; Andrade, M.; Zaratti, F. DNA damage and photosynthetic inhibition induced by solar ultraviolet radiation in tropical phytoplankton (Lake Titicaca, Bolivia). Eur. J. Phycol. 2001, 36, 157–166. [Google Scholar] [CrossRef]
- Alanoca, L.; Amouroux, D.; Monperrus, M.; Tessier, E.; Goni, M.; Guyoneaud, R.; Achá, D.; Gassie, C.; Audry, S.; Garcia, M.; et al. Diurnal variability and biogeochemical reactivity of mercury species in an extreme high altitude lake ecosystem of the Bolivian Altiplano. Environ. Sci. Pollut. Res. 2016, 23, 6919–6933. [Google Scholar] [CrossRef]
- Alanoca, L.; Guédron, S.; Amouroux, D.; Audry, S.; Monperrus, M.; Tessier, M.; Goix, S.; Achá, D.; Seyler, P.; Point, D. Synergistic effects of mining and urban effluents on the level and distribution of methylmercury in a shallow aquatic ecosystem of the Bolivian Altiplano. Environ. Sci. Process. Impacts 2016, 18, 1550–1560. [Google Scholar] [CrossRef]
- Tapia, J.; Audry, S.; van Beek, P. Natural and anthropogenic controls on particulate metal(loid) deposition in Bolivian highland sediments, Lake Uru Uru (Bolivia). Holocene 2020, 30, 428–440. [Google Scholar] [CrossRef]
- Sarret, G.; Guédron, S.; Achá, D.; Bureau, S.; Arnaud-Godet, F.; Tisserand, D.; Goni-Urriza, M.; Gassie, C.; Duwig, C.l.; Proux, O.; et al. Extreme Arsenic Bioaccumulation Factor Variability in Lake Titicaca, Bolivia. Sci. Rep. 2019, 9, 10626. [Google Scholar] [CrossRef] [Green Version]
- Tapia, J.; Audry, S. Control of early diagenesis processes on trace metal (Cu, Zn, Cd, Pb and U) and metalloid (As, Sb) behaviors in mining-and smelting-impacted lacustrine environments of the Bolivian Altiplano. Appl. Geochem. 2013, 31, 60–78. [Google Scholar] [CrossRef]
- Guédron, S.; Grimaldi, M.; Grimaldi, C.; Cossa, D.; Tisserand, D.; Charlet, L. Amazonian former gold mined soils as a source of methylmercury: Evidence from a small scale watershed in French Guiana. Wat. Res. 2011, 45, 2659–2669. [Google Scholar] [CrossRef] [Green Version]
- Guédron, S.; Point, D.; Achá, D.; Bouchet, S.; Baya, P.A.; Molina, C.I.; Tessier, E.; Monperrus, M.; Flores, M.; Fernandez Saavedra, P.; et al. Mercury contamination level and speciation inventory in the hydrosystem of Lake Titicaca: Current status and future trends. Environ. Pollut. 2017, 231, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Bloom, N.S.; Fitzgerald, W.F. Determination of volatile mercury species at the picogram level by low-temperature gas chromatography with cold-vapor atomic fluorescence detection. Anal. Chim. Acta 1988, 208, 151–161. [Google Scholar] [CrossRef]
- Cossa, D.; Averty, B.; Pirrone, N. The origin of methylmercury in open Mediterranean waters. Limnol. Oceanogr. 2009, 54, 837–844. [Google Scholar] [CrossRef]
- Guédron, S.; Duwig, C.; Prado, B.L.; Point, D.; Flores, M.G.; Siebe, C. (Methyl) Mercury, Arsenic, and Lead Contamination of the World’s Largest Wastewater Irrigation System: The Mezquital Valley (Hidalgo State-Mexico). Water Air Soil Pollut. 2014, 225, 1–19. [Google Scholar] [CrossRef]
- Achá, D.; Guédron, S.; Amouroux, D.; Point, D.; Lazarro, X.; Fernandez, P.; Sarret, G. Algal bloom exacerbates hydrogen sulfide and methylmercury contamination in the emblematic high-altitude Lake Titicaca. Geosciences 2018, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Monperrus, M.; Gonzalez, P.R.; Amouroux, D.; Alonso, J.I.G.; Donard, O.F.X. Evaluating the potential and limitations of double-spiking species-specific isotope dilution analysis for the accurate quantification of mercury species in different environmental matrices. Anal. Bioanal. Chem. 2008, 390, 655–666. [Google Scholar] [CrossRef]
- Sharif, A.; Monperrus, M.; Tessier, E.; Bouchet, S.; Pinaly, H.; Rodriguez-Gonzalez, P.; Maron, P.; Amouroux, D. Fate of mercury species in the coastal plume of the Adour River estuary (Bay of Biscay, SW France). Sci. Total Environ. 2014, 496, 701–713. [Google Scholar] [CrossRef]
- Small, J.M.; Hintelmann, H. Methylene blue derivatization then LC-MS analysis for measurement of trace levels of sulfide in aquatic samples. Anal. Bioanal. Chem. 2007, 387, 2881–2886. [Google Scholar] [CrossRef]
- Small, J.M.; Hintelmann, H. Sulfide and mercury species profiles in two Ontario boreal shield lakes. Chemosphere 2014, 111, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A. Early Diagenesis; Princeton University Press: Princeton, NJ, USA, 1980; p. 240. [Google Scholar]
- Boudreau, B.P. The diffusive tortuosity of fine-grained unlithified sediments. Geochim. Cosmochim. Acta 1996, 60, 3139–3142. [Google Scholar] [CrossRef]
- Rothenberg, S.E.; Ambrose, R.F.; Jay, J.A. Mercury cycling in surface water, pore water and sediments of Mugu Lagoon, CA, USA. Environ. Pollut. 2008, 154, 32–45. [Google Scholar] [CrossRef]
- Dill, C.; Kuiken, T.; Zhang, H.; Ensor, M. Diurnal variation of dissolved gaseous mercury (DGM) levels in a southern reservoir lake (Tennessee, USA) in relation to solar radiation. Sci. Total Environ. 2006, 357, 176–193. [Google Scholar] [CrossRef]
- Amyot, M.; Mierle, G.; Lean, D.; McQueen, D.J. Effect of solar radiation on the formation of dissolved gaseus mercury in temperate lakes. Geochim. Cosmochim. Acta 1997, 61, 975–987. [Google Scholar] [CrossRef]
- Soerensen, A.L.; Schartup, A.T.; Gustafsson, E.; Gustafsson, B.G.; Undeman, E.; Björn, E. Eutrophication Increases Phytoplankton Methylmercury Concentrations in a Coastal Sea -¸ A Baltic Sea Case Study. Environ. Sci. Technol. 2016, 50, 11787–11796. [Google Scholar] [CrossRef]
- Lei, P.; Nunes, L.M.; Liu, Y.-R.; Zhong, H.; Pan, K. Mechanisms of algal biomass input enhanced microbial Hg methylation in lake sediments. Environ. Int. 2019, 126, 279–288. [Google Scholar] [CrossRef]
- Knorr, K.H. DOC-dynamics in a small headwater catchment as driven by redox fluctuations and hydrological flow paths—Are DOC exports mediated by iron reduction/oxidation cycles? Biogeosciences 2013, 10, 891–904. [Google Scholar] [CrossRef] [Green Version]
- Feyte, S.P.; Gobeil, C.; Tessier, A.; Cossa, D. Mercury dynamics in lake sediments. Geochim. Cosmochim. Acta 2012, 82, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Hellal, J.; Guédron, S.; Huguet, L.; Schaefer, J.; Laperche, V.; Joulian, C.; Lanceleur, L.; Burnol, A.; Ghestem, J.-P.; Garrido, F.; et al. Mercury mobilization and speciation linked to bacterial iron oxide and sulfate reduction: A column study to mimic reactive transfer in an anoxic aquifer. J. Contam. Hydrol. 2015, 180, 56–68. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P. Aspects of bioavailability of mercury for methylation in pure cultures of Desulfobulbus propionicus (1pr3). Appl. Environ. Microbiol. 2001, 67, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Gill, G.A.; Bloom, N.S.; Cappellino, S.; Driscoll, C.T.; Dobbs, C.; McShea, L.; Mason, R.; Rudd, J.W.M. Sediment-water fluxes of mercury in Lavaca Bay, Texas. Environ. Sci. Technol. 1999, 33, 663–669. [Google Scholar] [CrossRef]
- Pickhardt, P.C.; Fisher, N.S. Accumulation of Inorganic and Methylmercury by Freshwater Phytoplankton in Two Contrasting Water Bodies. Environ. Sci. Technol. 2007, 41, 125–131. [Google Scholar] [CrossRef]
- Quiroga-Flores, R.; Guédron, S.; Achá, D. High methylmercury uptake by green algae in Lake Titicaca: Potential implications for remediation. Ecotoxicol. Environ. Saf. 2020, 207, 111256. [Google Scholar] [CrossRef]
- Point, D.; Bareille, G.; Pinaly, H.; Belin, C.; Donard, O.F.X. Multielemental speciation of trace elements in estuarine waters with automated on-site UV photolysis and resin chelation coupled to inductively coupled plasma mass spectrometry. Talanta 2007, 72, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Achá, D.; Pabon, C.A.; Hintelmann, H. Mercury methylation and hydrogen sulfide production among unexpected strains isolated from periphyton of two macrophytes of the Amazon. FEMS Microbiol. Ecol. 2012, 80, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Gascon Diez, E.; Loizeau, J.-L.; Cosio, C.; Bouchet, S.; Adatte, T.; Amouroux, D.; Bravo, A.G. Role of settling particles on mercury methylation in the oxic water column of freshwater systems. Environ. Sci. Technol. 2016, 50, 11672–11679. [Google Scholar] [CrossRef]
- Audry, S.; Blanc, G.; Schäfer, J.; Chaillou, G.N.L.; Robert, S.B. Early diagenesis of trace metals (Cd, Cu, Co, Ni, U, Mo, and V) in the freshwater reaches of a macrotidal estuary. Geochim. Cosmochim. Acta 2006, 70, 2264–2282. [Google Scholar] [CrossRef]
- Wanty, R.B.; Goldhaber, M.B. Thermodynamics and kinetics of reactions involving vanadium in natural systems: Accumulation of vanadium in sedimentary rocks. Geochim. Cosmochim. Acta 1992, 56, 1471–1483. [Google Scholar] [CrossRef]
- Graham, A.M.; Cameron-Burr, K.T.; Hajic, H.A.; Lee, C.; Msekela, D.; Gilmour, C.C. Sulfurization of Dissolved Organic Matter Increases Hg-Sulfide-Dissolved Organic Matter Bioavailability to a Hg-Methylating Bacterium. Environ. Sci. Technol. 2017, 51, 9080–9088. [Google Scholar] [CrossRef]
- Von Wachenfeldt, E.; Tranvik, L.J. Sedimentation in Boreal Lakes: The Role of Flocculation of Allochthonous Dissolved Organic Matter in the Water Column. Ecosystems 2008, 11, 803–814. [Google Scholar] [CrossRef]
- Yan, J.; Lazouskaya, V.; Jin, Y. Soil colloid release affected by dissolved organic matter and redox conditions. Vadose Zone J. 2016, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lanza, W.G.; Achá, D.; Point, D.; Masbou, J.; Alanoca, L.; Amouroux, D.; Lazzaro, X. Association of a Specific Algal Group with Methylmercury Accumulation in Periphyton of a Tropical High-Altitude Andean Lake. Arch. Environ. Contam. Toxicol. 2017, 72, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moye, H.A.; Miles, C.J.; Phlips, E.J.; Sargent, B.; Merritt, K.K. Kinetics and Uptake Mechanisms for Monomethylmercury between Freshwater Algae and Water. Environ. Sci. Technol. 2002, 36, 3550–3555. [Google Scholar] [CrossRef]
- Deng, L.; Fu, D.; Deng, N. Photo-induced transformations of mercury(II) species in the presence of algae, Chlorella vulgaris. J. Hazard. Mater. 2009, 164, 798–805. [Google Scholar] [CrossRef]
- Andrade, M.; Forno, R.; Palenque, E.; Zaratti, F. Estudio preliminar del efecto de altura sobre la radiación solar ultravioleta B. Rev. Bol. Fis. 1998, 4, 14. [Google Scholar]
- Blumthaler, M.; Ambach, W.; Rehwald, W. Solar UV-A and UV-B radiation fluxes at two alpine stations at different altitudes. Theor. Appl. Climatol. 1992, 46, 39–44. [Google Scholar] [CrossRef]
- Villafae, V.E.; Andrade, M.; Lairana, V.; Zaratti, F.; Helbling, E.W. Inhibition of phytoplankton photosynthesis by solar ultraviolet radiation: Studies in Lake Titicaca, Bolivia. Freshw. Biol. 1999, 42, 215–224. [Google Scholar] [CrossRef]
- Klapstein, S.J.; O’Driscoll, N.J. Methylmercury biogeochemistry in freshwater ecosystems: A review focusing on DOM and photodemethylation. Bull. Environ. Contam. Toxicol. 2018, 100, 14–25. [Google Scholar] [CrossRef]
- Oremland, R.S.; Culbertson, C.W.; Winfrey, M.R. Methylmercury decomposition in sediments and bacterial cultures: Involvement of methanogens and sulfate reducers in oxidative demethylation. Appl. Environ. Microbiol. 1991, 57, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klapstein, S.J.; Ziegler, S.E.; O’Driscoll, N.J. Methylmercury photodemethylation is inhibited in lakes with high dissolved organic matter. Environ. Pollut. 2018, 232, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Klapstein, S.J.; Ziegler, S.E.; Risk, D.A.; O’Driscoll, N.J. Quantifying the effects of photoreactive dissolved organic matter on methylmercury photodemethylation rates in freshwaters. Environ. Toxicol. Chem. 2017, 36, 1493–1502. [Google Scholar] [CrossRef]
- Jeremiason, J.D.; Portner, J.C.; Aiken, G.R.; Hiranaka, A.J.; Dvorak, M.T.; Tran, K.T.; Latch, D.E. Photoreduction of Hg (II) and photodemethylation of methylmercury: The key role of thiol sites on dissolved organic matter. Environ. Sci. Process. Impacts 2015, 17, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Kramer, J.R.; Bell, R.A.; Werstiuk, N.H. Protonolysis of the Hg− C Bond of chloromethylmercury and dimethylmercury. A DFT and QTAIM Study. J. Phys. Chem. A 2006, 110, 9451–9458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, Y.; Li, Y.; Cai, Y.; Liu, J. Critical role of natural organic matter in photodegradation of methylmercury in water: Molecular weight and interactive effects with other environmental factors. Sci. Total Environ. 2017, 578, 535–541. [Google Scholar] [CrossRef] [PubMed]
- SENAMHI, B. Servicio Nacional de Meteorología de Hidrología. La PazBoliv. Available online: http://www.senamhi.gob.bo (accessed on 25 August 2020).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guédron, S.; Achá, D.; Bouchet, S.; Point, D.; Tessier, E.; Heredia, C.; Rocha-Lupa, S.; Fernandez-Saavedra, P.; Flores, M.; Bureau, S.; et al. Accumulation of Methylmercury in the High-Altitude Lake Uru Uru (3686 m a.s.l, Bolivia) Controlled by Sediment Efflux and Photodegradation. Appl. Sci. 2020, 10, 7936. https://0-doi-org.brum.beds.ac.uk/10.3390/app10217936
Guédron S, Achá D, Bouchet S, Point D, Tessier E, Heredia C, Rocha-Lupa S, Fernandez-Saavedra P, Flores M, Bureau S, et al. Accumulation of Methylmercury in the High-Altitude Lake Uru Uru (3686 m a.s.l, Bolivia) Controlled by Sediment Efflux and Photodegradation. Applied Sciences. 2020; 10(21):7936. https://0-doi-org.brum.beds.ac.uk/10.3390/app10217936
Chicago/Turabian StyleGuédron, Stéphane, Dario Achá, Sylvain Bouchet, David Point, Emmanuel Tessier, Carlos Heredia, Stéfany Rocha-Lupa, Pablo Fernandez-Saavedra, Marizol Flores, Sarah Bureau, and et al. 2020. "Accumulation of Methylmercury in the High-Altitude Lake Uru Uru (3686 m a.s.l, Bolivia) Controlled by Sediment Efflux and Photodegradation" Applied Sciences 10, no. 21: 7936. https://0-doi-org.brum.beds.ac.uk/10.3390/app10217936